Importance of Punctual Monitoring to Evaluate the Health Effects of Airborne Particulate Matter
Abstract
1. Introduction
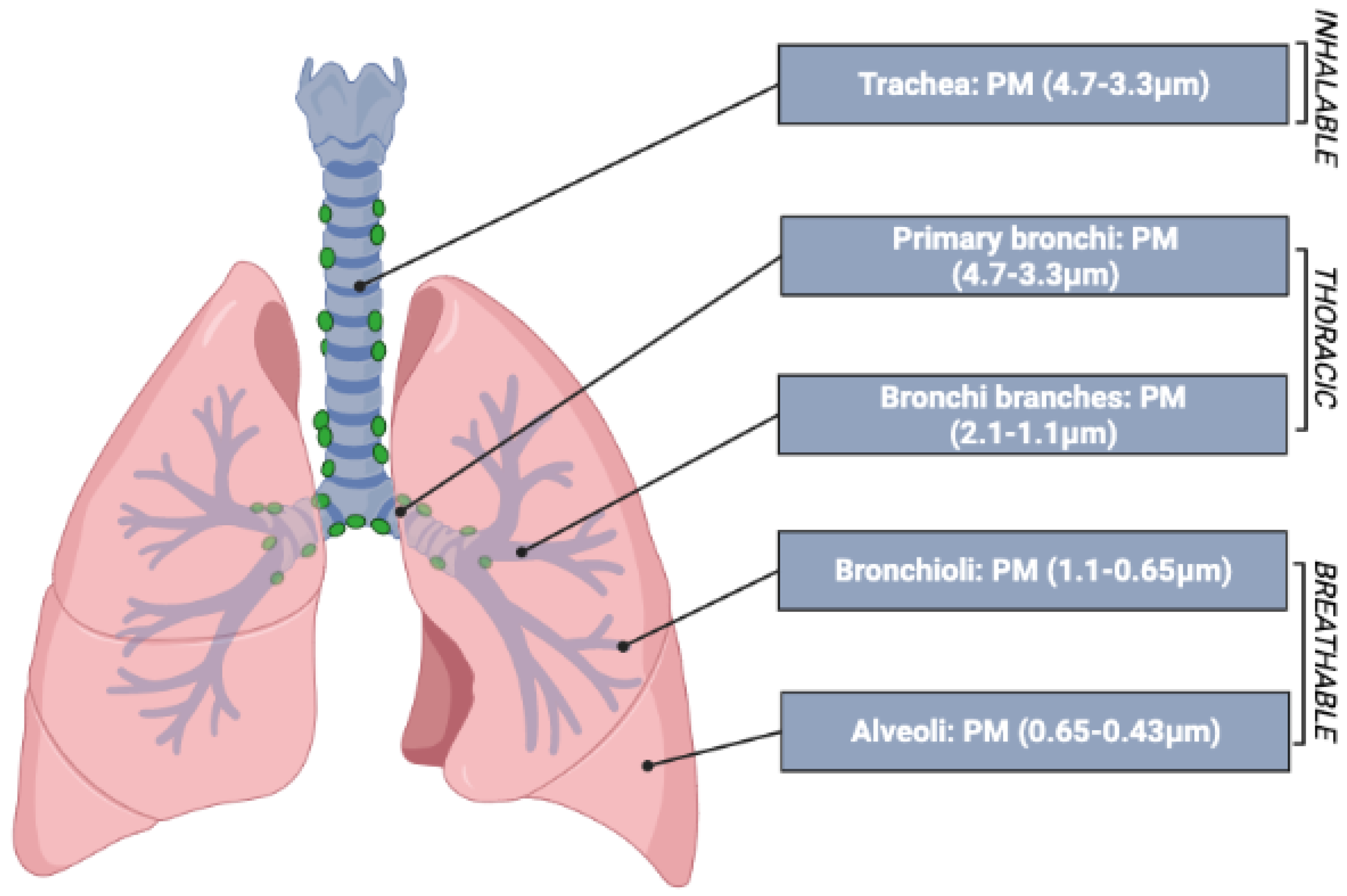
2. Particle Size
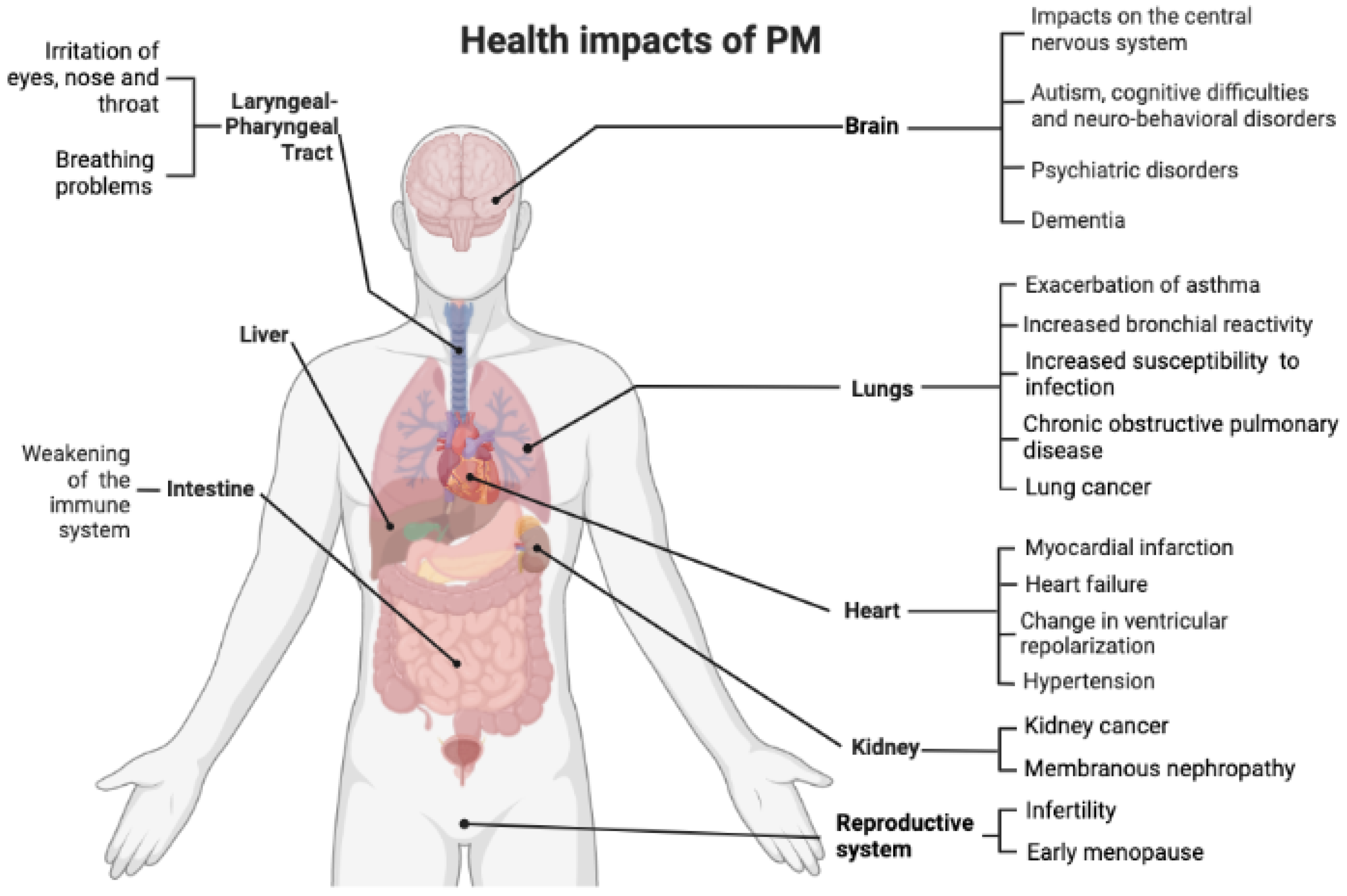
3. Health Effects of Exposure to Particulate Matter
- The inhalable fraction: includes all particles that manage to enter through the nostrils and mouth;
- The thoracic fraction: includes particles that manage to pass through the larynx and enter the lungs during inhalation, reaching the tracheo-bronchial region (including the trachea and the cilia);
- The respirable fraction: this includes particles small enough to reach the alveolar region, including the non-ciliated airways and alveolar sacs.
- -
- Epidemiological studies, which aim to identify possible associations between concentrations of particles in the atmosphere and effects such as mortality, acute effects, effects on sensitive individuals (children and the elderly), etc., may be cross-sectional, retrospective, or prospective [36]. Cross-sectional surveys are of limited scientific value as they compare health indicators and exposure to pollutants in two different populations living in different areas. The prospective ones are scientifically very robust but very difficult and complex to implement. Retrospective studies use the time-series method, evaluating mortality and daily pollution with a lag interval of usually 24 h. It is interesting to note that, despite their relative simplicity, time-series studies provide data very similar to prospective studies, although they underestimate the real impact of PM exposure on mortality by 10–20% [37];
- -
- Toxicological studies, mostly of an experimental nature, aim to identify and understand the biological mechanisms by which exposure to particles can cause harmful effects in humans.
3.1. Respiratory Diseases
- -
- The surface activity of the particles and the ability of the particle surface to generate free radicals;
- -
- The particle aggregation/disaggregation capacities and the concentration of particles on the alveolar surface once the particles have entered the respiratory tree;
- -
- The ability to act as a carrier for different chemicals.
3.2. COVID-19 and Atmospheric Particles
3.3. Cardiovascular Diseases
3.4. Neurological Damage and Nanoparticles
4. Risk Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation (WHO). Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide. In Air Quality Guidelines: Global Update 2005; WHO Regional Office for Europe Regional Publications: Copenhagen, Denmark, 2006. [Google Scholar]
- EPA United States, Environmental Protection Agency. Air Quality Designations for Particle Pollution. 2006. Available online: http://www.epa.gov/pmdesignations/ (accessed on 14 July 2022).
- European Environment Agency (EEA). Report No 09/2020: Air Quality in Europe; Publications Office of the European Union: Luxembourg, 2020; p. 110. Available online: https://www.eea.europa.eu//publications/air-quality-in-europe-2020-report (accessed on 14 July 2022).
- EU. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe. 2008, pp. 1–44. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vitgbgioeuzt (accessed on 14 July 2022).
- Morawska, L.; Ristovski, Z.; Jayaratne, E.R.; Keogh, D.U.; Ling, X. Ambient nano and ultrafine particles from motor vehicle emissions: Characteristics, ambient processing and implications on human exposure. Atmos. Environ. 2008, 42, 8113–8138. [Google Scholar] [CrossRef]
- Javed, W.; Iakovides, M.; Stephanou, E.G.; Wolfson, J.M.; Koutrakis, P.; Guo, B. Concentrations of aliphatic and polycyclic aromatic hydrocarbons in ambient PM2.5 and PM10 particulates in Doha, Qatar. J. Air Waste Manag. Assoc. 2019, 69, 162–177. [Google Scholar] [CrossRef]
- Zou, D.; Sun, Q.; Liu, J.; Xu, C.; Song, S. Seasonal source analysis of nitrogen and carbon aerosols of PM2.5 in typical cities of Zhejiang, China. Chemosphere 2022, 303, 135026. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Di-oxide, Sulfur Dioxide and Carbon Monoxide; WHO European Centre for Environment and Health: Bonn, Germany, 2021. Available online: https://www.who.int/publications/i/item/9789240034228 (accessed on 14 July 2022).
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.S.Z.S.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef]
- Kaiser, R.; Romieu, I.; Medina, S.; Schwartz, J.; Krzyzanowski, M.; Künzli, N. Air pollution attributable postneonatal infant mortality in U.S. metropolitan areas: A risk assessment study. Environ. Health 2004, 3, 4. [Google Scholar] [CrossRef]
- Lu, F.; Xu, D.; Cheng, Y.; Dong, S.; Guo, C.; Jiang, X.; Zheng, X. Systematic review and me-ta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015, 136, 196–204. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health im-pact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Environment-and-Health WHO/Europe. 2013. Available online: https://www.euro.who.int (accessed on 14 July 2022).
- Cowie, C.T.; Rose, N.; Gillett, R.; Walter, S.; Marks, G.B. Redistribution of traffic related air pollution associated with a new road tunnel. Environ. Sci. Technol. 2012, 46, 2918–2927. [Google Scholar] [CrossRef]
- De-Kok, T.M.C.M.; Driece, H.A.L.; Hogervorst, J.G.F.; Briede, J.J. Toxicological assessment of ambient and traffic-related particulate matter: A review of recent studies. Mutat. Res. 2006, 613, 103–122. [Google Scholar] [CrossRef]
- Johansson, C.; Norman, M.; Gidhagen, L. Spatial & temporal variations of PM10 and particle number concentrations in urban air. Environ. Monit. Assess. 2007, 127, 477–487. [Google Scholar]
- Abdul Shakor, A.S.; Pahrol, M.A.; Mazeli, M.I. Effects of Population Weighting on PM10 Concentration Estimation. J. Environ. Public Health 2020, 2020, 1561823. [Google Scholar] [CrossRef]
- Crouse, D.L.; Peters, P.A.; van Donkelaar, A.; Goldberg, M.S.; Villeneuve, P.J.; Brion, O.; Khan, S.; Odwa Atari, D.; Jerrett, M.; Pope, C.A.; et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentra-tions of fine particulate matter: A Canadian national-level cohort study. Environ. Health Perspect. 2012, 120, 708–714. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency; National Service Center for Environmental Publications (NSCEP). Altitude as a Factor in Air Pollution; EPA/600/9-78/015 (NTIS PB285645); United States Environmental Protection Agency: Washington, DC, USA, 1978. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=2000TAGZ.TXT (accessed on 16 September 2020).
- Löndahl, J.; Massling, A.; Pagels, J.; Swietlicki, E.; Vaclavik, E.; Loft, S. Size-resolved respiratory-tract deposition of fine and ul-trafine hydrophobic and hygroscopic aerosol particles during rest and exercise. Inhal. Toxicol. 2007, 19, 109–116. [Google Scholar] [CrossRef]
- Löndahl, J.; Pagels, J.; Swietlicki, E.; Zhou, J.; Ketzel, M.; Massling, A.; Bohgardb, M. A set-up for field studies of respiratory tract deposition of fine and ultrafine particles in humans. J. Aerosol Sci. 2006, 37, 1152–1163. [Google Scholar] [CrossRef]
- Fu, M.; Zheng, F.; Xu, X.; Niu, L. Advances of study on monitoring and evaluation of PM2. 5 pollution Meteorol. Disaster Reduct. Res. 2011, 34, 1–6. [Google Scholar]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health Part C 2008, 26, 339–362. [Google Scholar] [CrossRef]
- Fenoglio, I.; Greco, G.; Livraghi, S.; Fubibi, B. Non-UV-induced radical reactions at the surface of TiO2 nanoparticles that may trigger toxic responses. Chemistry 2009, 15, 4614–4621. [Google Scholar] [CrossRef]
- European Environment Agency. Air Quality in Europe-2018 Report; EEA Technical Report No 12/2018; EEA: Copenhagen, Denmark, 2018.
- Brunekreef, B.; Forsberg, B. Epidemiological evidence of effects of coarse airborne particles on health. Eur. Respir. J. 2005, 26, 309–318. [Google Scholar] [CrossRef]
- World Health Organization Media Centre. Available online: https://www.who.int/mediacentre/news/releases/2014/air-pollution/en/ (accessed on 3 April 2019).
- Iriti, M.; Piscitelli, P.; Missoni, E.; Miani, A. Air Pollution and Health: The Need for a Medical Reading of Environmental Monitoring Data. Int. J. Environ. Res. Public Health 2020, 17, 2174. [Google Scholar] [CrossRef]
- Anderson, H.R.; Spix, C.; Medina, S.; Schouten, J.P.; Castellsague, J.; Rossi, G.; Zmirou, D.; Touloumi, G.; Wojtyniak, B.; Ponka, A.; et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: Results from the APHEA project. Eur. Respir. J. 1997, 10, 1064–1071. [Google Scholar] [CrossRef]
- Burns, J.; Boogaard, H.; Polus, S.; Pfadenhauer, L.M.; Rohwer, A.C.; van Erp, A.M.; Turley, R.; Rehfuess, E. Interventions to reduce ambient particulate matter air pollution and their effect on health. Cochrane Database Syst. Rev. 2019, 5, CD010919. [Google Scholar] [CrossRef]
- Correia, A.W.; Pope, C.A., III; Dockery, D.W.; Wang, Y.; Ezzati, M.; Dominici, F. The effect of air pollution control on life expectancy in the United States: An analysis of 545 us counties for the period 2000 to 2007. Epidemiology 2013, 24, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Jerrett, M.; Burnett, R.T.; Ma, R.; Pope, C.A., III; Krewski, D.; Newbold, K.B.; Thurston, G.; Shi, Y.; Finkelstein, N.; Calle, E.E.; et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology 2005, 16, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Power, M.C.; Adar, S.D.; Yanosky, J.D.; Weuve, J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. NeuroToxicology 2016, 56, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Fitness Check of the Ambient Air Quality Directives; European Commission Working Document; European Commission: Brussels, Belgium, 2019; p. 427.
- Künzli, N.; Tager, I.B. Air pollution: From lung to heart. Swiss Med. Wkly. 2005, 135, 697–702. [Google Scholar] [PubMed]
- Dominici, F.; McDermott, A.; Daniels, M.; Zeger, S.L.; Samet, J.M. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: Mortality among residents of 90 cities. J. Toxicol. Environ. Health A 2005, 68, 1071–1092. [Google Scholar] [CrossRef]
- Izzotti, A.; Parodi, S.; Quaglia, A.; Faré, C.; Vercelli, M. The relationship between urban airborne pollution and mortality: Quantitative and qualitative aspects. Eur. J. Epidemiol. 2000, 16, 1027–1034. [Google Scholar] [CrossRef]
- Sioutas, C.; Delfino, R.J.; Singh, M. Exposure Assessment for Atmospheric Ultrafine Particles (UFPs) and Implications in Epidemiologic Research. Environ. Health Perspect. 2005, 113, 947–955. [Google Scholar] [CrossRef]
- Cyrys, J.; Stolzel, M.; Heinrich, J.; Kreyling, W.G.; Menzel, N.; Wittmaack, K.; Touch, T.; Wichmann, H.-E. Elemental composition and sources of fine and ultrafine ambient particles in Erfur, Germany. Sci. Total Environ. 2003, 305, 143–156. [Google Scholar] [CrossRef]
- Liu, L.; Urch, B.; Poon, R.; Szyszkowicz, M.; Speck, M.; Gold, D.R.; Wheeler, A.J.; Scott, J.A.; Brook, J.R.; Thorne, P.S.; et al. Effects of ambient coarse, fine, and ultrafine particles and their biological constituents on systemic biomarkers: A controlled human exposure study. Environ. Health Perspect. 2015, 123, 534–540. [Google Scholar] [CrossRef]
- van Eeden, S.F.; Akata, K. Macrophages-the immune effector guardians of the lung: Impact of corticosteroids on their functional responses. Clin. Sci. 2020, 134, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Döring, A.; Wichmann, H.-E.; Koenig, W. Increased plasma viscosity during an air pollution episode: A link to mor-tality? Lancet 1997, 349, 1582–1587. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, X.; Lin, H.; Zhao, M.; Liu, W.; Li, W.; Han, L.; Ma, Q.; Dong, C.; Li, Y.; et al. Adipose mesenchymal stem cell-derived antioxidative extracellular vesicles exhibit anti-oxidative stress and immunomodulatory effects under PM2.5 exposure. Toxicology 2021, 447, 152627. [Google Scholar] [CrossRef]
- Lee, H.-C.; Lin, T.-H. Air Pollution Particular Matter and Atherosclerosis. Acta Cardiol. Sin. 2017, 33, 646–647. [Google Scholar]
- Parodi, S.; Vercelli, M.; Garrone, E.; Fontana, V.; Izzotti, A. Ozone air pollution and daily mortality in Genoa, Italy between 1993 and 1996. Public Health 2005, 119, 844–850. [Google Scholar] [CrossRef]
- Liu, X.; Lian, H.; Ruan, Y.; Liang, R.; Zhao, X.; Routledge, M.; Fan, Z. Association of Exposure to particular matter and Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2015, 12, 12924–12940. [Google Scholar] [CrossRef]
- Wu, J.; Tou, F.; Yang, Y.; Liu, C.; Hower, J.C.; Baalousha, M.; Wang, G.; Liu, M.; Hochella, M.F., Jr. Metal-Containing Nanoparticles in Low-Rank Coal-Derived Fly Ash from China: Characterization and Implications toward Human Lung Toxicity. Environ. Sci. Technol. 2021, 55, 6644–6654. [Google Scholar] [CrossRef]
- Cass, G.R.; Hughes, L.A.; Bhave, P.; Kleeman, M.J.; Allen, J.O.; Salmon, L.G. The Chemical Composition of Atmospheric Ultrafine Particles. Philos. Trans. R. Soc. A 2000, 358, 2581–2592. [Google Scholar] [CrossRef]
- Dunne, A. Inflammasome activation: From inflammatory disease to infection. Biochem. Soc. Trans. 2011, 39, 669–673. [Google Scholar] [CrossRef]
- Costabile, F.; Birmili, W.; Klose, S.; Tuch, T.; Wehner, B.; Wiedensohler, A.; Franck, U.; Konig, K.; Sonntag, A. Spatio-temporal variability and principal components of the particle number size distribution in an urban atmosphere. Atmos. Chem. Phys. 2009, 9, 3163–3195. [Google Scholar] [CrossRef]
- Spyratos, D.; Sioutas, C.; Tsiotsios, A.; Haidich, A.-B.; Chloros, D.; Triantafyllou, G.; Sichletidis, L. Effects of particulate air pollution on nasal and lung function development among Greek children: A 19-year cohort study. Int. J. Environ. Health Res. 2015, 25, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Glencross, D.A.; Ho, T.-R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air pollution and its effects on the immune system. Free. Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef]
- Chen, J.; Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 2020, 143, 105974. [Google Scholar] [CrossRef] [PubMed]
- Schmid, O.; Möller, W.; Semmler-Behnke, M.; Ferron, G.A.; Karg, E.; Lipka, J.; Schulz, H.; Kreyling, W.G.; Stoeger, T. Biomarkers. Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers 2009, 14 (Suppl. 1), 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, Y.; Ding, Y.; Zhang, P.; He, X.; Zhang, Z. Toxicity of Two Different Size Ceria Nanoparticles to Mice After Repeated Intranasal Instillation. Nanosci. Nanotechnol. 2019, 19, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 42390–42404. [Google Scholar] [CrossRef]
- Lippmann, M.; Yeates, D.B.; Albert, R.E. Deposition, retention, and clearance of inhaled particles. Br. J. Ind. Med. 1980, 37, 337–362. [Google Scholar] [CrossRef]
- Becker, H.M.; Bertschinger, M.M.; Rogler, G. Microparticles and their impact on intestinal immunity. Dig. Dis. 2012, 30 (Suppl. 3), 47–54. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- Song, S.; Paek, D.; Park, C.; Lee, C.; Lee, J.-H.; Yu, S.-D. Exposure to ambient ultrafine particles and urinary 8-hydroxyl-2-deoxyguanosine in children with and without eczema. Sci. Total Environ. 2013, 458–460, 408–413. [Google Scholar] [CrossRef]
- Park, J.; Kwon, O.-H.; Yoon, C.; Park, M. Estimates of particulate matter inhalation doses during three-dimensional printing: How many particles can penetrate into our body? Indoor Air 2021, 31, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.P. Cigarette smoke and calcium conspire to impair CFTR function in airway epithelia. Channels 2014, 8, 172–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiu, E.Y.C.; Leung, N.H.L.; Cowling, B.J. Controversy around airborne versus droplet transmission of respiratory viruses: Implication for infection prevention. Curr. Opin. Infect. Dis. 2019, 32, 372–379. [Google Scholar] [CrossRef]
- Tung, N.T.; Cheng, P.-C.; Chi, K.-H.; Hsiao, T.-C.; Jones, T.; BéruBé, K.; Ho, K.-F.; Chuang, H.-C. Particulate matter and SARS-CoV-2: A possible model of COVID-19 transmission. Sci. Total Environ. 2021, 750, 141532. [Google Scholar] [CrossRef]
- Martin, J.; Bello, D.; Bunker, K.; Shafer, M.; Christiani, D.; Woskie, S.; Demokritou, P. Occupational exposure to nano-particles at commercial photocopy centers. J. Hazard. Mater. 2015, 298, 351–360. [Google Scholar] [CrossRef]
- Bendtsen, K.M.; Gren, L.; Malmborg, V.B.; Shukla, P.C.; Tunér, M.; Essig, Y.J.; Krais, A.M.; Clausen, P.A.; Berthing, T.; Loeschner, K.; et al. Particle characterization and toxicity in C57BL/6 mice following instillation of five different diesel exhaust particles designed to differ in physicochemical properties. Part. Fibre Toxicol. 2020, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- HSE Health and Safety Executive. Health Effects of Particles Produced for Nanotechnologies. Hazard Assessment Document EH75/6. 2004. Available online: https://www.hse.gov.uk/nanotechnology/healtheffects.pdf (accessed on 14 July 2022).
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D.W. Reduction in Fine Particulate Air Pollution and Mortality Extended Follow-up of the Harvard Six Cities Study. Am. J. Respir. Crit. Care Med. 2006, 173, 667–672. [Google Scholar] [CrossRef]
- EPA United States Environmental Protection Agency. National Air Quality and Emissions Trends Report. 1999. Available online: https://www.epa.gov/sites/default/files/2017-11/documents/trends_report_1999.pdf (accessed on 14 July 2022).
- Sicard, P.; Agathokleous, E.; De Marco, A.; Paoletti, E.; Calatayud, V. Urban population exposure to air pollution in Europe over the last decades. Environ. Sci. Eur. 2021, 33, 28. [Google Scholar] [CrossRef]
- Zhong, J.; Karlsson, O.; Wang, G.; Li, J.; Guo, Y.; Lin, X.; Zemplenyi, M.; Sanchez-Guerra, M.; Trevisi, L.; Urch, B.; et al. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc. Natl. Acad. Sci. USA 2017, 114, 3503–3508. [Google Scholar] [CrossRef] [PubMed]
- Hautekiet, P.; Nawrot, T.S.; Janssen, B.G.; Martens, D.S.; De Clercq, E.M.; Dadvand, P.; Plusquin, M.; Bijnens, E.M.; Saenen, N.D. Child buccal telomere length and mitochondrial DNA content as biomolecular markers of ageing in association with air pollution. Environ. Int. 2021, 147, 106332. [Google Scholar] [CrossRef] [PubMed]
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and COVID-19: The Role of Particulate Matter in the Spread and Increase of COVID-19’s Morbidity and Mortality. Int. J. Environ. Res. Public Health 2020, 17, 4487. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Global perspective of COVID-19 epidemiology for a full-cycle pandemic. Eur. J. Clin. Investig. 2020, 50, e13423. [Google Scholar] [CrossRef]
- Pivato, A.; Amoruso, I.; Formenton, G.; Di Maria, F.; Bonato, T.; Vanin, S.; Marion, A.; Baldovin, T. Evaluating the presence of SARS-CoV-2 RNA in particulate matter during the peak of COVID-19 in Padua, northern Italy. Sci. Total Environ. 2021, 784, 147129. [Google Scholar] [CrossRef]
- La Rosa, G.; Mancini, P.; Bonanno Ferraro, G.; Veneri, C.; Iaconelli, M.; Bonadonna, L.; Lucentini, L.; Suffredini, E. SARS-CoV-2 has been circulating in northern Italy since December 2019: Evidence from environmental monitoring. Sci. Total Environ. 2021, 750, 141711. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.; Kim, W.J.; Choi, Y.H.; Yang, S.-R.; Hong, S.-H. Diesel Particulate Matter 2.5 Induces Epithelial-to-Mesenchymal Transition and Upregulation of SARS-CoV-2 Receptor during Human Pluripotent Stem Cell-Derived Alveolar Organoid Development. Int. J. Environ. Res. Public Health 2020, 17, 8410. [Google Scholar] [CrossRef]
- Izzotti, A.; Fracchia, E.; Au, W.; Colombo, M.; Pfeffer, U.; Emionite, L.; Pavan, S.; Miotto, D.; Lova, P.; Grasselli, E.; et al. Prevention of COVID-19 Infection and Related Complications by Ozonized Oils. J. Pers. Med. 2021, 11, 226. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Q.; Zhang, Y.; Yan, M.; Liu, H.; Zhang, N.; Ran, W.; Cao, J. Impacts of primary emissions and secondary aerosol formation on air pollution in an urban area of China during the COVID-19 lockdown. Environ. Int. 2021, 150, 106426. [Google Scholar] [CrossRef]
- Marris, C.R.; Kompella, S.N.; Miller, M.R.; Incardona, J.P.; Brette, F.; Hancox, J.C.; Sørhus, E.; Shiels, H.A. Polyaromatic hydro-carbons in pollution: A heart-breaking matter. J. Physiol. 2020, 598, 227–247. [Google Scholar] [CrossRef]
- Peralta, A.A.; Schwartz, J.; Gold, D.R.; Coull, B.; Koutrakis, P. Associations between PM2.5 metal components and QT interval length in the Normative Aging Study. Environ. Res. 2021, 195, 110827. [Google Scholar] [CrossRef] [PubMed]
- Englert, N. Fine particles and human-health—A review of epidemiological studies. Toxicol. Lett. 2004, 149, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Frampton, M.W. Systemic and cardiovascular effects of airway injury and inflammation: Ultrafine particle exposure in humans. Environ. Health Perspect. 2001, 109 (Suppl. 4), 529–532. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine Particulate Air Pollution and Hospital Admission for Cardiovascular and Respiratory Diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Bai, L.; Weichenthal, S.; Kwong, J.C.; Burnett, R.T.; Hatzopoulou, M.; Jerrett, M.; Van Donkelaar, A.; Martin, R.V.; Van Ryswyk, K.; Lu, H.; et al. Associations of long-term exposure to ultrafine particles and nitrogen dioxide with increased in-cidence of congestive heart failure and acute myocardial infarction. Am. J. Epidemiol. 2019, 188, 151–159. [Google Scholar] [CrossRef]
- Woo, Y.R.; Park, S.-Y.; Choi, K.; Hong, E.S.; Kim, S.; Kim, H.S. Air Pollution and Atopic Dermatitis (AD): The Impact of Particulate Matter (PM10) on an AD Mouse-Model. Int. J. Mol. Sci. 2020, 21, 6079. [Google Scholar] [CrossRef]
- Shehab, M.A.; Pope, F.D. Effects of short-term exposure to particulate matter air pollution on cognitive performance. Sci. Rep. 2019, 9, 8237. [Google Scholar] [CrossRef]
- Carugno, M.; Palpella, D.; Ceresa, A.; Pesatori, A.C.; Buoli, M. Short-term air pollution exposure is associated with lower severity and mixed features of manic episodes in hospitalized bipolar patients: A cross-sectional study in Milan, Italy. Environ. Res. 2021, 196, 110943. [Google Scholar] [CrossRef]
- Heusser, K.; Tank, J.; Holz, O.; May, M.; Brinkmann, J.; Engeli, S.; Diedrich, A.; Framke, T.; Koch, A.; Großhenning, A.; et al. Ultrafine particles and ozone perturb norepinephrine clearance rather than centrally generated sympathetic activity in humans. Sci. Rep. 2019, 9, 3641. [Google Scholar] [CrossRef]
- Santacatalina, M.; Reche, C.; Minguillón, M.C.; Escrig, A.; Sanfelix, V.; Carratalá, A.; Nicolás, J.F.; Yubero, E.; Crespo, J.; Alastuey, A.; et al. Impact of fugitive emissions in ambient PM levels and composition: A case study in Southeast Spain. Sci. Total Environ. 2010, 408, 4999–5009. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzotti, A.; Spatera, P.; Khalid, Z.; Pulliero, A. Importance of Punctual Monitoring to Evaluate the Health Effects of Airborne Particulate Matter. Int. J. Environ. Res. Public Health 2022, 19, 10587. https://doi.org/10.3390/ijerph191710587
Izzotti A, Spatera P, Khalid Z, Pulliero A. Importance of Punctual Monitoring to Evaluate the Health Effects of Airborne Particulate Matter. International Journal of Environmental Research and Public Health. 2022; 19(17):10587. https://doi.org/10.3390/ijerph191710587
Chicago/Turabian StyleIzzotti, Alberto, Paola Spatera, Zumama Khalid, and Alessandra Pulliero. 2022. "Importance of Punctual Monitoring to Evaluate the Health Effects of Airborne Particulate Matter" International Journal of Environmental Research and Public Health 19, no. 17: 10587. https://doi.org/10.3390/ijerph191710587
APA StyleIzzotti, A., Spatera, P., Khalid, Z., & Pulliero, A. (2022). Importance of Punctual Monitoring to Evaluate the Health Effects of Airborne Particulate Matter. International Journal of Environmental Research and Public Health, 19(17), 10587. https://doi.org/10.3390/ijerph191710587







