Progress in the Preparation and Modification of Zinc Ferrites Used for the Photocatalytic Degradation of Organic Pollutants
Abstract
:1. Introduction
2. Synthesis of ZnFe2O4
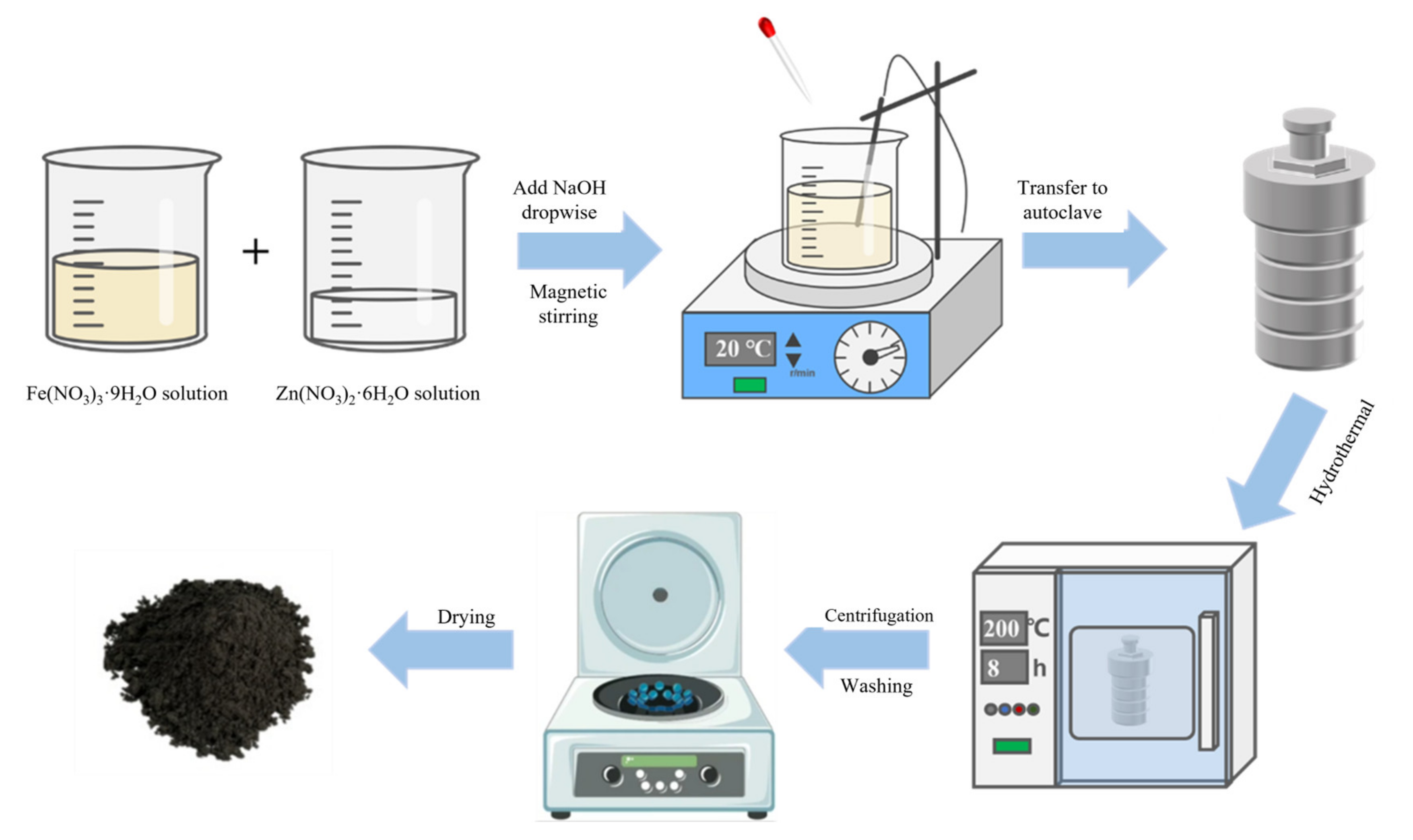
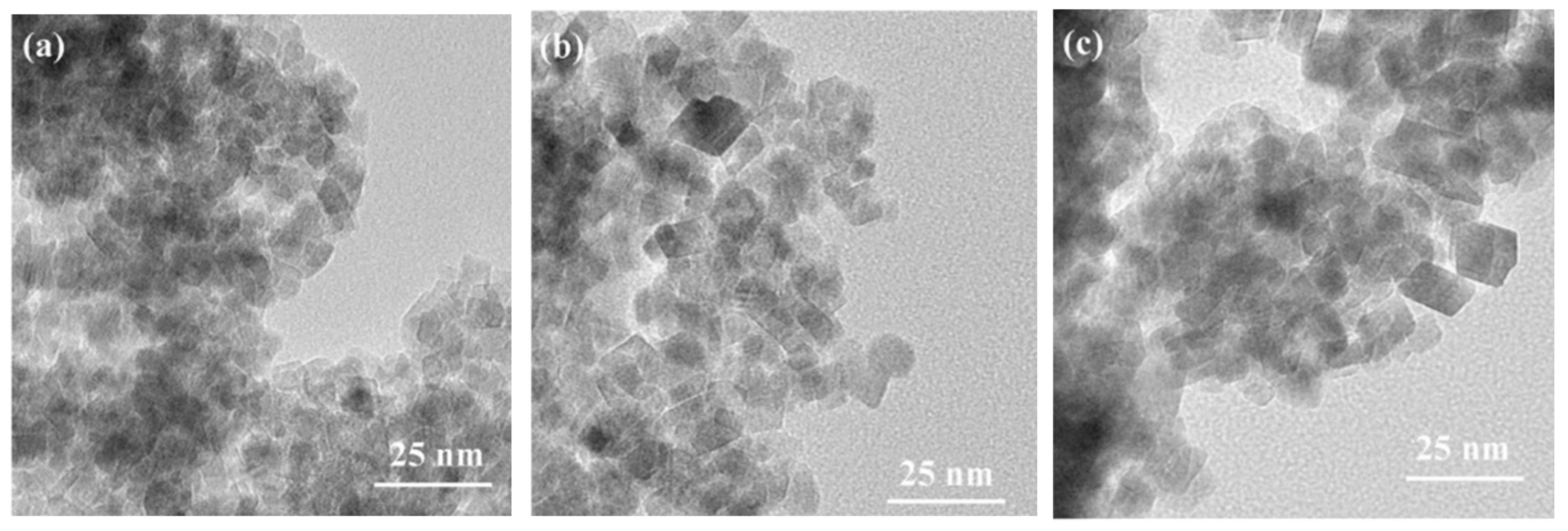
2.1. Hydrothermal Synthesis/Solvothermal Process
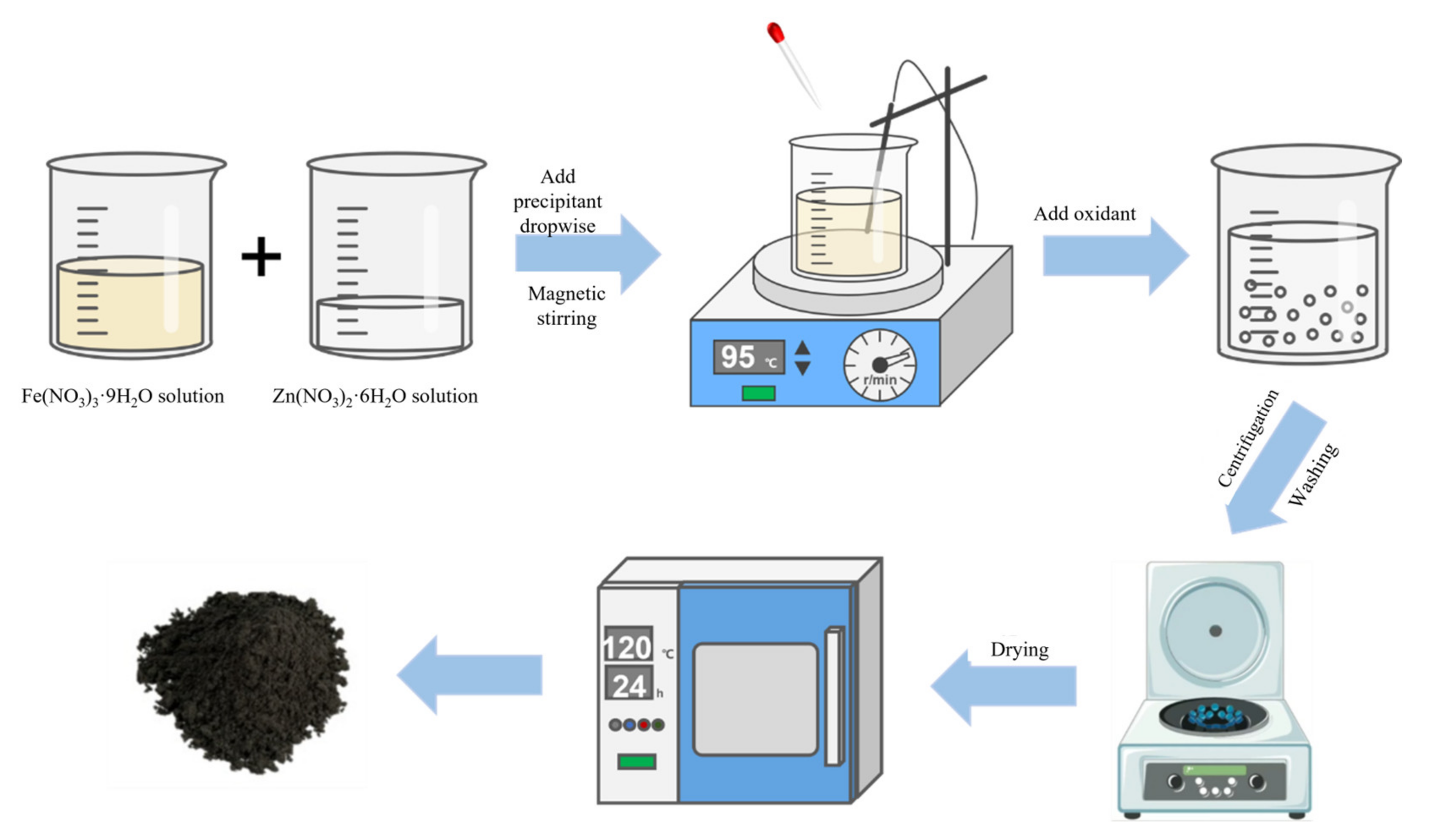
2.2. Co-Precipitation Technique
2.3. Sol–Gel Method
2.4. Biosynthesis Method
2.5. Other Synthesis Methods
2.5.1. Microwave-Assisted Method
2.5.2. Ball Milling Method
2.5.3. Sonochemical Method
3. Modification of ZnFe2O4-Based Photocatalysts
3.1. Elemental Doping
3.2. Composite Modification
3.2.1. Composites of ZnFe2O4 and Conducting Polymers
3.2.2. ZnFe2O4 Composite Using Metal Oxides
3.2.3. ZnFe2O4 Composite Using Metal Sulfides
3.2.4. ZnFe2O4 Composites Using Carbon-Based Materials
3.3. Morphological Modification
3.3.1. One-Dimensional ZnFe2O4
3.3.2. Two-Dimensional ZnFe2O4
3.3.3. Three-Dimensional ZnFe2O4
3.4. Pollutant Degradation Performance of Photocatalysts Prepared Using Different Modification Methods
3.4.1. Organic Dyes
3.4.2. Phenols
3.4.3. Antibiotics
4. Conclusions
- (1)
- Developing innovative preparation methods. Hydrothermal, co-precipitation, and sol–gel methods are relatively mature, but they are expensive and energy-intensive. Expanding the biosynthesis concept to each preparation method and the development of green methods could reduce the particle size of the nanoparticles and improve their active sites at the same time, with low cost, simple operation, and no chemical pollution. It is possible to improve the biosynthesis methods by studying trace minerals or enzymes, and further research into the synthesis mechanisms can be conducted based on the enzymes and minerals present in plants.
- (2)
- Expanding materials in a modified way. In order to better suit the practical applications, a variety of methods such as doping and composite modification may be used to improve the performance and stability of single ZnFe2O4 photocatalysts. By doping different elements with defects or combining them with strong redox-capable nanomaterials, heterojunctions can be formed to adjust the material band gap, improve carrier separation, and reduce carrier recombination, thereby improving the photochemical performance.
- (3)
- There is a need for further investigation into the light absorption mechanisms of different morphologies. At present, the research on the morphological modification of ZnFe2O4 is relatively limited, which mainly focuses on one-dimensional modification. Some studies have shown that the selection of different dimensional modifications leads to an exponential increase in the light absorption capacity. An in-depth understanding of the light absorption and photocatalytic mechanisms of multidimensional materials is needed. Research can be conducted on a number of excellent three-dimensional structures such as hollow multi-shell structures, nanosheet arrays, etc.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, A.; Kumari, M.; Swati; Kumar, R.; Iqbal, K.; Thakur, I.S. Persistent organic pollutants in the environment: Risk assessment, hazards, and mitigation strategies. Bioresour. Technol. Rep. 2022, 19, 101143. [Google Scholar] [CrossRef]
- Liu, H.; Ren, X.; Chen, L. Synthesis and characterization of magnetic metal–organic framework for the adsorptive removal of Rhodamine B from aqueous solution. J. Ind. Eng. Chem. 2016, 34, 278–285. [Google Scholar] [CrossRef]
- Sellaoui, L.; Kehili, M.; Lima, E.C.; Thue, P.S.; Bonilla-Petriciolet, A.; Lamine, A.B.; Dotto, G.L.; Erto, A. Adsorption of phenol on microwave-assisted activated carbons: Modelling and interpretation. J. Mol. Liq. 2019, 274, 309–314. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef]
- Garcia-Rosero, H.; Romero-Cano, L.A.; Aguilar-Aguilar, A.; Bailon-Garcia, E.; Carvalho, A.P.; Perez-Cadenas, A.F.; Carrasco-Marin, F. Adsorption and thermal degradation of Atenolol using carbon materials: Towards an advanced and sustainable drinking water treatment. J. Water Process Eng. 2022, 49, 102987. [Google Scholar] [CrossRef]
- Rao, T.; Ma, X.; Yang, Q.; Cheng, S.; Ren, G.; Wu, Z.; Sires, I. Upgrading the peroxi-coagulation treatment of complex water matrices using a magnetically assembled mZVI/DSA anode: Insights into the importance of ClO radical. Chemosphere 2022, 303 Pt 1, 134948. [Google Scholar] [CrossRef]
- Sayin, F.E.; Karatas, O.; Ozbay, I.; Gengec, E.; Khataee, A. Treatment of real printing and packaging wastewater by combination of coagulation with Fenton and photo-Fenton processes. Chemosphere 2022, 306, 135539. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, X.; Zhuo, N.; Zhu, G.; Cai, Z. Interaction-sedimentation strategy for highly efficient removal of refractory humic substances in biologically treated wastewater effluent: From mechanistic investigation to full-scale application. J. Hazard. Mater. 2021, 418, 126145. [Google Scholar] [CrossRef]
- Rebosura, M., Jr.; Salehin, S.; Pikaar, I.; Keller, J.; Sharma, K.; Yuan, Z. The impact of primary sedimentation on the use of iron-rich drinking water sludge on the urban wastewater system. J. Hazard. Mater. 2021, 402, 124051. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Chen, Z.; Zhu, J.; Chen, G. A Comprehensive Review on Forward Osmosis Water Treatment: Recent Advances and Prospects of Membranes and Draw Solutes. Int. J. Environ. Res. Public Health 2022, 19, 8215. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.B.; Ren, L.F.; Shao, J.H.; Xia, L.; Zhao, Y. An integrated separation technology for high fluoride-containing wastewater treatment: Fluoride removal, membrane fouling behavior and control. J. Clean. Prod. 2022, 349, 131225. [Google Scholar] [CrossRef]
- Liu, T.; Yao, B.; Luo, Z.; Li, W.; Li, C.; Ye, Z.; Gong, X.; Yang, J.; Zhou, Y. Applications and influencing factors of the biochar-persulfate based advanced oxidation processes for the remediation of groundwater and soil contaminated with organic compounds. Sci. Total Environ. 2022, 836, 155421. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.F.; Morent, R.; Wang, N.; Wang, Y.X.; Peng, B.F.; Jiang, N.; Lu, N.; Li, J. Degradation of sulfamethoxazole (SMX) by water falling film DBD Plasma/Persulfate: Reactive species identification and their role in SMX degradation. Chem. Eng. J. 2022, 431, 133916. [Google Scholar] [CrossRef]
- McKercher, L.J.; Messer, T.L.; Mittelstet, A.R.; Comfort, S.D. A biological and chemical approach to restoring water quality: A case study in an urban eutrophic pond. J. Environ. Manag. 2022, 318, 115463. [Google Scholar] [CrossRef] [PubMed]
- Changotra, R.; Rajput, H.; Dhir, A. Treatment of real pharmaceutical wastewater using combined approach of Fenton applications and aerobic biological treatment. J. Photoch. Photobio. A 2019, 376, 175–184. [Google Scholar] [CrossRef]
- Kumaravel, S.; Thiripuranthagan, S.; Vembuli, T.; Erusappan, E.; Durai, M.; Sureshkumar, T.; Durai, M. Fabrication of mesoporous WO3-SBA-15 catalysts and enhanced photocatalytic degradation of harmful dyes. Optik 2021, 235, 166599. [Google Scholar] [CrossRef]
- Erusappan, E.; Thiripuranthagan, S.; Radhakrishnan, R.; Durai, M.; Kumaravel, S.; Vembuli, T.; Kaleekkal, N.J. Fabrication of mesoporous TiO2/PVDF photocatalytic membranes for efficient photocatalytic degradation of synthetic dyes. J. Environ. Chem. Eng. 2021, 9, 105776. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, Y.X.; Li, M.W.; Zhang, J.; Qin, J.N.; Li, Y.X.; Wang, C.Y. Thermal coupled photocatalysis over Pt/g-C3N4 for selectively reducing CO2 to CH4 via cooperation of the electronic metal-support interaction effect and the oxidation state of Pt. Appl. Catal. B-Environ. 2021, 298, 120565. [Google Scholar] [CrossRef]
- Lin, C.S.; Liu, H.Y.; Guo, M.; Zhao, Y.H.; Su, X.; Zhang, P.Y.; Zhang, Y.F. Plasmon-induced broad spectrum photocatalytic overall water splitting: Through non-noble bimetal nanoparticles hybrid with reduced graphene oxide. Colloids Surf. A 2022, 646, 128962. [Google Scholar] [CrossRef]
- Wei, P.F.; Zhang, P.Y.; Zhang, Y.F.; Li, X.M. Highly efficient photocatalytic overall water splitting on plasmonic Cu6Sn5/polyaniline nanocomposites. J. Colloid Interface Sci. 2022, 609, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Elmongy, H.; Madrakian, T.; Abdel-Rehim, M. Nanomaterials as sorbents for sample preparation in bioanalysis: A review. Anal. Chim. Acta 2017, 958, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Miao, W.S.; Zhang, Y.D.; Gao, H.J.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Nkambule, T.T.; Mamba, B.B. Spinel ferrite nanoparticles and nanocomposites for biomedical applications and their toxicity. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110314. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Shokrollahi, H. The role of cobalt ferrite magnetic nanoparticles in medical science. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1–8. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic nanocarriers: Evolution of spinel ferrites for medical applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar] [CrossRef]
- Sutka, A.; Gross, K.A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuat. B Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Peng, Y.T.; Tang, H.M.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y.Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Qin, H.; He, Y.; Xu, P.; Huang, D.; Wang, Z.; Wang, H.; Wang, Z.; Zhao, Y.; Tian, Q.; Wang, C. Spinel ferrites (MFe2O4): Synthesis, improvement and catalytic application in environment and energy field. Adv. Colloid Interface Sci. 2021, 294, 102486. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Maletin, M.; Moshopoulou, E.G.; Kontos, A.G.; Devlin, E.; Delimitis, A.; Zaspalis, V.; Nalbandian, L.; Srdic, V.V. Synthesis and structural characterization of In-doped ZnFe2O4 nanoparticles. J. Eur. Ceram. Soc. 2007, 27, 4391–4394. [Google Scholar] [CrossRef]
- Yao, J.H.; Li, Y.W.; Li, X.H.; Le, S.R. First-Principles Investigation on the Electronic Structure and Stability of In-Substituted ZnFe2O4. Metall. Mater. Trans. A 2014, 45, 3686–3693. [Google Scholar] [CrossRef]
- Ueda, K.; Tsuji, M.; Ohyama, J.; Satsuma, A. Active coordination sites of Co spinel oxides for NO reduction by CO. Catal. Today 2022, in press. [CrossRef]
- Makovec, D.; Kodre, A.; Arcon, I.; Drofenik, M. Structure of manganese zinc ferrite spinel nanoparticles prepared with co-precipitation in reversed microemulsions. J. Nanopart. Res. 2009, 11, 1145–1158. [Google Scholar] [CrossRef]
- Lazarevic, Z.Z.; Jovalekic, C.; Ivanovski, V.N.; Recnik, A.; Milutinovic, A.; Cekic, B.; Romcevic, N.Z. Characterization of partially inverse spinel ZnFe2O4 with high saturation magnetization synthesized via soft mechanochemically assisted route. J. Phys. Chem. Solids 2014, 75, 869–877. [Google Scholar] [CrossRef]
- Hoque, S.M.; Srivastava, C.; Venkatesha, N.; Anil Kumar, P.S.; Chattopadhyay, K. Superparamagnetic behaviour and T-1, T-2 relaxivity of ZnFe2O4 nanoparticles for magnetic resonance imaging. Philos. Mag. 2013, 93, 1771–1783. [Google Scholar] [CrossRef]
- Pund, S.N.; Nagwade, P.A.; Nagawade, A.V.; Thopate, S.R.; Bagade, A.V. Preparation techniques for zinc ferrites and their applications: A review. Mater. Today Proc. 2022, 60, 2194–2208. [Google Scholar] [CrossRef]
- Sonu; Sharma, S.; Dutta, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.; Nguyen, V.H.; VanLe, Q.; Singh, P. An overview of heterojunctioned ZnFe2O4 photocatalyst for enhanced oxidative water purification. J. Environ. Chem. Eng. 2021, 9, 105812. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Zhou, N.; Du, Y.; Zeng, X.; Zhou, S.; He, Q.; Wu, G. In-situ growth of core-shell ZnFe2O4 @ porous hollow carbon microspheres as an efficient microwave absorber. J. Colloid Interface Sci. 2021, 581 Pt B, 475–484. [Google Scholar] [CrossRef]
- Mohanty, D.; Mallick, P.; Biswal, S.K.; Behera, B.; Mohapatra, R.K.; Behera, A.; Satpathy, S.K. Investigation of structural, dielectric and electrical properties of ZnFe2O4 composite. Mater. Today Proc. 2020, 33, 4971–4975. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Liu, D.; Lin, G.; Wan, J.W.; Jiang, H.; Lai, X.Y.; Hao, S.J.; Liu, X.H. Lychee-like ZnO/ZnFe2O4 core-shell hollow microsphere for improving acetone gas sensing performance. Ceram. Int. 2020, 46, 5960–5967. [Google Scholar] [CrossRef]
- Pradeep, A.; Priyadharsini, P.; Chandrasekaran, G. Structural, magnetic and electrical properties of nanocrystalline zinc ferrite. J. Alloys Compd. 2011, 509, 3917–3923. [Google Scholar] [CrossRef]
- Chandel, N.; Sharma, K.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Singh, P. Magnetically separable ZnO/ZnFe2O4 and ZnO/CoFe2O4 photocatalysts supported onto nitrogen doped graphene for photocatalytic degradation of toxic dyes. Arab. J. Chem. 2020, 13, 4324–4340. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Sun, Y.Q.; Gao, D.Z.; Xu, Y.Y. Quasi-cube ZnFe2O4 nanocrystals: Hydrothermal synthesis and photocatalytic activity with TiO2 (Degussa P25) as nanocomposite. Mater. Res. Bull. 2010, 45, 755–760. [Google Scholar] [CrossRef]
- Wu, S.K.; Shen, X.P.; Zhu, G.X.; Zhou, H.; Ji, Z.Y.; Chen, K.M.; Yuan, A.H. Synthesis of ternary Ag/ZnO/ZnFe2O4 porous and hollow nanostructures with enhanced photocatalytic activity. Appl. Catal. B Environ. 2016, 184, 328–336. [Google Scholar] [CrossRef]
- Riaz, K.; Nadeem, S.; Chrouda, A.; Iqbal, S.; Mohyuddin, A.; Ul Hassan, S.; Javed, M.; BaQais, A.; Tamam, N.; Aroosh, K.; et al. Coupling of Se-ZnFe2O4 with rGO for spatially charged separated nanocomposites as an efficient photocatalyst for degradation of organic pollutants in natural sunlight. Colloids Surf. A 2022, 649, 129332. [Google Scholar] [CrossRef]
- Wang, X.D.; Dai, Y.X.; Tian, C.; Zhang, H.Z.; Li, X.W.; Liu, W.L.; Li, W.B.; Kuang, S.P.; Tong, H.T. Boosted photocatalytic removal of Cr(VI) using MoS2 modified g-C3N4/ ZnFe2O4 magnetic heterojunction composites. Process Saf. Environ. 2022, 162, 72–82. [Google Scholar]
- Tang, L.; Liu, L.; Chen, Q.; Yang, F.; Quan, X. The construction and performance of photocatalytic-fuel-cell with Fe-MoS2/reduced graphene oxide@carbon fiber cloth and ZnFe2O4/Ag/Ag3VO4@carbon felt as photo electrodes. Electrochim. Acta 2020, 362, 137037. [Google Scholar] [CrossRef]
- Fu, Y.M.; Tang, Y.B.; Shi, W.L.; Chen, F.Y.; Guo, F.; Hao, C.C. Preparation of rambutan-shaped hollow ZnFe2O4 sphere photocatalyst for the degradation of tetracycline by visible-light photocatalytic persulfate activation. Mater. Chem. Phys. 2022, 286, 126176. [Google Scholar] [CrossRef]
- Sharafinia, S.; Farrokhnia, A.; Lemraski, E.G.; Rashidi, A. Decoration of ZnFe2O4 and UiO-66 over g-C3N4 as magnetically novel reusable visible light photocatalyst for degradation of Rh–B. Opt. Mater. 2022, 132, 112838. [Google Scholar] [CrossRef]
- Amin, A.M.M.; Soliman, Y.M.M.; El-Dek, S.I.; Ahmed, Y.M.Z.; Zaki, A.H. Valorization of industrial iron and zinc sludges for the synthesis of ZnFe2O4 ceramics. J. Magn. Magn. Mater. 2022, 544, 168681. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on Tio2 Surfaces—Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Savunthari, K.V.; Shanmugam, S. Effect of co-doping of bismuth, copper and cerium in zinc ferrite on the photocatalytic degradation of bisphenol A. J. Taiwan Inst. Chem. E 2019, 101, 105–118. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, B.; Li, X.; Wang, X.; Huang, K.; Chen, Z. MOF-derived magnetically recoverable Z-scheme ZnFe2O4/Fe2O3 perforated nanotube for efficient photocatalytic ciprofloxacin removal. Chem. Eng. J. 2022, 430, 132728. [Google Scholar] [CrossRef]
- Zhu, X.S.; Cao, C.X.; Su, S.B.; Xia, A.L.; Zhang, H.Y.; Li, H.L.; Liu, Z.Y.; Jin, C.G. A comparative study of spinel ZnFe2O4 ferrites obtained via a hydrothermal and a ceramic route: Structural and magnetic properties. Ceram. Int. 2021, 47, 15173–15179. [Google Scholar] [CrossRef]
- Lestari, K.R.; Yoo, P.; Kim, D.H.; Liu, C.; Lee, B.W. ZnFe2O4 Nanoparticles Prepared Using the Hydrothermal and the Sol-gel Methods. J. Korean Phys. Soc. 2015, 66, 651–655. [Google Scholar] [CrossRef]
- Kurian, J.; Mathew, M.J. Structural, optical and magnetic studies of CuFe2O4, MgFe2O4 and ZnFe2O4 nanoparticles prepared by hydrothermal/solvothermal method. J. Magn. Magn. Mater. 2018, 451, 121–130. [Google Scholar] [CrossRef]
- Yoo, P.S.; Lee, B.W.; Liu, C. Effects of pH Value, Reaction Time, and Filling Pressure on the Hydrothermal Synthesis of ZnFe2O4 Nanoparticles. IEEE Trans. Magn. 2015, 51, 2003004. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, K.D.M.; Nguyen, T.A.; No, K. The synthesis of zinc ferrite spinel: Determination of pH value in the co-precipitation step. Ceram. Int. 2022, 48, 4090–4095. [Google Scholar] [CrossRef]
- Somvanshi, S.B.; Khedkar, M.V.; Kharat, P.B.; Jadhav, K.M. Influential diamagnetic magnesium (Mg2+) ion substitution in nano-spinel zinc ferrite (ZnFe2O4): Thermal, structural, spectral, optical and physisorption analysis. Ceram. Int. 2020, 46, 8640–8650. [Google Scholar] [CrossRef]
- Sonia, L.C.; Victory, M.; Phanjoubam, S. A Comparative Study of the Properties of Zinc Ferrite Nanoparticles Synthesized by Different Techniques for Nanofluid Preparation. Integr. Ferroelectr. 2020, 204, 100–111. [Google Scholar] [CrossRef]
- Prasad, B.B.V.S.V.; Ramesh, K.V.; Srinivas, A. Structural and Magnetic Studies of Nano-crystalline Ferrites MFe2O4 (M = Zn, Ni, Cu, and Co) Synthesized via Citrate Gel Autocombustion Method. J. Supercond. Nov. Magn. 2017, 30, 3523–3535. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, N.; Bhushan Das, S.; Singh, R.K.; Sarkar, K.; Kumar, M. Sol-gel assisted synthesis and tuning of structural, photoluminescence, magnetic and multiferroic properties by annealing temperature in nanostructured zinc ferrite. Mater. Today Proc. 2021, 47, 6242–6248. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Amrutha, V.S.; Anantharaju, K.S.; Prasanna, D.S.; Rangappa, D.; Shetty, K.; Nagabhushana, H.; Ashwini, K.; Vidya, Y.S.; Darshan, G.P. Enhanced Sunlight driven photocatalytic performance and visualization of latent fingerprint by green mediated ZnFe2O4-RGO nanocomposite. Arab. J. Chem. 2020, 13, 1449–1465. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Murali, K.R. Biosynthesis of zinc ferrite (ZnFe2O4) nanoparticles using flower extract of nyctanthes arbor-tristis and their photocatalytic activity. Ferroelectrics 2020, 555, 1–14. [Google Scholar] [CrossRef]
- Surendra, B.S.; Nagaswarupa, H.P.; Hemashree, M.U.; Khanum, J. Jatropha extract mediated synthesis of ZnFe2O4 nanopowder: Excellent performance as an electrochemical sensor, UV photocatalyst and an antibacterial activity. Chem. Phys. Lett. 2020, 739, 136980. [Google Scholar] [CrossRef]
- Rahmayeni; Febrialita, R.; Stiadi, Y.; Putri, Y.E.; Sofyan, N.; Zulhadjri. Simbang Darah (Iresine herbstii) extract mediated hydrothermal method in the synthesis of zinc ferrite spinel nanoparticles used for photocatalysis and antibacterial applications. J. Environ. Chem. Eng. 2021, 9, 105140. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Danyliuk, N.; Kotsyubynsky, V.; Shumskaya, A.; Kaniukov, E.; Ghfar, A.A.; Naushad, M.; Shyichuk, A. Eco-friendly synthesis of cobalt-zinc ferrites using quince extract for adsorption and catalytic applications: An approach towards environmental remediation. Chemosphere 2022, 294, 133565. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.G.; Chaudhary, L.S.; Sakhare, P.A.; Dongale, T.D.; Patil, P.S.; Sheikh, A.D. Impact of collected sunlight on ZnFe2O4 nanoparticles for photocatalytic application. J. Colloid Interface Sci. 2018, 527, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Naik, H.S.B.; Nagaraju, G.; Viswanath, R.; Rashmi, S.K.; Kumar, M.V. Sugarcane juice mediated eco-friendly synthesis of visible light active zinc ferrite nanoparticles: Application to degradation of mixed dyes and antibacterial activities. Mater. Chem. Phys. 2018, 212, 351–362. [Google Scholar] [CrossRef]
- Gao, S.; Feng, D.; Chen, F.; Shi, H.; Chen, Z. Multi-functional well-dispersed pomegranate-like nanospheres organized by ultrafine ZnFe2O4 nanocrystals for high-efficiency visible-light-Fenton catalytic activities. Colloids Surf. A 2022, 648, 129282. [Google Scholar] [CrossRef]
- Ju, Y.M.; Liu, R.L.; Ji, G.X.; Su, L.H.; Qiao, J.Q.; Xing, W.L.; Fan, D.L.; Zhao, K.Q.; Dionysiou, D.D. Novel strategy for enhanced visible light-responsive photoactivity of ZnFe2O4 with a single-mode microwave combustion process: Primary parameters. Chem. Eng. J. 2022, 440, 135551. [Google Scholar] [CrossRef]
- Yang, H.; Hao, H.; Zhao, Y.; Hu, Y.; Min, J.; Zhang, G.; Bi, J.; Yan, S.; Hou, H. An efficient construction method of S-scheme Ag2CrO4/ZnFe2O4 nanofibers heterojunction toward enhanced photocatalytic and antibacterial activity. Colloids Surf. A 2022, 641, 128603. [Google Scholar] [CrossRef]
- Arimi, A.; Megatif, L.; Granone, L.I.; Dillert, R.; Bahnemann, D.W. Visible-light photocatalytic activity of zinc ferrites. J. Photoch. Photobio. A 2018, 366, 118–126. [Google Scholar] [CrossRef]
- Janani, B.; Syed, A.; Sruthi, L.; Sivaranjani, P.R.; Elgorban, A.M.; Bahkali, A.H.; Zaghloul, N.S.S.; Badawy, M.M.; Das, A.; Khan, S.S. Visible light driven photocatalytic activity and efficient antibacterial activity of ZnFe2O4 decorated CdO nanohybrid heterostructures synthesized by ultrasonic-assisted method. Colloids Surf. A 2021, 628, 127307. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar]
- Krishnan, R.; Shibu, S.N.; Poelman, D.; Badyal, A.K.; Kunti, A.K.; Swart, H.C.; Menon, S.G. Recent advances in microwave synthesis for photoluminescence and photocatalysis. Mater. Today Commun. 2022, 32, 103890. [Google Scholar]
- Sarkar, P.; De, S.R.D.; Neogi, S. Microwave assisted facile fabrication of dual Z-scheme g-C3N4/ZnFe2O4/Bi2S3 photocatalyst for peroxymonosulphate mediated degradation of 2,4,6-Trichlorophenol: The mechanistic insights. Appl. Catal. B. Environ. 2022, 307, 121165. [Google Scholar]
- Tamaddon, F.; Mosslemin, M.H.; Asadipour, A.; Gharaghani, M.A.; Nasiri, A. Microwave-assisted preparation of ZnFe2O4@methyl cellulose as a new nano-biomagnetic photocatalyst for photodegradation of metronidazole. Int. J. Biol. Macromol. 2020, 154, 1036–1049. [Google Scholar] [PubMed]
- Zhang, Y.; Wu, Y.; Qin, Q.; Wang, F.; Chen, D. A study of the mechanism of microwave-assisted ball milling preparing ZnFe2O4. J. Magn. Magn. Mater. 2016, 409, 6–9. [Google Scholar]
- Lemine, O.M.; Bououdina, M.; Sajieddine, M.; Al-Saie, A.M.; Shafi, M.; Khatab, A.; Al-hilali, M.; Henini, M. Synthesis, structural, magnetic and optical properties of nanocrystalline ZnFe2O4. Physica B 2011, 406, 1989–1994. [Google Scholar]
- Rameshbabu, R.; Ramesh, R.; Kanagesan, S.; Karthigeyan, A.; Ponnusamy, S. Synthesis and Study of Structural, Morphological and Magnetic Properties of ZnFe2O4 Nanoparticles. J. Supercond. Novel Magn. 2013, 27, 1499–1502. [Google Scholar]
- Surinwong, S.; Rujiwatra, A. Ultrasonic cavitation assisted solvothermal synthesis of superparamagnetic zinc ferrite nanoparticles. Particuology 2013, 11, 588–593. [Google Scholar]
- Patil, K.; Kadam, S.; Lokhande, P.; Balgude, S.; More, P. The effects of cobalt and magnesium co-doping on the structural and magnetic properties of ZnFe2O4 synthesized using a sonochemical process. Solid State Commun. 2021, 337, 114435. [Google Scholar]
- Raja, V.R.; Karthika, A.; Kirubahar, S.L.; Suganthi, A.; Rajarajan, M. Sonochemical synthesis of novel ZnFe2O4/CeO2 heterojunction with highly enhanced visible light photocatalytic activity. Solid State Ion. 2019, 332, 55–62. [Google Scholar]
- Son, N.; Lee, J.; Yoon, T.; Kang, M. Design for a longer photoinduced charge separation and improved visible-light-driven H2 generation through structure reversal and oxygen vacancies via Ni substitution into ZnFe2O4 spinel. Ceram. Int. 2021, 47, 20317–20334. [Google Scholar]
- Abu El-Fadl, A.; Hassan, A.M.; Kassem, M.A. Tunable cationic distribution and structure-related magnetic and optical properties by Cr3+ substitution for Zn2+ in nanocrystalline Ni-Zn ferrites. Results Phys. 2021, 28, 104622. [Google Scholar]
- Wang, W.D.; Li, N.; Hong, K.Q.; Guo, H.W.; Ding, R.; Xia, Z.H. Z-scheme recyclable photocatalysts based on flower-like nickel zinc ferrite nanoparticles/ZnO nanorods: Enhanced activity under UV and visible irradiation. J. Alloys Compd. 2019, 777, 1108–1114. [Google Scholar]
- Lemziouka, H.; Boutahar, A.; Moubah, R.; Omari, L.H.; Bahhar, S.; Abid, M.; Lassri, H. Synthesis, structural, optical and dispersion parameters of La-doped spinel zinc ferrites ZnFe2-xLaxO4 (x = 0.00, 0.001, 0.005, 0.01 and 0.015). Vacuum 2020, 182, 109780. [Google Scholar]
- Tholkappiyan, R.; Vishista, K. Influence of lanthanum on the optomagnetic properties of zinc ferrite prepared by combustion method. Phys. B Condens. Matter 2014, 448, 177–183. [Google Scholar]
- Shoba, M.; Kaleemulla, S. Structural, optical and dielectric studies of Er substituted zinc ferrite nanospheres. J. Phys. Chem. Solids 2017, 111, 447–457. [Google Scholar]
- Musa, M.A.; Xu, D.; Sun, F.; Shao, H.; Dong, X.T.; Azis, R.S.; Ugya, A.Y.; Ari, H.A. Electrospun ZnFe2O4/Al: ZnFe2O4 nanofibers for degradation of RhB via visible light photocatalysis and photo-Fenton processes. J. Mater. Sci. Mater. Electron. 2022, 33, 2375–2385. [Google Scholar]
- Reyes-Rodríguez, P.Y.; Ávila-Orta, C.A.; Andrade-Guel, M.; Cortés-Hernández, D.A.; Herrera-Guerrero, A.; Cabello-Alvarado, C.; Sánchez-Fuentes, J.; Ramos-Martínez, V.H.; Valdez-Garza, J.A.; Hurtado-López, G.F. Synthesis and characterization of magnetic nanoparticles Zn1-xMgxFe2O4 with partial substitution of Mg2+ (x= 0.0, 0.25, 0.5, 0.75 and 1.0) for adsorption of uremic toxins. Ceram. Int. 2020, 46, 27913–27921. [Google Scholar]
- Peymani-Motlagh, S.M.; Moeinian, N.; Rostami, M.; Fasihi-Ramandi, M.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Eghbali-Arani, M.; Ganjali, M.R.; Jesionowski, T.; Ehrlich, H.; et al. Effect of Gd3+-, Pr3+- or Sm3+-substituted cobalte-zinc ferrite on photodegradation of methyl orange and cytotoxicity tests. J. Rare Earth 2019, 37, 1288–1295. [Google Scholar]
- Borhan, A.I.; Slatineanu, T.; Iordan, A.R.; Palamaru, M.N. Influence of chromium ion substitution on the structure and properties of zinc ferrite synthesized by the sol-gel auto-combustion method. Polyhedron 2013, 56, 82–89. [Google Scholar]
- Li, J.; Li, X.; Chen, X.; Yin, Z.; Li, Y.; Jiang, X. In situ construction of yolk-shell zinc ferrite with carbon and nitrogen co-doping for highly efficient solar light harvesting and improved catalytic performance. J. Colloid Interface Sci. 2019, 554, 91–102. [Google Scholar]
- Sonu; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar]
- Das, K.K.; Patnaik, S.; Mansingh, S.; Behera, A.; Mohanty, A.; Acharya, C.; Parida, K.M. Enhanced photocatalytic activities of polypyrrole sensitized zinc ferrite/graphitic carbon nitride n-n heterojunction towards ciprofloxacin degradation, hydrogen evolution and antibacterial studies. J. Colloid Interface Sci. 2020, 561, 551–567. [Google Scholar] [PubMed]
- Zhang, R.; Han, Q.; Li, Y.; Zhang, T.Q.; Liu, Y.; Zeng, K.L.; Zhao, C. Solvothermal synthesis of a peony flower-like dual Z-scheme PANI/BiOBr/ZnFe2O4 photocatalyst with excellent photocatalytic redox activity for organic pollutant under visible-light. Sep. Purif. Technol. 2020, 234, 116098. [Google Scholar]
- Wang, J.; Zhang, Q.; Deng, F.; Luo, X.B.; Dionysiou, D.D. Rapid toxicity elimination of organic pollutants by the photocatalysis of environment-friendly and magnetically recoverable step-scheme SnFe2O4/ZnFe2O4 nano-heterojunctions. Chem. Eng. J. 2020, 379, 122264. [Google Scholar]
- AL-Shwaiman, H.A.; Akshhayya, C.; Syed, A.; Bahkali, A.H.; Elgorban, A.M.; Das, A.; Varma, R.S.; Khan, S.S. Fabrication of intimately coupled CeO2/ZnFe2O4 nano-heterojunction for visible-light photocatalysis and bactericidal application. Mater. Chem. Phys. 2022, 279, 125759. [Google Scholar]
- Akshhayya, C.; Okla, M.K.; Thomas, A.M.; AL-ghamdi, A.A.; Abdel-Maksoud, M.A.; Almunqedhi, B.; AbdElgawad, H.; Raju, L.L.; Khan, S.S. Insights into photocatalytic mechanism for the rational design of p-n heterojunction by decorating mesoporous SnS2 over ZnFe2O4 nanocomposite for accelerated visible light photocatalysis. Mater. Chem. Phys. 2022, 277, 125464. [Google Scholar]
- Singh, C.; Goyal, A.; Malik, R.; Bansal, S.; Singhal, S. Envisioning the attachment of CdS nanoparticles on the surface of MFe2O4 (M = Zn, Co and Ni) nanocubes: Analysis of structural, optical, magnetic and photocatalytic properties. J. Alloys Compd. 2017, 695, 351–363. [Google Scholar]
- Piaskowski, K.; Zarzycki, P.K. Carbon-Based Nanomaterials as Promising Material for Wastewater Treatment Processes. Int. J. Environ. Res. Public Health 2020, 17, 5862. [Google Scholar]
- Jun, B.M.; Elanchezhiyan, S.S.; Yoon, Y.; Wang, D.J.; Kim, S.; Prabhu, S.M.; Park, C.M. Accelerated photocatalytic degradation of organic pollutants over carbonate-rich lanthanum-substituted zinc spinel ferrite assembled reduced graphene oxide by ultraviolet (UV)-activated persulfate. Chem. Eng. J. 2020, 393, 124733. [Google Scholar]
- Baynosa, M.L.; Mady, A.H.; Nguyen, V.Q.; Kumar, D.R.; Sayed, M.S.; Tuma, D.; Shim, J.J. Eco-friendly synthesis of recyclable mesoporous zinc ferrite@reduced graphene oxide nanocomposite for efficient photocatalytic dye degradation under solar radiation. J. Colloid Interface Sci. 2020, 561, 459–469. [Google Scholar]
- Wang, W.R.; Guo, S.S.; Zhang, D.X.; Yang, Z. One-pot hydrothermal synthesis of reduced graphene oxide/zinc ferrite nanohybrids and its catalytic activity on the thermal decomposition of ammonium perchlorate. J. Saudi Chem. Soc. 2019, 23, 133–140. [Google Scholar]
- Gao, Y.; Wang, W.L.; Xu, M.; Hao, Y.; Zou, D.L. The magnetically recoverable C-ZnFe2O4 as a microwave absorption catalyst for the degradation of norfloxacin by persulfate: Mechanism, degradation pathway, toxicity, and stability. J. Environ. Chem. Eng. 2022, 10, 107434. [Google Scholar] [CrossRef]
- Ghazkoob, N.; Shoushtari, M.Z.; Kazeminezhad, I.; Baghal, S.M.L. Investigation of structural, magnetic, optical and photocatalytic properties of zinc ferrite nanowires/bismuth vanadate composite. J. Alloys Compd. 2022, 900, 163467. [Google Scholar] [CrossRef]
- Liu, H.Y.; Han, C.H.; Shao, C.L.; Yang, S.; Li, X.W.; Li, B.; Li, X.H.; Ma, J.G.; Liu, Y.C. ZnO/ZnFe2O4 Janus Hollow Nanofibers with Magnetic Separability for Photocatalytic Degradation of Water-Soluble Organic Dyes. ACS Appl. Nano Mater. 2019, 2, 4879–4890. [Google Scholar] [CrossRef]
- Chen, Y.J.; Jiang, H.Y.; Li, L.G.; Wang, Q.; Du, L.Z.; Liu, X.; Tian, G.H. Hierarchical NiS decorated CuO@ZnFe2O4 nanoarrays as advanced photocathodes for hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 6174–6183. [Google Scholar] [CrossRef]
- Cao, Y.; Lei, X.Y.; Chen, Q.L.; Kang, C.; Li, W.X.; Liu, B.J. Enhanced photocatalytic degradation of tetracycline hydrochloride by novel porous hollow cube ZnFe2O4. J. Photoch. Photobio. A 2018, 364, 794–800. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Ni, Q.Q. Carbon nanotube template-assisted synthesis of zinc ferrite nanochains. Mater. Chem. Phys. 2010, 124, 1029–1033. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Deng, W.; Chen, L.; Courtois, J.; Tian, Q.; Zhang, X.W.; Almasy, L.; Yan, M.H.; Xiong, K. Langmuir-Blodgett-assembled monolayer zinc ferrite nanoparticle film with unique photogenerated charge carrier separation efficiency and charge transfer behavior. Appl. Surf. Sci. 2020, 534, 147646. [Google Scholar] [CrossRef]
- Chen, Y.F.; Spoddig, D.; Ziese, M. Epitaxial thin film ZnFe2O4: A semi-transparent magnetic semiconductor with high Curie temperature. J. Phys. D Appl. Phys. 2008, 41, 205004. [Google Scholar] [CrossRef]
- Luo, X.; Duan, Z.F.; Zhu, Y.; Ni, J.Y.; Li, Y.L.; Zhang, J.Y.; Chen, Y.R.; Wang, X.H.; Zhao, G.Y. Enhanced visible-light catalytic activity of micro-patterned ZnFe2O4/Fe3+-TiO2 heterojunction composite thin films prepared by photolithography-assisted chemical solution deposition. Mater. Res. Bull. 2022, 155, 111951. [Google Scholar] [CrossRef]
- Erusappan, E.; Thiripuranthagan, S.; Durai, M.; Kumaravel, S.; Vembuli, T. Photocatalytic performance of visible active boron nitride supported ZnFe2O4 (ZnFe2O4/BN) nanocomposites for the removal of aqueous organic pollutants. N. J. Chem. 2020, 44, 7758–7770. [Google Scholar] [CrossRef]
- Wang, G.J.; Li, Z.C.; Li, M.Y.; Feng, Y.M.; Li, W.; Lv, S.S.; Liao, J.C. Synthesizing vertical porous ZnO nanowires arrays on Si/ITO substrate for enhanced photocatalysis. Ceram. Int. 2018, 44, 1291–1295. [Google Scholar] [CrossRef]
- Zhu, C.Z.; He, Q.Y.; Wang, W.K.; Du, F.; Yang, F.; Chen, C.X.; Wang, C.H.; Wang, S.B.; Duan, X.G. S-scheme photocatalysis induced by ZnIn2S4 nanoribbons-anchored hierarchical CeO2 hollow spheres for boosted hydrogen evolution. J. Colloid Interface Sci. 2022, 620, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.H.; Wang, H.C.; Wu, Y.; Lv, L.P.; Chen, S.Q.; Sun, W.W.; Wang, Y. Covalent-Induced Heterostructure of Covalent-Organic Frameworks and MXene as Advanced Electrodes with Motivated Pseudocapacitance Performance. Chemelectrochem 2022, 9, e202200340. [Google Scholar] [CrossRef]
- Geng, Q.H.; Wang, H.C.; Wang, J.L.; Hong, J.; Sun, W.W.; Wu, Y.; Wang, Y. Boosting the Capacity of Aqueous Li-Ion Capacitors via Pinpoint Surgery in Nanocoral-Like Covalent Organic Frameworks. Small Methods 2022, 6, 2200314. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, Y.A.; Teng, G.X.; Wang, L.P.; Jin, X.D.; Qiang, Z.W.; Ma, W.G. Fabrication of a Double-Shell Ag/AgCl/G-ZnFe2O4 Nanocube with Enhanced Light Absorption and Superior Photocatalytic Antibacterial Activity. ACS Appl. Mater. Interfaces 2020, 12, 29883–29898. [Google Scholar] [CrossRef]
- Dihom, H.R.; Al-Shaibani, M.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Sharma, A.; Khamidun, M.H.B. Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: A critical review. J. Water Process Eng. 2022, 47, 102705. [Google Scholar] [CrossRef]
- Chahar, D.; Taneja, S.; Bisht, S.; Kesarwani, S.; Thakur, P.; Thakur, A.; Sharma, P.B. Photocatalytic activity of cobalt substituted zinc ferrite for the degradation of methylene blue dye under visible light irradiation. J. Alloys Compd. 2021, 851, 156878. [Google Scholar] [CrossRef]
- Shakil, M.; Inayat, U.; Khalid, N.R.; Tanveer, M.; Gillani, S.S.A.; Tariq, N.H.; Shah, A.; Mahmood, A.; Dahshan, A. Enhanced structural, optical, and photocatalytic activities of Cd-Co doped Zn ferrites for degrading methyl orange dye under irradiation by visible light. J. Phys. Chem. Solids 2022, 161, 110419. [Google Scholar] [CrossRef]
- Sun, X.F.; Wang, C.W.; Li, Y.B.; Wang, W.G.; Wei, J. Treatment of phenolic wastewater by combined UF and NF/RO processes. Desalination 2015, 355, 68–74. [Google Scholar] [CrossRef]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Mady, A.H.; Baynosa, M.L.; Tuma, D.; Shim, J.-J. Heterogeneous activation of peroxymonosulfate by a novel magnetic 3D γ-MnO2@ZnFe2O4/rGO nanohybrid as a robust catalyst for phenol degradation. Appl. Catal. B 2019, 244, 946–956. [Google Scholar] [CrossRef]
- Mathur, P.; Sanyal, D.; Callahan, D.L.; Conlan, X.A.; Pfeffer, F.M. Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environ-ment and human health. Environ. Pollut. 2021, 291, 118233. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Rana, G.; Kumar, A.; Sharma, G.; Vo, D.V.N.; AlGarni, T.S.; Naushad, M.; ALOthman, Z.A. Nanostructured magnetic inverse spinel Ni-Zn ferrite as environmental friendly visible light driven photo-degradation of levofloxacin. Chem. Eng. Res. Des. 2021, 175, 85–101. [Google Scholar] [CrossRef]
- Vinosha, P.A.; Vinsla, J.V.A.; Madhavan, J.; Devanesan, S.; AlSalhi, M.S.; Nicoletti, M.; Xavier, B. Impact of dysprosium doped (Dy) zinc ferrite (ZnFe2O4) nanocrystals in photo-fenton exclusion of recalcitrant organic pollutant. Environ. Res. 2022, 203, 111913. [Google Scholar] [CrossRef]
- Ignat, M.; Rotaru, R.; Samoila, P.; Sacarescu, L.; Timpu, D.; Harabagiu, V. Relationship between the component synthesis order of zinc ferrite–titania nanocomposites and their performances as visible light-driven photocatalysts for relevant organic pollutant degradation. Comptes Rendus Chim. 2018, 21, 263–269. [Google Scholar] [CrossRef]
- Makofane, A.; Motaung, D.E.; Hintsho-Mbita, N.C. Photocatalytic degradation of methylene blue and sulfisoxazole from water using biosynthesized zinc ferrite nanoparticles. Ceram. Int. 2021, 47, 22615–22626. [Google Scholar] [CrossRef]
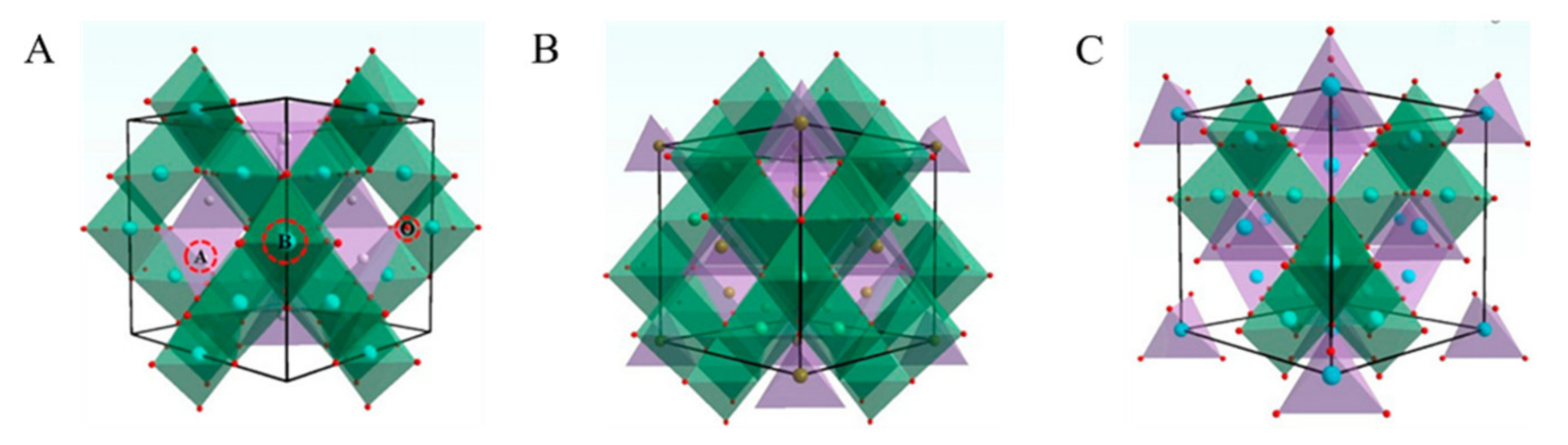

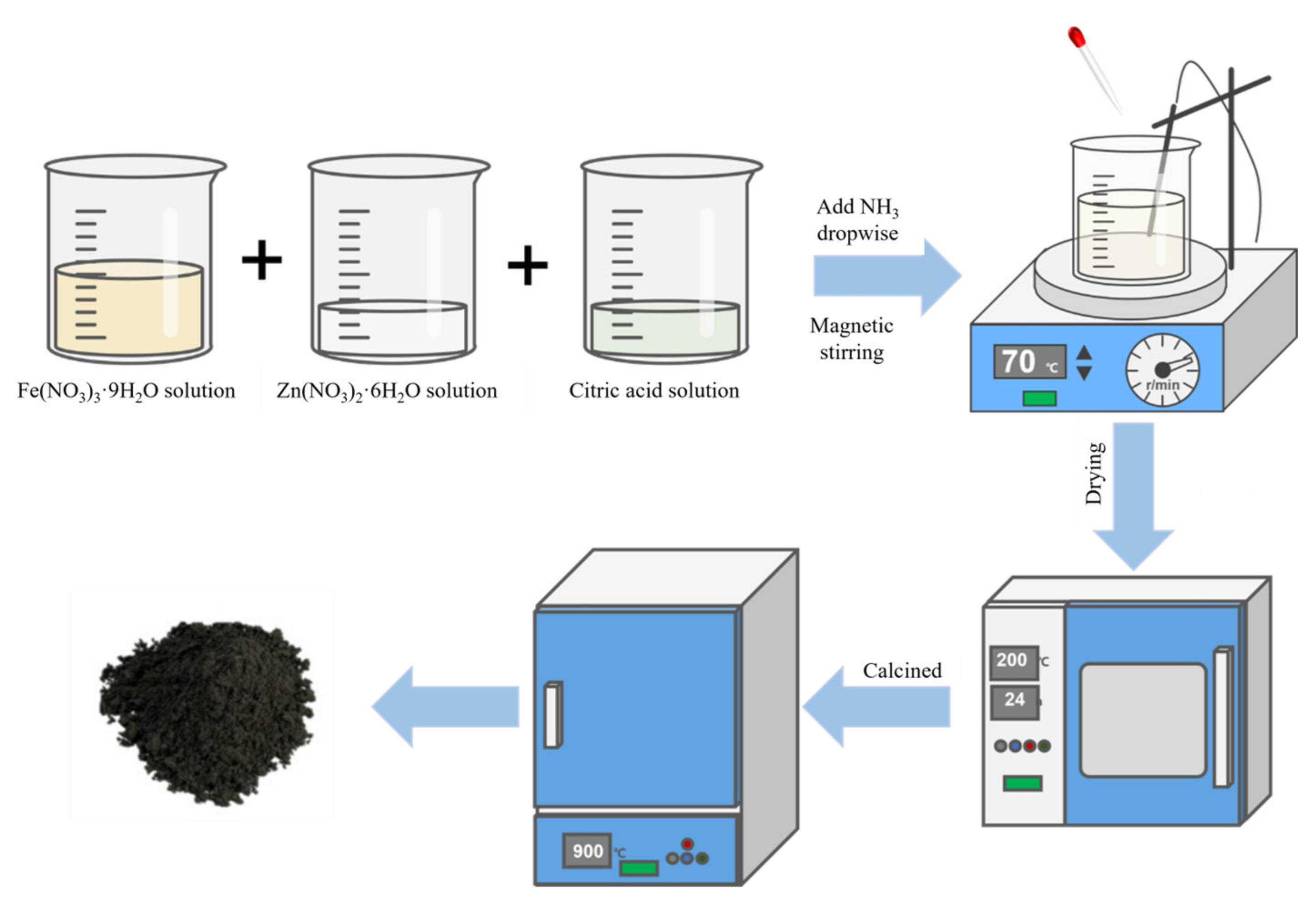
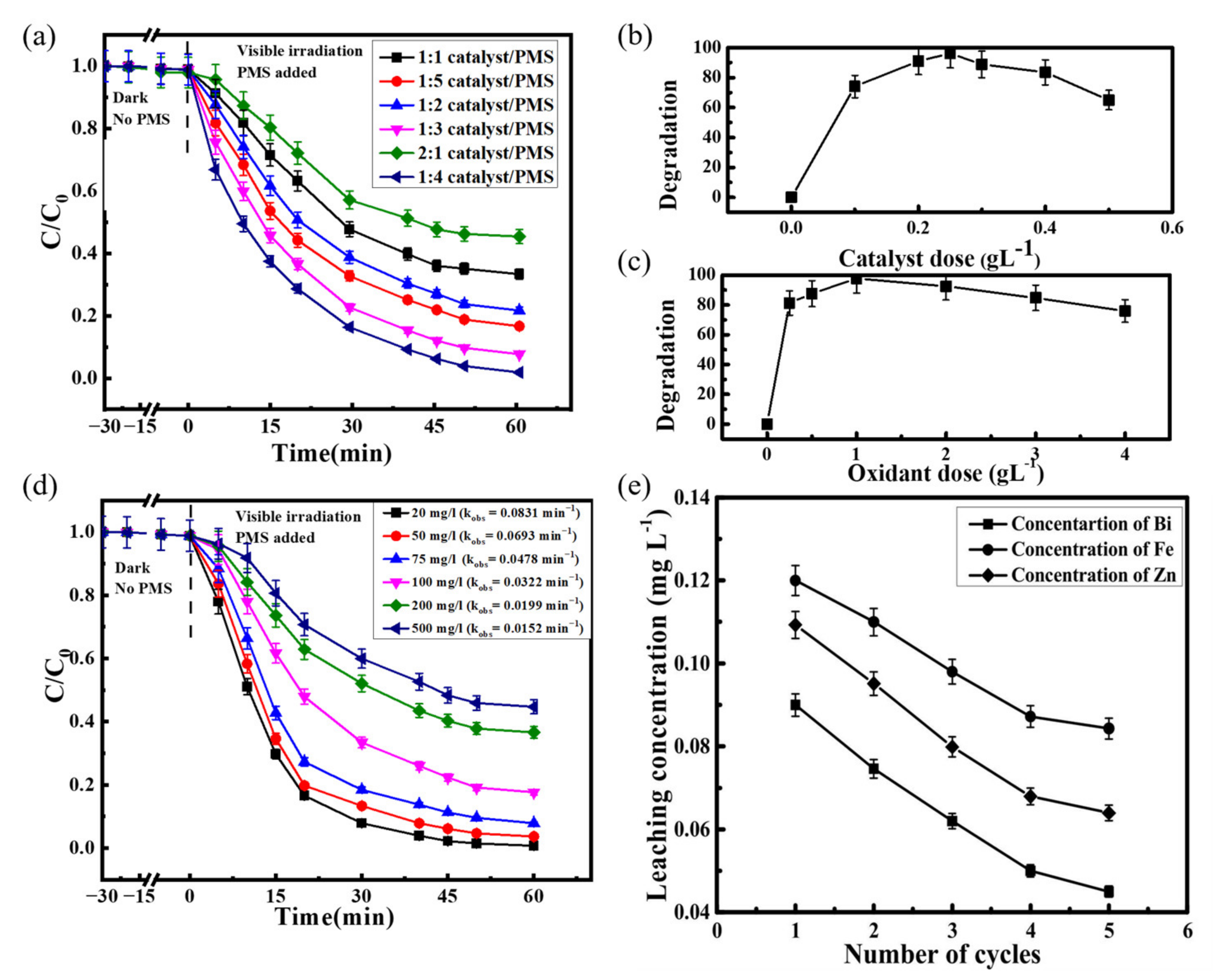
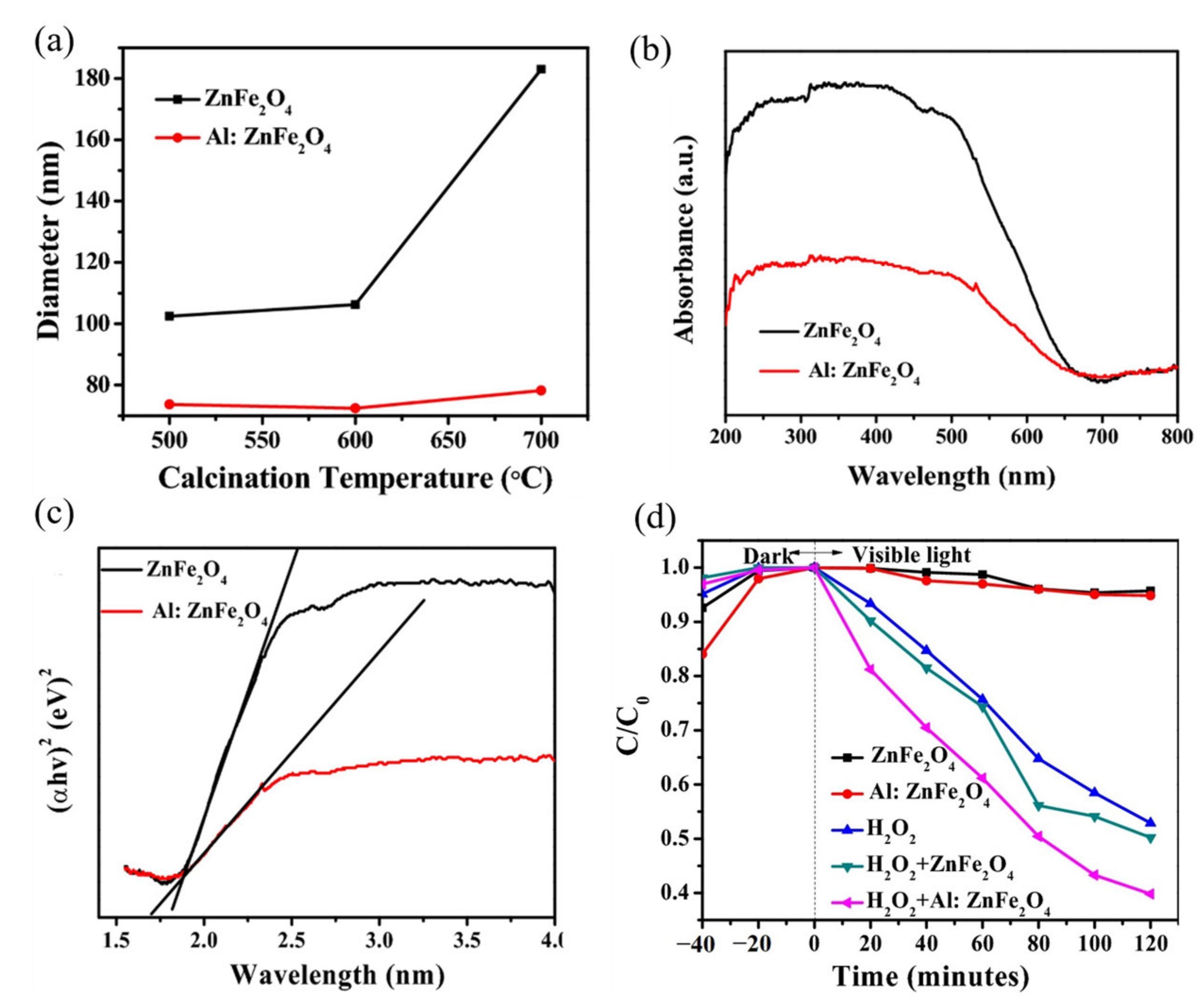
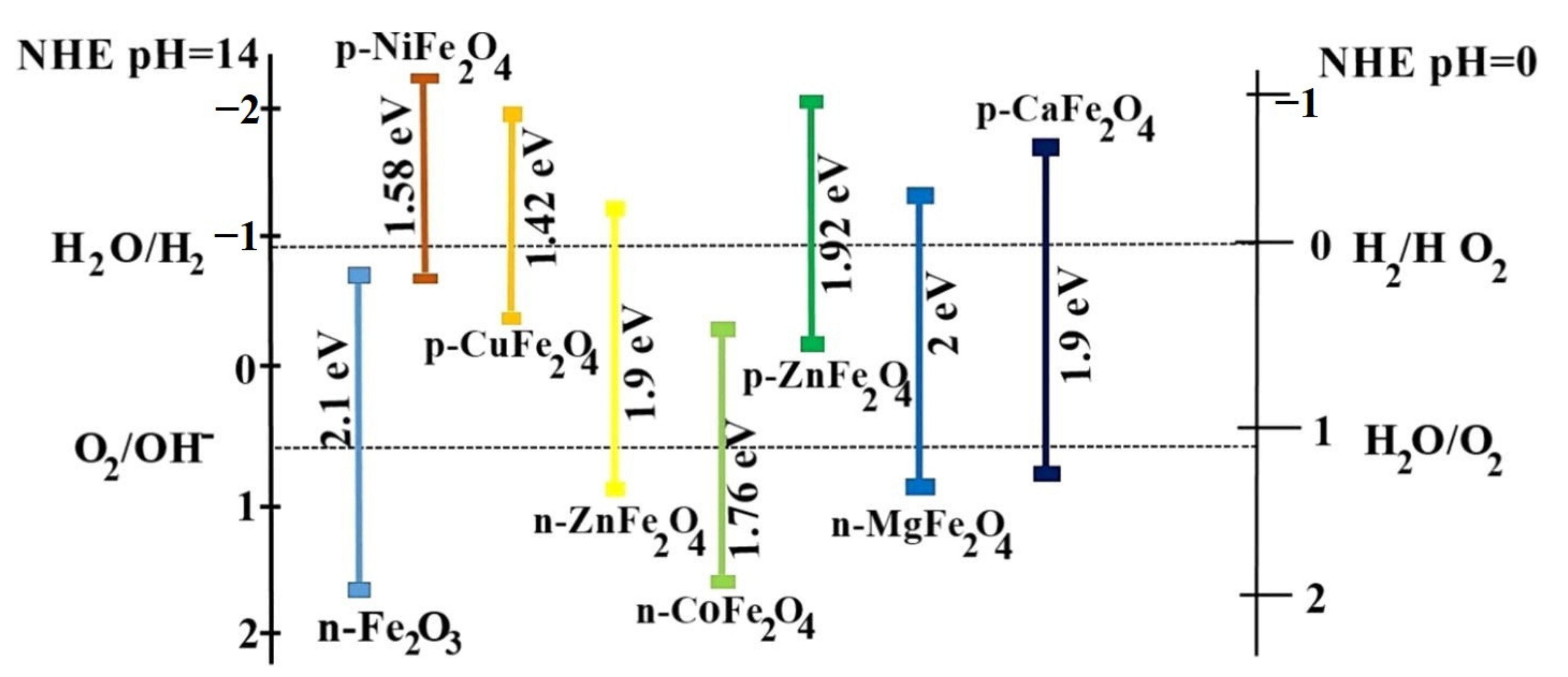

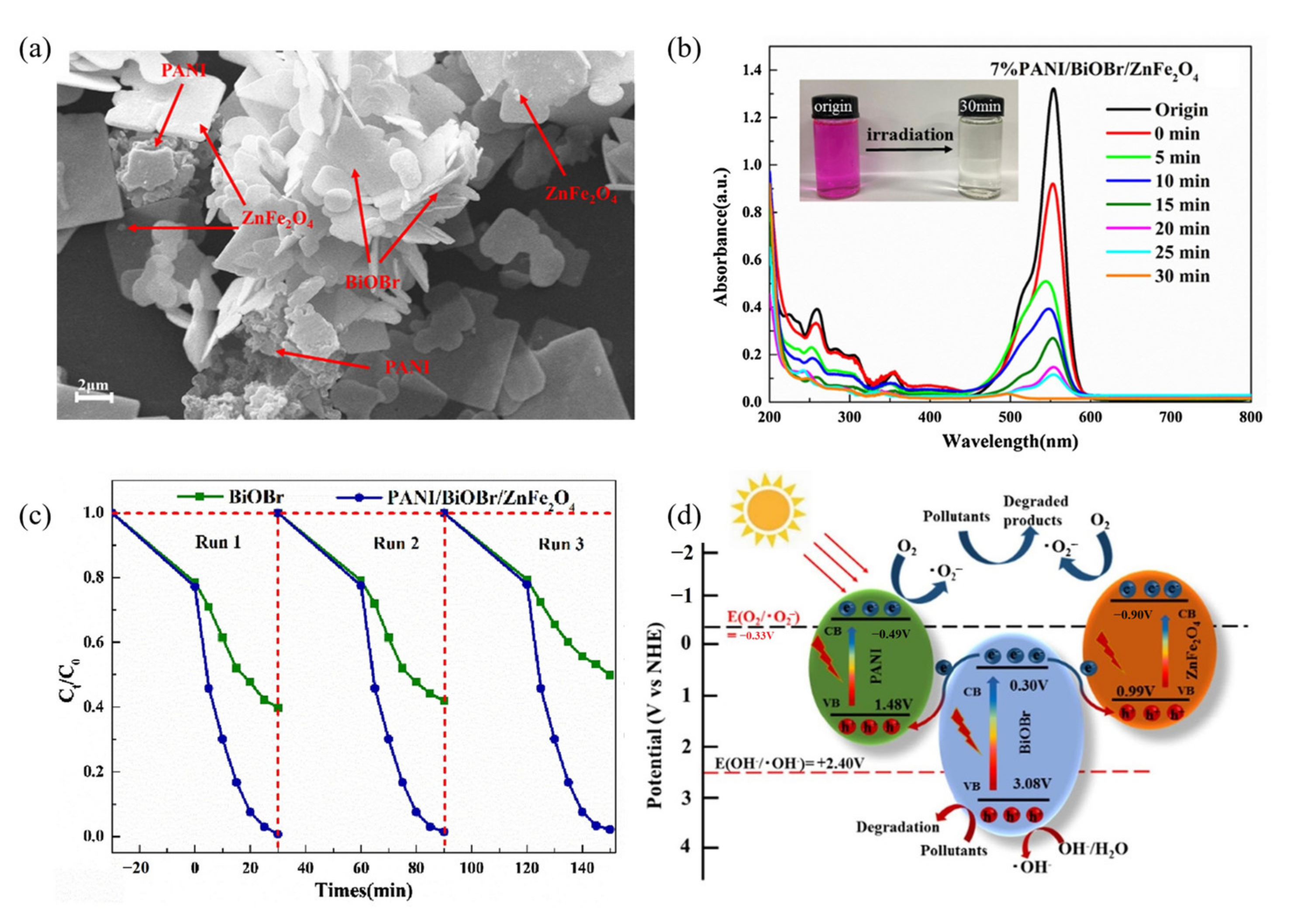

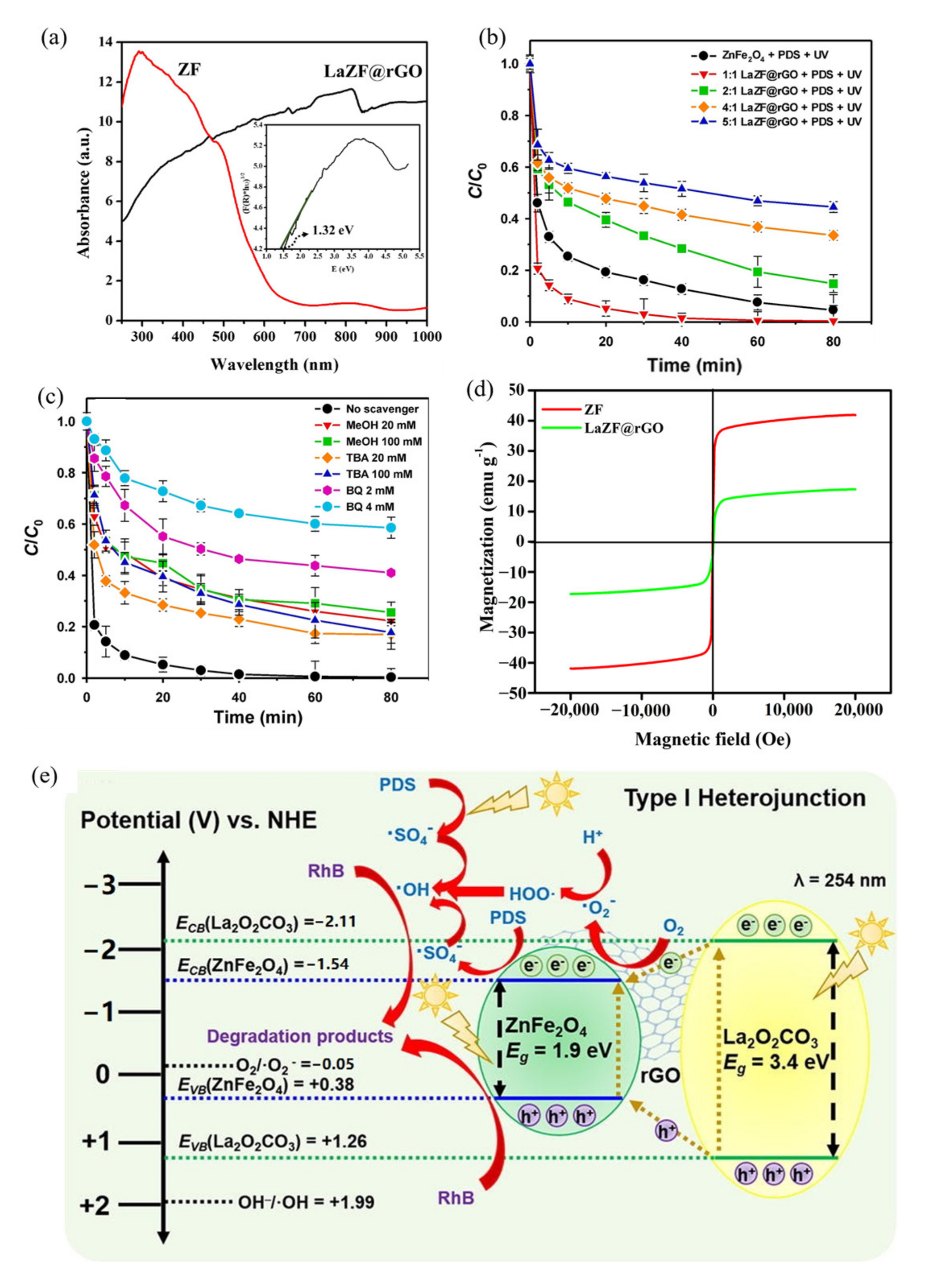
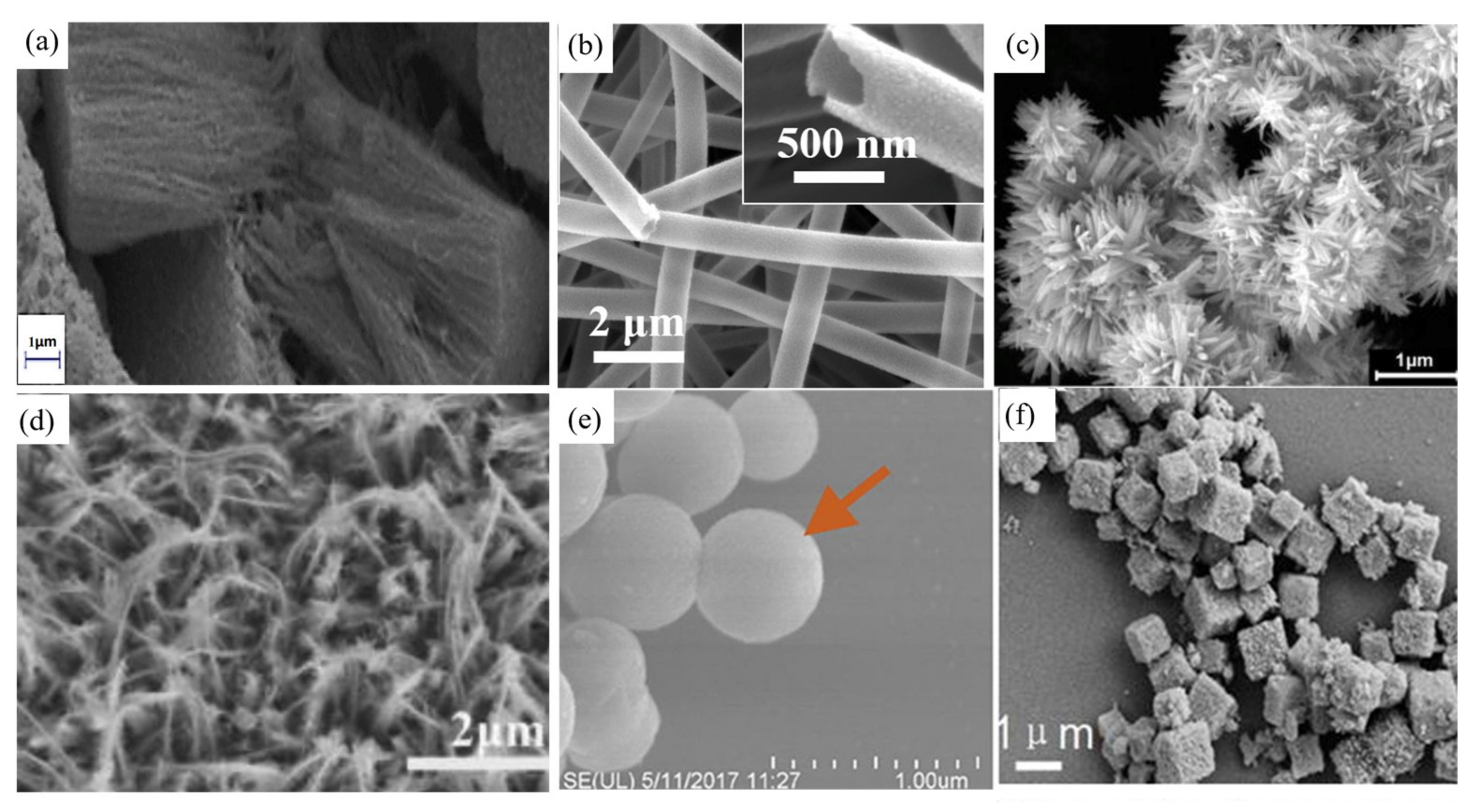
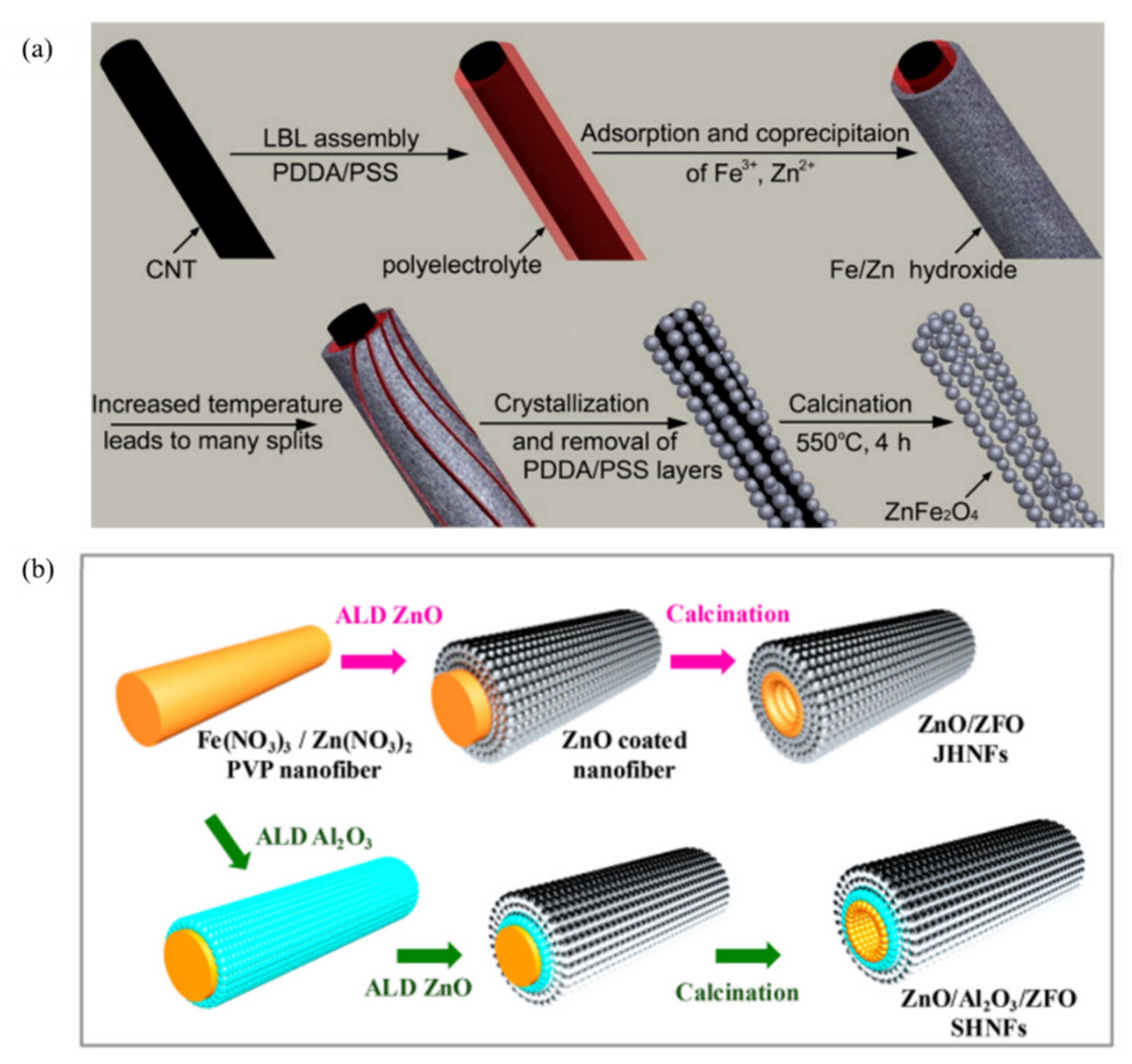
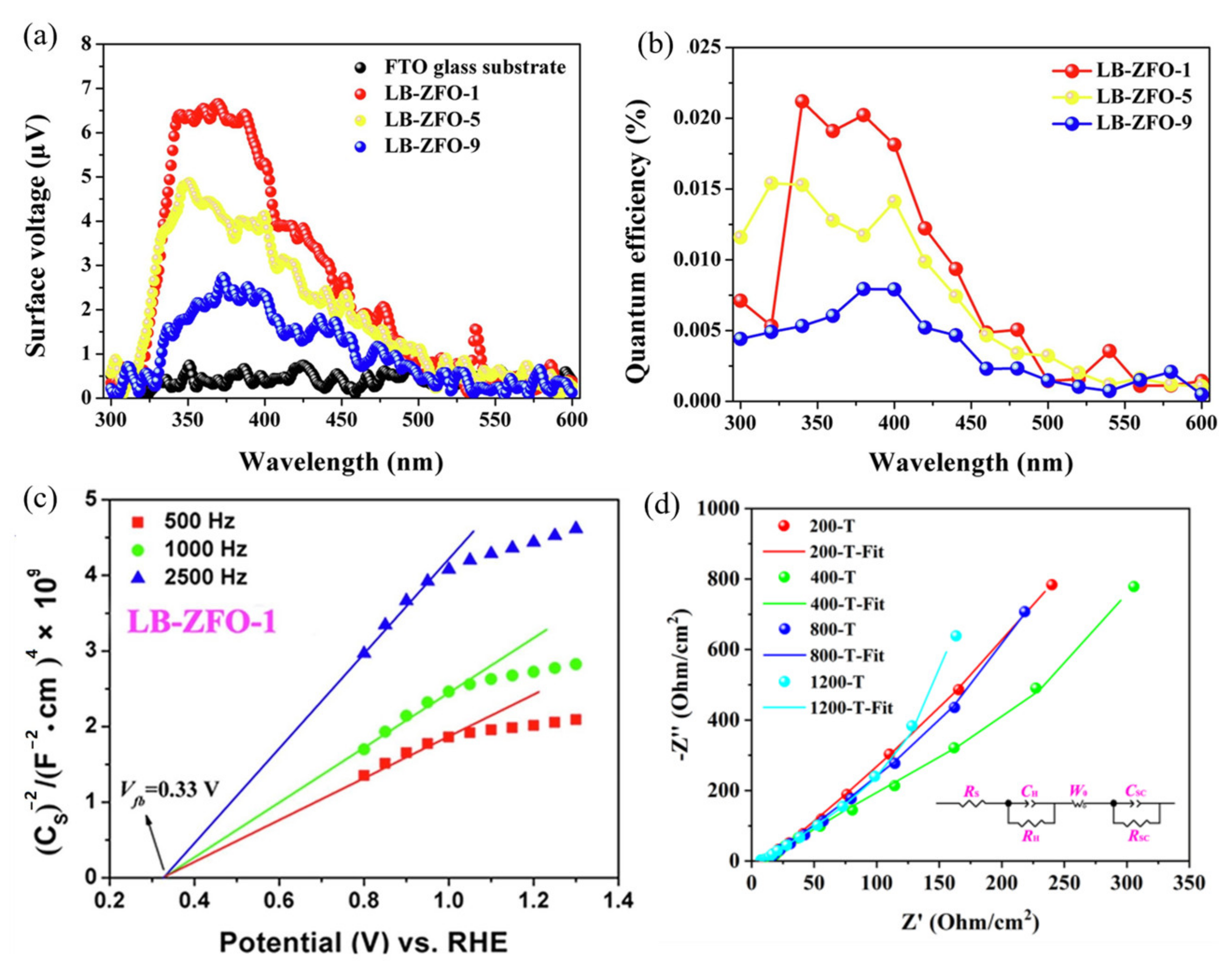
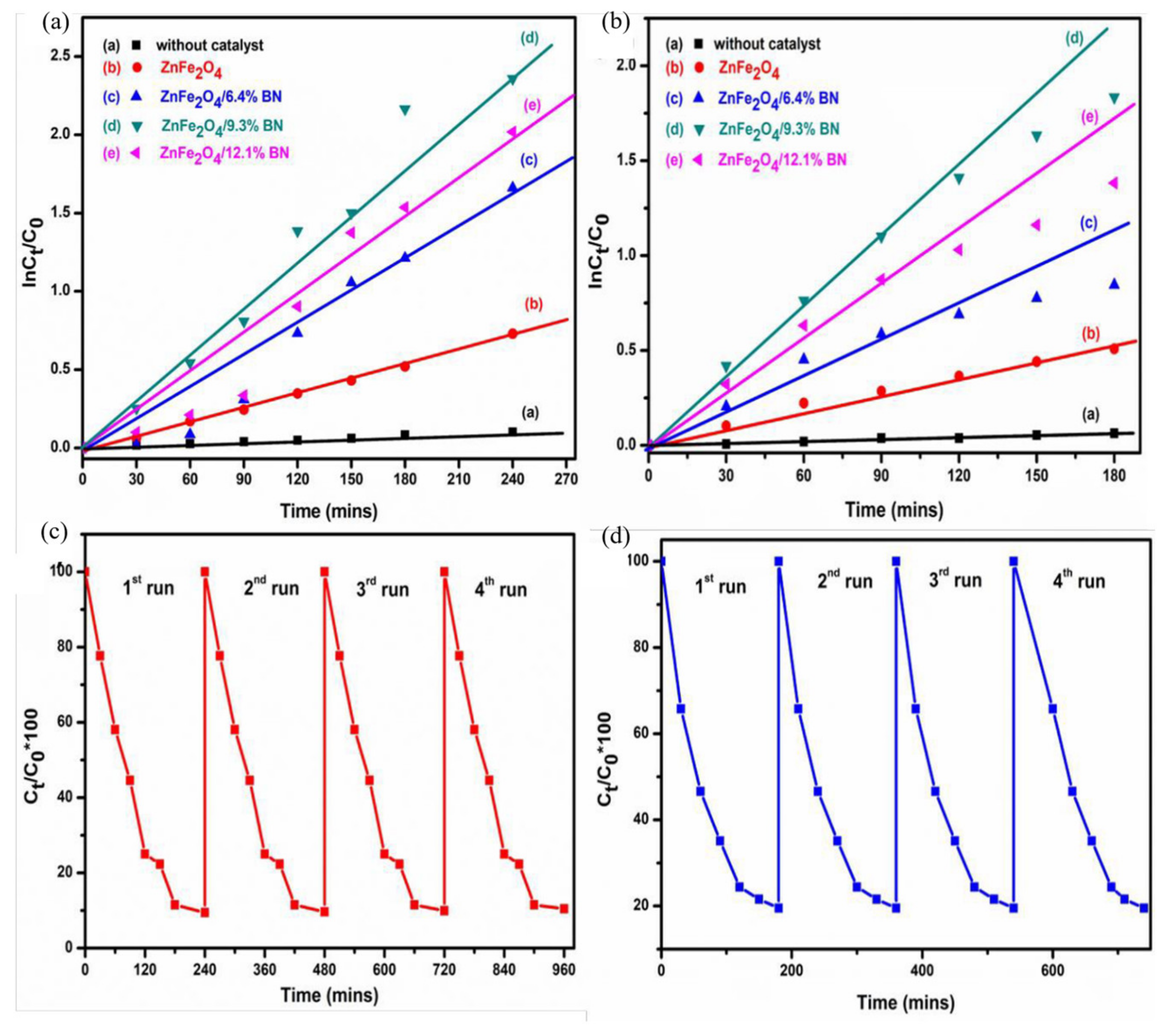

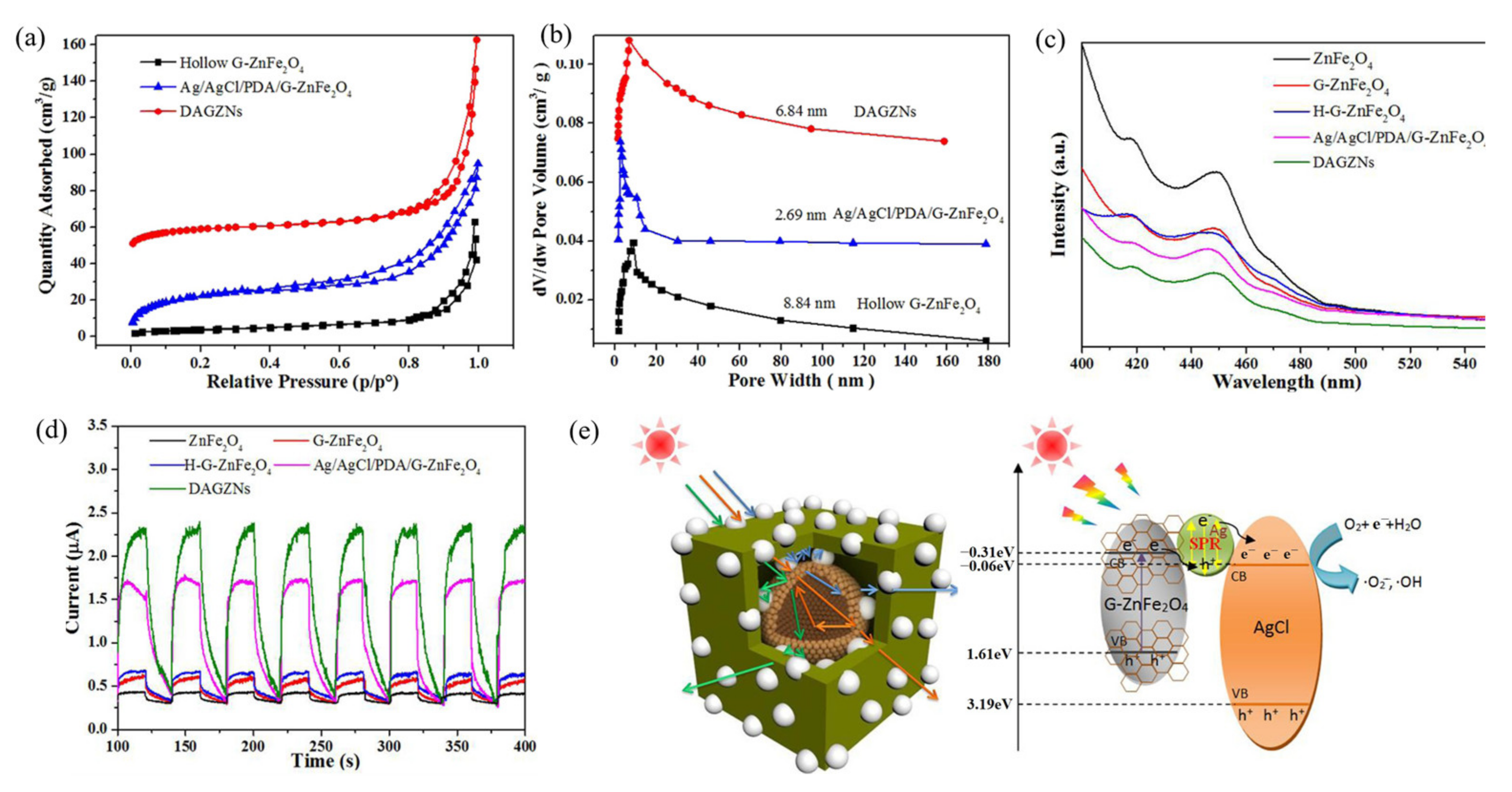
| ZnFe2O4 Dose (g/L) | Synthesis Method | Pollutants and Concentrations (mg/L) | Time (min) and Degradation Efficiency (%) | Refs. |
|---|---|---|---|---|
| 0.2 | Biosynthesis method (Nyctanthes arbor-tristis flower extract) | RhB, 5 | 30, 100 | 2019 [68] |
| 0.5 | Co-precipitation | Methylene Blue (MB), 10 | 50, 99 | 2018 [72] |
| —— | Biosynthesis method (Jatropha extract) | Sudan, —— | ——, 98 | 2020 [69] |
| —— | Biosynthesis method (Simbang Darah (Iresine herbstii) extract) | Direct Red 81, 35 | 120, 99.66 | 2021 [70] |
| 0.5 | Biosynthesis method (Sugarcane juice) | MB, 5 & RhB, 5 | 120, 98 & 95 | 2018 [73] |
| 0.6 | —— | Terramycin, 40 | 60, 94.68 | 2022 [74] |
| 2 | A single-mode microwave combustion process | RhB, 10 | 60, 5 | 2022 [75] |
| 0.3 | Electrospinning and co-precipitation | MB, 5 | 60, 29.7% | 2022 [76] |
| 1 | Hydrothermal | MB, 2 | 240, 20 | 2018 [77] |
| 0.1 | Ultra-sonication assisted chemical precipitation method | MB, 25 | 160, 66.4% | 2021 [78] |
| Method | Preparation Principles | Advantage | Shortcoming |
|---|---|---|---|
| Microwave-assisted method | Using microwave heating, energy is directly transferred to the reactant particles through chemical interaction with electromagnetic radiation, providing a thermally uniform nucleation environment. | The product has a very fine particle size, a high heating rate, high purity, low thermal inertia, and low cost. | Complex equipment, different reactors are needed to apply high pressure, and the reactors take a long time to react. |
| Ball milling method | Dry or wet milling of the metal salt mixture in a ball mill breaks up and combines the particles repeatedly. | Low cost, simple operation, no use of toxic and expensive solutions, prevention of particle agglomeration, potential for large-scale production. | Low product purity, risk of material contamination during milling, long production cycle, special equipment required. |
| Vapor deposition method | Using thermal decomposition, precursor gases are converted to thin films, which are deposited on substrates. | It has good conformal coverage on complex structures and can deposit various materials such as metals, oxides, semiconductors, etc. | Crystallinity of the deposited layer is usually columnar and not bendable. |
| Sonochemical method | Mechanical waves act on the fluid, producing a sharp motion that results in tiny bubbles that compress the solvent and sparse it, thereby speeding up chemical reactions. | Low energy consumption; quick response; uniform size; simple and economical. | The product has a low magnetic moment and poor crystallinity. |
| Pulsed laser method | Different substrates are bombarded with laser light, and the bombarded substances are deposited. | High deposition rate, short test cycle, easy cleaning. | Molten particles generated by the laser acting on the target can cause contamination of the material and uneven film thickness. |
| Ceramic method | After the two metal oxides (ZnO and Fe2O3) are mixed and ground into fine particles, they are pressed into particles using a press and sintered at a high temperature. | —— | Some of the reactants and intermediate products remain. The purity is not high, the particle size is large, the reaction temperature is extremely high, the limitations are obvious, and the energy consumption is large. |
| Spray pyrolysis | The raw materials are first dissolved and then atomized and volatilized to obtain the product. | As raw materials are thermally decomposed during the preparation process, the reaction temperature is low, operation is quick and simple, and washing and grinding are unnecessary. | Equipment is easily corroded by polluted reaction gases. |
| Researcher | A Site | B Site | Modified Catalyst | Eg/eV | Refs. |
|---|---|---|---|---|---|
| Son et al. | Ni | -- | ZnFe2O4 | 1.81 | [89] |
| Zn0.75Ni0.25Fe2O4 | 1.73 | ||||
| Zn0.4Ni0.6Fe2O4 | 1.72 | ||||
| Somvanshi et al. | Mg | -- | ZnFe2O4 | 2.39 | [62] |
| Zn0.5Mg0.5Fe2O4 | 1.96 | ||||
| Fadl et al. | Cr, Ni | -- | Ni0.4Zn0.5Cr0.1Fe2O4 | 3.9 | [90] |
| Ni0.4Zn0.3Cr0.3Fe2O4 | 3.85 | ||||
| Ni0.4Cr0.6Fe2O4 | 3.78 | ||||
| Abdo et al. | Co, Cu | Y | Co0.5Cu0.25Zn0.25Fe2.0O4 | 1.57 | [91] |
| Co0.5Cu0.25Zn0.25Y0.06Fe1.94O4 | 1.54 | ||||
| Co0.5Cu0.25Zn0.25Y0.1Fe1.9O4 | 1.52 | ||||
| Lemziouka et al. | -- | La | ZnFe1·99La0·01O4 | 1.945 | [92] |
| Tholkappiyan et al. | -- | La | ZnFe1·94La0·06O4 | 1.97 | [93] |
| Shoba et al. | -- | Er | ZnFe2O4 | 1.85 | [94] |
| ZnFe1.8Er0.2O4 | 1.93 | ||||
| ZnFe1.6Er0.4O4 | 1.96 |
| Sr. No. | Catalyst and Dose (g/L) | Modification Method | Light Source | Pollutants and Concentrations (mg/L) | Time (min) and Degradation Efficiency (%) | Stability | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | Co0.5Zn0.5Fe2O4, 0.05 | Elemental doping (A site) | 300 W Tungsten lamp | MB, 10 | 60 min, 77% | —— | [127] |
| 2 | Ni0.1Zn0.9Fe2O4, 0.4 | Elemental doping (A site) | 500 W Xenon lamp | Levofloxacin, 10 | 90 min, 98.6% | Five cycles 100% | [133] |
| 3 | Zn0.9Dy0.1Fe2O4, 1 | Elemental doping (A site) | 125 W Mercury lamp | MB, 20 | 45 min, 97.3% | Five cycles 100% | [134] |
| 4 | Co0.5Cu0.25Zn0.25Y0.1Fe1.9O4, 0.5 | Elemental doping (AB site) | 250 W Mercury lamp and 500 W Xenon lamp | MB, 20 | 60 min, 95% | Five cycles 99% | [91] |
| 5 | Zn0.5Cu0.5Fe1.95Ce0.05O4,1 | Elemental doping (AB site) | 250 W Mercury lamp and 500 W Tungsten lamp | Bisphenol A, 10 | 180 min, 91.3% | Three cycles 97% | [55] |
| 6 | Cd0.75Zn0.25Co0.25Fe1.75O4, 0.2 | Elemental doping (AB site) | 400 W Mercury lamp | MO, 2 | 120 min, 82% | Six cycles 100% | [128] |
| 7 | ZFCN@20PPY, 0.2 | Compound modification (conducting polymers) | Solar light | Ciprofloxacin, 20 | 120 min, 92% | Four cycles 100% | [101] |
| 8 | PANI/BiOBr/ZnFe2O4, 1 | Compound modification (conducting polymers) | Mercury lamp | RhB, 20 | 60 min, 99% | Three cycles 98% | [102] |
| 9 | ZnFe2O4/SnFe2O4, 0.3 | Compound modification (metal oxide) | 300 W Xenon lamp | Pharmaceutical wastewater, —— | 720 min, 77.5% | Four cycles 100% | [103] |
| 10 | ZnFe2O4/TiO2, 0.83 | Compound modification (metal oxide) | 150 W Mercury lamp | RhB, 50 | 10 min, 97.87% | —— | [135] |
| 11 | ZnFe2O4/Fe2O3, 0.5 | Compound modification (metal oxide) | 300 W Xenon lamp | Ciprofloxacin, 10 | 180 min, 96.5% | Five cycles 100% | [56] |
| 12 | CeO2/ZnFe2O4, 0.15 | Compound modification (metal oxide) | 500 W Tungsten lamp | MB, 25 | 90 min, 90.48% | Six cycles 92% | [104] |
| 13 | γ-MnO2@ZnFe2O4/rGO, 0.2 | Compound modification (metal oxide and carbon material) | —— | Phenol, 20 | 30 min, 100% | Five cycles 100% | [131] |
| 14 | ZnFe2O4/SnS2, 0.03 | Compound modification (metal sulfide) | 500 W Tungsten lamp | MB, 25 | 45 min, 91.78% | Six cycles 100% | [105] |
| 15 | ZnFe2O4/CdS, 1 | Compound modification (metal sulfide) | Visible light | RhB, 5 | 120 min, 100% | Three cycles 100% | [106] |
| 16 | LaZF@rGO, 0.25 | Compound modification (carbon material) | 11 W Tungsten lamp | RhB, 30 | 60 min, 100% | Four cycles 91% | [108] |
| 17 | ZnFe2O4@rGO, 0.5 | Compound modification (carbon material) | 150 W One-sun solar simulator | MB, 10 | 60 min, 94% | Three cycles 91% | [109] |
| 18 | C-ZnFe2O4, 1.5 | Compound modification (carbon material) | —— | Noroxin, 5 | 40 min, 86.2% | Four cycles 98% | [111] |
| 19 | ZnFe2O4, —— | Morphology modification (nanosheets) | —— | Sudan red, —— | 120 min, 67% | —— | [69] |
| 20 | ZnFe2O4, 0.25 | Morphology modification (nano stave) | 150 W UV Osram lamp | MB, 5 | 45 min, 99.8% | Five cycles 100% | [136] |
| 21 | ZnFe2O4/BiVO4, 0.6 | Morphology modification (nanowires) | 100 W Xenon lamp | CR, 20 | 105 min, 100% | —— | [112] |
| 22 | ZnO/Al2O3/ZnFe2O4, 1 | Morphology modification (nanofibers) | 150 W Xenon lamp | MB, 10 | 120 min, 90% | —— | [113] |
| 23 | ZFO/FTO, —— | Morphology modification (films) | 350 W Xenon lamp | MB, 100 | 19 min, 100% | Ten cycles 100% | [119] |
| 24 | ZnFe2O4, 0.5 | Morphology modification (hollow cube) | 300 W Xenon lamp | Tetracycline hydrochloride, 40 | 20 min, 80% | —— | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhu, Y.; Chen, Z.; Wu, S.; Fang, X.; Yao, Y. Progress in the Preparation and Modification of Zinc Ferrites Used for the Photocatalytic Degradation of Organic Pollutants. Int. J. Environ. Res. Public Health 2022, 19, 10710. https://doi.org/10.3390/ijerph191710710
Zhu J, Zhu Y, Chen Z, Wu S, Fang X, Yao Y. Progress in the Preparation and Modification of Zinc Ferrites Used for the Photocatalytic Degradation of Organic Pollutants. International Journal of Environmental Research and Public Health. 2022; 19(17):10710. https://doi.org/10.3390/ijerph191710710
Chicago/Turabian StyleZhu, Jinyuan, Yingying Zhu, Zhen Chen, Sijia Wu, Xiaojian Fang, and Yan Yao. 2022. "Progress in the Preparation and Modification of Zinc Ferrites Used for the Photocatalytic Degradation of Organic Pollutants" International Journal of Environmental Research and Public Health 19, no. 17: 10710. https://doi.org/10.3390/ijerph191710710
APA StyleZhu, J., Zhu, Y., Chen, Z., Wu, S., Fang, X., & Yao, Y. (2022). Progress in the Preparation and Modification of Zinc Ferrites Used for the Photocatalytic Degradation of Organic Pollutants. International Journal of Environmental Research and Public Health, 19(17), 10710. https://doi.org/10.3390/ijerph191710710







