Increased Oral Care Needs and Third Molar Symptoms in Women with Gestational Diabetes Mellitus: A Finnish Gestational Diabetes Case–Control Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Characteristics
3.2. Oral Health
3.3. Oral Health and Background Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Working Group Set Up by the Finnish Medical Society Duodecim, the Finnish Diabetes Association and the Finnish Gynecologist Association. Helsinki: The Finnish Medical Society Duodecim, Gestational Diabetes Mellitus, Current Care Guidelines. 2009. Available online: https://www.kaypahoito.fi (accessed on 30 May 2022).
- Pirkola, J.; Pouta, A.; Bloigu, A.; Miettola, S.; Hartikainen, A.-L.; Järvelin, M.-R.; Vaäräsmäki, M. Prepregnancy Overweight and Gestational Diabetes as Determinants of Subsequent Diabetes and Hypertension After 20-Year Follow-Up. Obstet. Gynecol. Surv. 2010, 65, 439–440. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- National Institute for Health and Welfare, Finland. Medical Birth Register. 2019. Available online: http://urn.fi/URN:NBN:fi-fe2020112092125 (accessed on 31 May 2022).
- Bernstein, J.; Lee-Parritz, A.; Quinn, E.; Ameli, O.; Craig, M.; Heeren, T.; Iverson, R.; Jack, B.; McCloskey, L. After Gestational Diabetes: Impact of Pregnancy Interval on Recurrence and Type 2 Diabetes. BioResearch Open Access 2019, 8, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Rayanagoudar, G.; Hashi, A.A.; Zamora, J.; Khan, K.S.; Hitman, G.A.; Thangaratinam, S. Quantification of the type 2 diabetes risk in women with gestational diabetes: A systematic review and meta-analysis of 95,750 women. Diabetologia 2016, 59, 1403–1411. [Google Scholar] [CrossRef]
- Kinane, D.F. Causation and pathogenesis of periodontal disease. Periodontology 2001, 25, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Working Group Set Up by the Finnish Medical Society Duodecim and the Finnish Dentist Society Apollonia. Helsinki: The Finnish Medical Society Duodecim Periodontitis. Current Care Guidelines. 2019. Available online: www.kaypahoito.fi (accessed on 31 May 2022).
- Kocher, T.; König, J.; Borgnakke, W.; Pink, C.; Meisel, P. Periodontal complications of hyperglycemia/diabetes mellitus: Epidemiologic complexity and clinical challenge. Periodontology 2018, 78, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidin, A.; Makrilakis, K.; Taylor, R. Periodontitis and Diabetes: A Two-Way Relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Simpson, T.C.; E Clarkson, J.; Worthington, H.V.; MacDonald, L.; Weldon, J.C.; Needleman, I.; Iheozor-Ejiofor, Z.; Wild, S.H.; Qureshi, A.; Walker, A.; et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst. Rev. 2022, 2022, 4714. [Google Scholar] [CrossRef]
- Abariga, S.A.; Whitcomb, B.W. Periodontitis and Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Observational Studies. BMC Pregnancy Childbirth 2016, 16, 344-z. [Google Scholar] [CrossRef]
- Estevens Lima, R.P.; Cyrino, R.M.; de Carvalho Dutra, B.; Oliveira da Silveira, J.; Martins, C.C.; Miranda Cota, L.O.; Costa, F.O. Association between Periodontitis and Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis. J. Periodontol. 2016, 8, 48–57. [Google Scholar] [CrossRef]
- Ventä, I.; Ylipaavalniemi, P.; Turtola, L. Clinical Outcome of Third Molars in Adults Followed during 18 Years. J. Oral Maxillofac. Surg. 2004, 62, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Moss, K.L.; Ruvo, A.T.; Offenbacher, S.; Beck, J.D.; Mauriello, S.M.; White, R.P. Third Molars and Progression of Periodontal Pathology During Pregnancy. J. Oral Maxillofac. Surg. 2007, 65, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Kornman, K.; Working Group 3 of the Joint EFP/AAP Workshop. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S164–S169. [Google Scholar] [CrossRef]
- Keikkala, E.; Mustaniemi, S.; Koivunen, S.; Kinnunen, J.; Viljakainen, M.; Männistö, T.; Ijäs, H.; Puta, A.; Kaaja, R.; Erikson, J.G.; et al. Cohort Profile: The Finnish Gestational Diabetes (FinnGeDi) Study. Int. J. Epidemiol. 2020, 49, 762–763. [Google Scholar] [CrossRef]
- Finnish Government. Goverment Decree on Maternity Clinic and Child Welfare Clinic Servicies, School and Student Health Care and Preventive Oral and Dental Care for Children and Young People. Available online: https://www.finlex.fi (accessed on 15 November 2021).
- Finnish National Maternity Guideline Committee. Maternity Guideline. Recommendations for Maternity Clinic. Available online: http://Urn.fi/URN:ISBN:978-952-245-972-5 (accessed on 16 November 2021).
- Sanz, M.; Del Castillo, A.M.; Jepsen, S.; Juanatey, J.R.G.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Working Group Set Up by the Finnish Medical Society Duodecim and the Finnish Dentist Society Apollonia. Helsinki: The Finnish Medical Society Duodecim. Caries (Management). Current Care Guidelines. Available online: https://www.kaypahoito.fi (accessed on 11 August 2022).
- Sankilampi, U.; Hannila, M.-L.; Saari, A.; Gissler, M.; Dunkel, L. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann. Med. 2013, 45, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.B.; Jeppe-Jensen, D.; Petersen, P.E. Self-reported gingival conditions and self-care in the oral health of Danish women during pregnancy. J. Clin. Periodontol. 2003, 30, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Chokwiriyachit, A.; Dasanayake, A.P.; Suwannarong, W.; Hormdee, D.; Sumanonta, G.; Prasertchareonsuk, W.; Wara-Aswapati, N.; Combellick, J.; Pitiphat, W. Periodontitis and Gestational Diabetes Mellitus in Non-Smoking Females. J. Periodontol. 2013, 84, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.R.; Romito, G.; Dib, A.S. Periodontal disease in gestational and type 1 diabetes mellitus pregnant women. Oral Dis. 2011, 17, 515–521. [Google Scholar] [CrossRef]
- Saito, T.; Shimazaki, Y.; Kiyohara, Y.; Kato, I.; Kubo, M.; Iida, M.; Yamashita, Y. Relationship between obesity, glucose tolerance, and periodontal disease in Japanese women: The Hisayama study. J. Periodontal. Res. 2005, 40, 346–353. [Google Scholar] [CrossRef]
- De Castilhos, E.D.; Horta, B.L.; Gigante, D.P.; Demarco, F.; Peres, K.G.D.A.; Peres, M.A. Association between obesity and periodontal disease in young adults: A population-based birth cohort. J. Clin. Periodontol. 2012, 39, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A.; Mirra, D.; Schütz, C.; Sculean, A.; Lang, N.P.; Walter, C.; Salvi, G.E. Bleeding on Probing as it relates to smoking status in patients enrolled in supportive periodontal therapy for at least 5 years. J. Clin. Periodontol. 2015, 42, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Working Group Set Up by the Finnish Medical Society Duodecim and the Finnish Dentist Society Apollonia. Helsinki: The Finnish Medical Society Duodecim. Third Molar. Current Care Guidelines. Available online: https://www.kaypahoito.fi (accessed on 16 November 2021).
- Szymczak-Pajor, I.; Wenclewska, S.; Śliwińska, A. Metabolic Action of Metformin. Pharmaceuticals 2022, 15, 810. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, G.E.; Sundberg, H.E.; Fjellstrom, C.A.; Wikblad, K.F. Type 2 diabetes and oral health: A comparison between diabetic and non-diabetic subjects. Diabetes Res. Clin. Pract. 2000, 50, 27–34. [Google Scholar] [CrossRef]
- World Health Organization. The 1st International Conference on Health Promotion, Ottawa. 1986. Available online: https://www.who.int/teams/health-promotion/enhanced-wellbeing/first-global-conference (accessed on 14 March 2022).
- Boggess, K.A.; Urlaub, D.M.; Massey, K.E.; Moos, M.-K.; Matheson, M.B.; Lorenz, C. Oral Hygiene Practices and Dental Service Utilization Among Pregnant Women. J. Am. Dent. Assoc. 2010, 141, 553–561. [Google Scholar] [CrossRef]
- Poulsen, H.; Meurman, J.H.; Kautiainen, H.; Heikkinen, A.M.; Huvinen, E.; Koivusalo, S.; Eriksson, J.G. Oral Health in Women with a History of High Gestational Diabetes Risk. Dent. J. 2019, 7, 92. [Google Scholar] [CrossRef] [Green Version]
- Thl.fi. Maternity Clinic. Available online: https://thl.fi/fi/web/lapset-nuoret-ja-perheet/peruspalvelut/aitiys_ja_lastenneuvola/aitiysneuvola (accessed on 15 November 2021).



| Characteristic | Controls | n | GDM | n | p Value |
|---|---|---|---|---|---|
| Age at delivery (y) (mean, SD) | 29.4 (5.0) | 935 | 32.0 (5.3) | 1030 | <0.001 |
| Pre-pregnancy BMI (kg/m2) (median, IQR) | 22.8 (20.8–25.6) | 935 | 26.0 (23.8–31.6) | 1029 | <0.001 |
| Weight gain during pregnancy (kg) (mean, SD) | 14.9 (5.0) | 908 | 12.4 (5.7) | 948 | <0.001 |
| Parity (median, IQR) | 0 (0–1) | 935 | 1 (0–2) | 1030 | <0.05 |
| Primiparous | 475 (50.8%) | 935 | 437 (42.4%) | 1030 | <0.001 |
| Early-onset GDM a | - | - | 295 (28.6%) | 976 | |
| Smoking during pregnancy | 143 (15.3%) | 935 | 166 (16.1%) | 1030 | >0.05 F |
| Education | 935 | 1030 | <0.05 F | ||
| Basic or less | 42 (4.5%) | 68 (6.6%) | |||
| Secondary | 426 (45.6%) | 486 (47.2%) | |||
| Lower-level tertiary | 231 (24.7%) | 270 (26.2%) | |||
| Upper-level tertiary | 236 (25.2%) | 206 (20.0%) | |||
| Asthma | 77 (8.6%) | 900 | 112 (11.4%) | 981 | <0.05 F |
| Insomnia and/or mental disorders | 102 (11.3%) | 905 | 142 (14.5%) | 977 | <0.05 F |
| Chronic hypertension b | 47 (5.0%) | 935 | 168 (16.3%) | 1029 | <0.001 F |
| Gestational hypertension c and/or pre-eclampsia d | 177 (16.6%) | 935 | 304 (26.6%) | 1029 | <0.001 F |
| Gestational age at delivery (weeks) (median, IQR) | 40.3 (39.4–41.1) | 935 | 39.7 (38.7–40.6) | 1030 | <0.001 |
| Induction of labor | 342 (32.1%) | 935 | 515 (44.9%) | 1030 | <0.001 F |
| Cesarean section | 116 (12.4%) | 935 | 212 (20.6%) | 1030 | <0.001 F |
| Mean birth weight (SD) (g) (mean, SD) | 3600 (496) | 935 | 3700 (507) | 1030 | <0.05 |
| Birth weight SD score e (mean, SD) | −0.10 (0.98) | 935 | 0.25 (1.11) | 1030 | <0.001 |
| LGA e | 28 (2.6%) | 935 | 64 (5.6%) | 1030 | <0.001 F |
| Characteristic | Controls | n | Pharmacologically Treated GDM f | n | Diet-Treated GDM | n |
|---|---|---|---|---|---|---|
| Age at delivery (y) (mean, SD) | 29.4 (5.0) | 935 | 33.7 (5.5) */‡ | 196 | 31.7 (5.3) * | 805 |
| Pre-pregnancy BMI (kg/m2) (median, IQR) | 22.8 (20.8–25.6) | 935 | 29.0 (24.6–34.4) */‡ | 196 | 26.6 (23.6–30.9) * | 804 |
| Weight gain during pregnancy (kg) (mean, SD) | 14.9 (5.0) | 908 | 10.7 (6.3) */‡ | 167 | 12.7 (5.5) * | 756 |
| Parity (median, IQR) | 0 (0–1) | 935 | 1 (0–2) */§ | 196 | 0 (0–2) | 805 |
| Primiparous | 475 (50.8%) | 935 | 65 (33.2%) */§/F/F | 196 | 362 (45.0%) †/F | 805 |
| Early-onset GDM a | - | - | 119 (58.9%) ‡/F | 177 | 202 (23.5%) | 776 |
| Smoking during pregnancy | 143 (15.3%) | 935 | 26 (13.3%) F/F | 196 | 136 (16.9%) | 805 |
| Education | 935 | 196 | F | 805 | ||
| Basic or less | 42 (4.5%) | 13 (6.6%) | 51 (6.3%) | |||
| Secondary | 426 (45.6%) | 108 (55.1%) | 363 (45.1%) | |||
| Lower-level tertiary | 231 (24.7%) | 42 (21.4%) | 221 (27.5%) | |||
| Upper-level tertiary | 236 (25.2%) | 33 (16.8%) | 170 (21.1%) | |||
| Asthma | 77 (8.6%) | 900 | 23 (12.2%) | 189 | 86 (11.2%) | 766 |
| Insomnia and/or mental disorders | 102 (11.3%) | 905 | 37 (19.5%) †/§ | 190 | 103 (13.5%) | 761 |
| Chronic hypertension b | 47 (5.0%) | 935 | 36 (18.4%) * | 196 | 123 (15.3%) */F | 804 |
| Gestational hypertension c and/or pre-eclampsia d | 177 (16.6%) | 935 | 61 (27.6%) * | 196 | 234 (26.2%) */F | 804 |
| Gestational age at delivery (weeks) (median, IQR) | 40.3 (39.4−41.1) | 935 | 39.1 (38.3–39.8) */‡ | 196 | 39.9 (39.0−40.7) * | 805 |
| Induction of labor | 342 (32.1%) | 935 | 143 (64.7%) */‡/F/F | 196 | 355 (39.6%) * | 805 |
| Cesarean section | 116 (12.4%) | 935 | 51 (26.0%) */‡ | 196 | 152 (18.9%) */F | 805 |
| Mean birth weight (SD) (g) (mean, SD) | 3600 (496) | 935 | 3700 (494) † | 196 | 3600 (501) † | 805 |
| Birth weight SD score e (mean, SD) | −0.10 (0.98) | 935 | 0.53 (1.30) */‡ | 196 | 0.19 (1.03) * | 805 |
| LGA e | 28 (2.6%) | 935 | 25 (11.3%) */‡ | 196 | 36 (4.0%) F | 805 |
| Characteristic | Controls | n | Recurrent GDM f | n | First-Onset GDM g | n |
|---|---|---|---|---|---|---|
| Age at delivery (years) (mean, SD) | 29.4 (5.0) | 935 | 33.8 (5.5) */‡ | 233 | 31.5 (5.2) * | 797 |
| Pre-pregnancy BMI (kg/m2) (median, IQR) | 22.8 (20.8–25.6) | 935 | 28.4 (24.8−33.2) */‡ | 233 | 26.6 (23.5−31.0) * | 796 |
| Weight gain during pregnancy (kg) (mean, SD) | 14.9 (5.0) | 908 | 11.2 (5.7) */§ | 204 | 12.7 (5.7) * | 744 |
| Parity (median, IQR) | 0 (0−1) | 935 | 2 (1−3) */‡ | 233 | 0 (0−1) † | 797 |
| Primiparous | 475 (50.8%) | 935 | 1 (0.4%) */‡/F/F | 233 | 436 (54.7%) | 797 |
| Early-onset GDM a | - | 141 (60.5%) ‡/F | 211 | 154 (19.3%) | 765 | |
| Smoking during pregnancy | 143 (15.3%) | 935 | 30 (12.9%) F/F | 233 | 136 (17.1%) F | 797 |
| Education | 935 | 233 | F | 797 | ||
| Basic or less | 42 (4.5%) | 17 (7.3%) | 51 (6.4%) | |||
| Secondary | 426 (45.6%) | 129 (55.4%) | 357 (44.8%) | |||
| Lower-level tertiary | 231 (24.7%) | 51 (21.9%) | 219 (27.5%) | |||
| Upper-level tertiary | 236 (25.2%) | 36 (15.5%) | 170 (21.3%) | |||
| Asthma | 77 (8.6%) | 900 | 22 (10.0%) F | 220 | 90 (11.8%) † | 761 |
| Insomnia and/or mental disorders | 102 (11.3%) | 905 | 31 (14.0%) F | 221 | 111 (14.7%) † | 756 |
| Chronic hypertension b | 47 (5.0%) | 935 | 40 (17.2%) * | 232 | 128 (16.1%) */F | 797 |
| Gestational hypertension c and/or pre-eclampsia d | 177 (16.6%) | 935 | 56 (22.1%) †/F | 232 | 224 (28.1%) */F | 797 |
| Gestational age at delivery (weeks) (median, IQR) | 40.3 (39.4−41.1) | 935 | 39.3 (38.3−40.1) */‡ | 233 | 39.7 (38.9−40.6) * | 797 |
| Induction of labor | 342 (32.1%) | 935 | 137 (53.9%) */‡ | 233 | 331 (41.5%) * | 797 |
| Cesarean section | 116 (12.4%) | 935 | 29 (12.4%) ‡/F | 233 | 183 (23.0%) */F | 797 |
| Mean birth weight (SD) (g) (mean, SD) | 3600 (496) | 935 | 3700 (485) */§ | 233 | 3700 (503) † | 797 |
| Birth weight SD score e (mean, SD) | −0.10 (0.98) | 935 | 0.39 (1.11) * | 233 | 0.23 (1.12) * | 797 |
| LGA e | 28 (2.6%) | 935 | 21 (8.3%) * | 233 | 40 (5.0%) †/F | 797 |
| Parameters | Control (n = 935) | GDM (n = 1030) | Subgroups of GDM | |||
|---|---|---|---|---|---|---|
| Pharmacologically Treated GDM a (n = 196) | Diet-Treated GDM (n = 805) | Recurrent GDM b (n = 233) | First-Onset GDM c (n = 797) | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
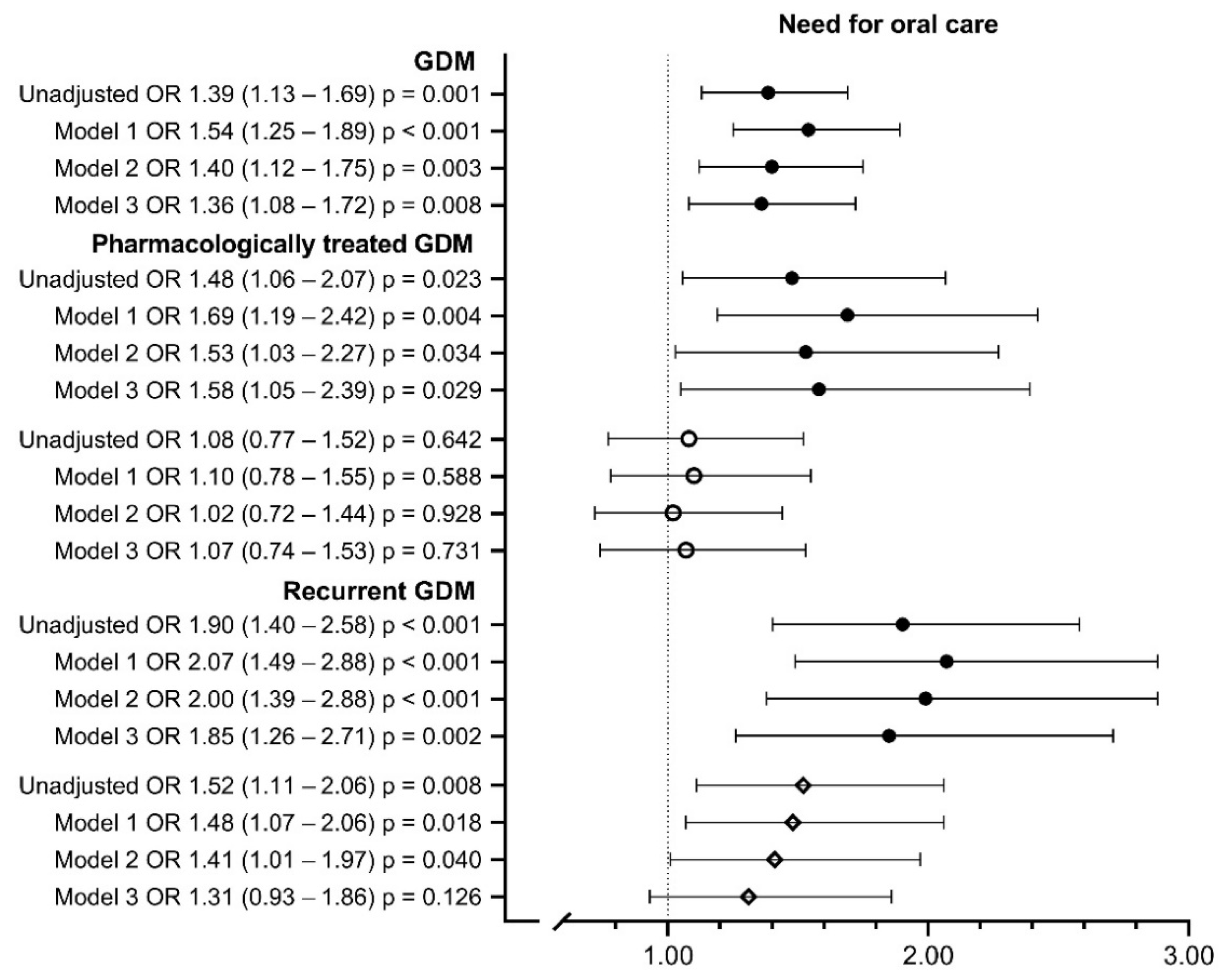
| Need for oral care | ||||||
| High or intermediate | 229 (24.5%) | 319 (31.1%) */F | 63 (32.5%) * | 247 (30.7%) * | 88 (38.1%) †/‡ | 231 (29%) * |
| Low, very low or no | 698 (74.7%) | 702 (68.4%) | 130 (67%) | 552 (68.7%) | 141 (61%) | 561 (70.5%) |
| Cannot say | 7 (0.7%) | 6 (0.6%) | 1 (0.5%) | 5 (0.6%) | 2 (0.9%) | 4 (0.5%) |
| Total | 934 (100%) | 1027 (100%) | 194 (100%) | 804 (100%) | 231 (100%) | 796 (100%) |
| Removed third molar | ||||||
| Mean (SD) | 1.8 (1.6) | 2.0 (1.6) * | 2.1 (1.6) * | 2.0 (1.6) * | 2.3 (1.6) †/§ | 1.9 (1.6) |
| Yes | 581 (62.4%) | 694 (67.6%) * | 135 (68.9%) */F/F | 539 (67.2%) */F | 170 (73.3%) */‡/F/F | 524 (65.9%) F |
| No | 344 (36.9%) | 323 (31.4%) | 58 (29.6%) | 256 (31.9%) | 60 (25.9%) | 263 (33.1%) |
| Cannot say | 6 (0.6%) | 10 (1%) | 3 (1.5%) | 7 (0.9%) | 2 (0.9%) | 8 (1%) |
| Total | 931 (100%) | 1027 (100%) | 196 (100%) | 802 (100%) | 232 (100%) | 795 (100%) |
| Third molar symptoms | ||||||
| Yes | 335 (36.1%) | 410 (39.9%) F | 92 (46.9%) */‡ | 305 (38%) | 96 (41.2%) | 314 (39.5%) |
| No | 567 (61.2%) | 594 (57.8%) | 99 (50.5%) | 482 (60.1%) | 130 (55.8%) | 464 (58.4%) |
| Cannot say | 25 (2.7%) | 23 (22.2%) | 5 (2.6%) | 15 (1.9%) | 7 (3%) | 16 (2%) |
| Total | 925 (100%) | 1027 (100%) | 196 (100%) | 802 (100%) | 233 (100%) | 794 (100%) |
| Gingival bleeding | ||||||
| Weekly or more often | 90 (9.6%) | 107 (10.4%) F | 21 (10.7%) | 81 (10.1%) | 19 (8.2%) F/F | 88 (11.1%) |
| Rarely | 837 (89.7%) | 910 (88.8%) | 175 (89.3%) | 712 (89.0%) | 212 (91.4%) | 698 (88.0%) |
| Cannot say | 6 (0.6%) | 8 (0.8%) | 0 (0) | 7 (0.9%) | 1 (0.4%) | 7 (0.9%) |
| Total | 933 (100%) | 1025 (100%) | 196 (100%) | 800 (100%) | 232 (100%) | 793 (100%) |
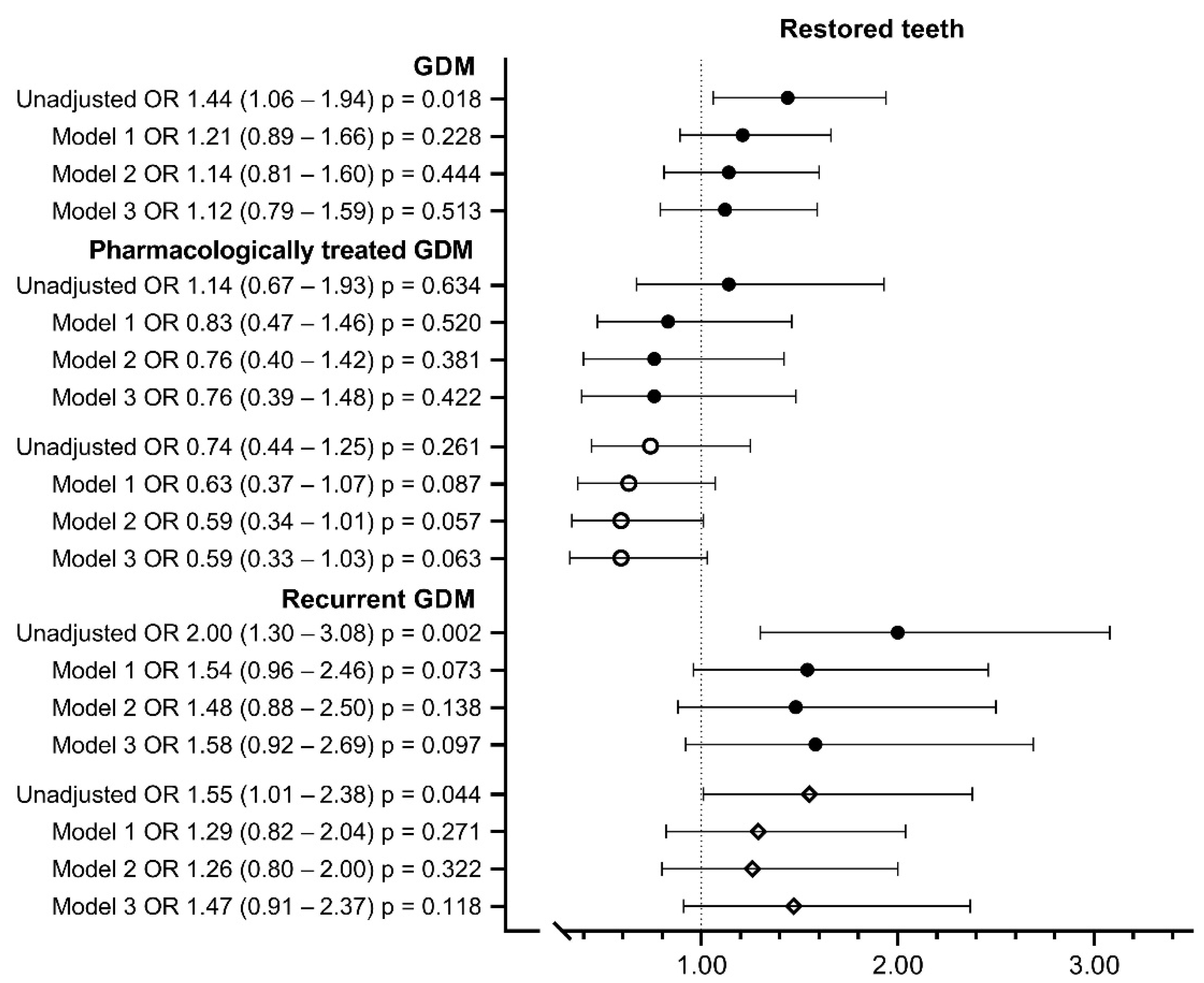
| Restored teeth | ||||||
| >10 | 78 (8.4%) | 120 (11.7%) */F | 19 (9.8%) F | 99 (12.3%) */F | 35 (15.2%) * | 85 (10.7%) |
| 0–10 | 784 (85.2%) | 839 (81.7%) | 168 (86.6%) | 650 (81%) | 176 (76.2%) | 663 (83.4%) |
| Cannot say | 69 (7.4%) | 67 (6.5%) | 7 (3.6%) | 54 (6.7%) | 20 (8.7%) | 47 (5.9%) |
| Total | 931 (100%) | 1026 (100%) | 195 (100%) | 803 (100%) | 231 (100%) | 795 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pukkila, J.; Mustaniemi, S.; Lingaiah, S.; Lappalainen, O.-P.; Kajantie, E.; Pouta, A.; Kaaja, R.; Eriksson, J.G.; Laivuori, H.; Gissler, M.; et al. Increased Oral Care Needs and Third Molar Symptoms in Women with Gestational Diabetes Mellitus: A Finnish Gestational Diabetes Case–Control Study. Int. J. Environ. Res. Public Health 2022, 19, 10711. https://doi.org/10.3390/ijerph191710711
Pukkila J, Mustaniemi S, Lingaiah S, Lappalainen O-P, Kajantie E, Pouta A, Kaaja R, Eriksson JG, Laivuori H, Gissler M, et al. Increased Oral Care Needs and Third Molar Symptoms in Women with Gestational Diabetes Mellitus: A Finnish Gestational Diabetes Case–Control Study. International Journal of Environmental Research and Public Health. 2022; 19(17):10711. https://doi.org/10.3390/ijerph191710711
Chicago/Turabian StylePukkila, Jenni, Sanna Mustaniemi, Shilpa Lingaiah, Olli-Pekka Lappalainen, Eero Kajantie, Anneli Pouta, Risto Kaaja, Johan G. Eriksson, Hannele Laivuori, Mika Gissler, and et al. 2022. "Increased Oral Care Needs and Third Molar Symptoms in Women with Gestational Diabetes Mellitus: A Finnish Gestational Diabetes Case–Control Study" International Journal of Environmental Research and Public Health 19, no. 17: 10711. https://doi.org/10.3390/ijerph191710711
APA StylePukkila, J., Mustaniemi, S., Lingaiah, S., Lappalainen, O.-P., Kajantie, E., Pouta, A., Kaaja, R., Eriksson, J. G., Laivuori, H., Gissler, M., Vääräsmäki, M., & Keikkala, E. (2022). Increased Oral Care Needs and Third Molar Symptoms in Women with Gestational Diabetes Mellitus: A Finnish Gestational Diabetes Case–Control Study. International Journal of Environmental Research and Public Health, 19(17), 10711. https://doi.org/10.3390/ijerph191710711






