A Study of Sr Sorption Behavior in Claystone from a Candidate High-Level Radioactive Waste Geological Disposal Site under the Action of FeOOH Colloids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Methods
2.3. Data Analysis Methods
2.4. Characterization
3. Results and Discussion
3.1. Factors Influencing Sr Sorption onto the Sorbent
3.1.1. Effect of Colloid Amount
3.1.2. Effect of Solid Content
3.1.3. Effect of the pH
3.2. Interaction Mechanism
3.2.1. Sorption Kinetics Analysis
3.2.2. Microstructure Analysis
3.2.3. Mineral Species Analysis
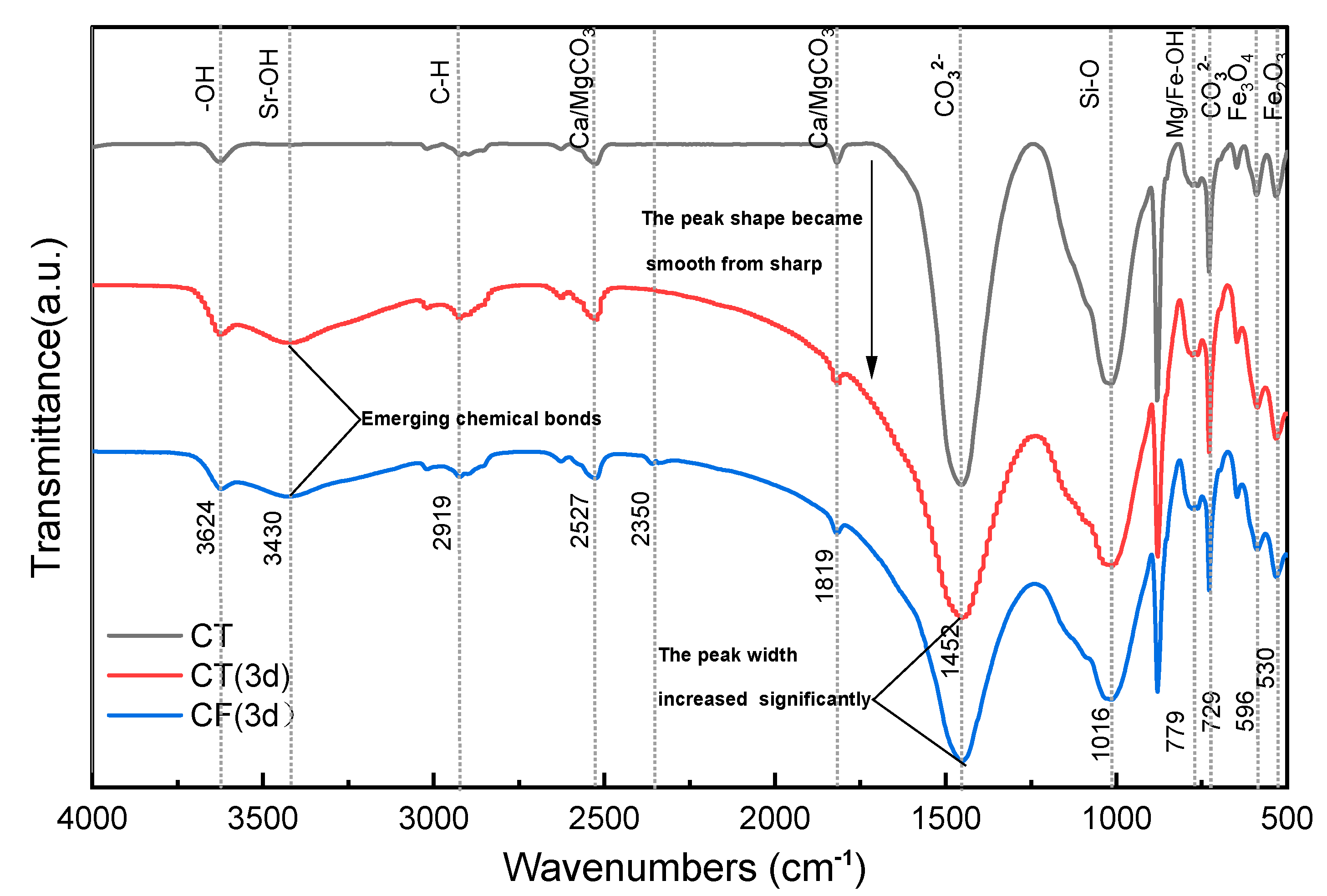
3.2.4. Functional Group Analysis
3.2.5. Chemical Bond Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prăvălie, R.; Bandoc, G. Nuclear energy: Between global electricity demand, worldwide decarbonisation imperativeness, and planetary environmental implications. J. Environ. Manag. 2018, 209, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hou, D.; Zhang, J.; O’Connor, D.; Li, G.; Gu, Q.; Li, S.; Liu, P. Environmental and socio-economic sustainability appraisal of contaminated land remediation strategies: A case study at a mega-site in China. Sci. Total Environ. 2018, 610–611, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, Y.; Sand, W.; Wang, X. Effects of deep geological environments for nuclear waste disposal on the hydrogen entry into titanium. Int. J. Hydrogen Energy 2019, 44, 12200–12214. [Google Scholar] [CrossRef]
- Chapman, N.; Hooper, A. The disposal of radioactive wastes underground. Proc. Geol. Assoc. 2012, 123, 46–63. [Google Scholar] [CrossRef]
- Grarnbow, B.; Bretesche, S. Geological disposal of nuclear waste: II. From laboratory data to the safety analysis—Addressing societal concerns. Appl. Geochem. 2014, 49, 247–258. [Google Scholar] [CrossRef]
- Sellin, P.; Leupin, O.X. The Use of Clay as an Engineered Barrier in Radioactive-Waste Management—A Review. Clays Clay Miner. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, P.; Liu, X.; Xiang, L.; Dai, C.; Rao, G. Mineral composition and geochemical characteristics of claystones in the Suhongtu area. Inner Mongolia J. Geol. 2019, 43, 253–262. [Google Scholar]
- Faghihian, H.; Iravani, M.; Moayed, M. Application of PAN-NaY Composite for CS+ and SR2+ Adsorption: Kinetic and Thermodynamic Studies. Environ. Prog. Sustain. Energy 2015, 34, 999–1008. [Google Scholar] [CrossRef]
- Ma, F.; Li, Z.; Zhao, H.; Geng, Y.; Zhou, W.; Li, Q.; Zhang, L. Potential application of graphene oxide membranes for removal of Cs(I) and Sr(II) from high level-liquid waste. Sep. Purif. Technol. 2017, 188, 523–529. [Google Scholar] [CrossRef]
- Cabrera, W.E.; Schrooten, I.; De Broe, M.E.; D’Haese, P.C. Strontium and bone. J. Bone Min. Res. 1999, 14, 661–668. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Peng, J.; Zhai, M.; Li, J.; Wei, G. Adsorption and desorption of Sr(II) ions in the gels based on polysaccharide derivates. J. Hazard. Mater. 2009, 171, 820–826. [Google Scholar] [CrossRef]
- Salam, M.A.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; Abukhadra, M.R. Insight into the role of the zeolitization process in enhancing the adsorption performance of kaolinite/diatomite geopolymer for effective retention of Sr (II) ions; batch and column studies. J. Environ. Manag. 2021, 294, 112984. [Google Scholar] [CrossRef]
- Yu, S.; Mei, H.; Chen, X.; Tan, X.; Ahmad, B.; Alsaedi, A.; Hayat, T.; Wang, X. Impact of environmental conditions on the sorption behavior of radionuclide 90 Sr(II) on Na-montmorillonite. J. Mol. Liq. 2015, 203, 39–46. [Google Scholar] [CrossRef]
- Xu, D.; Zuo, R.; Han, K.; Ding, F.; Jin, S.; Zhao, X.; Shi, R.; Wang, J. Sorption of Sr in granite under typical colloidal action. J. Contam. Hydrol. 2020, 233, 103659. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Watson, E.B. Partitioning of strontium between calcite and fluid. Geochem. Geophys. Geosyst. 2006, 7, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; He, Y.; Zhang, K.-N.; Chen, Y.-G.; Ye, W.-M. Montmorillonite alteration and its influence on Sr (II) adsorption on GMZ bentonite. Environ. Earth Sci. 2021, 80, 791. [Google Scholar] [CrossRef]
- Chen, X.; Peng, S.; Wang, J. Retention profile and kinetics characteristics of the radionuclide 90-Sr (II) onto kaolinite. J. Radioanal. Nucl. Chem. Artic. 2014, 303, 509–519. [Google Scholar] [CrossRef]
- Lihareva, N.; Dimowa, L.; Petrov, O.; Tzvetanova, Y.; Atanasova-Vladimirova, S. Study of the kinetics and mechanism of Sr2+ sorption by clinoptilolite. J. Radioanal. Nucl. Chem. Artic. 2019, 321, 31–38. [Google Scholar] [CrossRef]
- Kasar, S.; Kumar, S.; Saha, A.; Tomar, B.S.; Bajpai, R.K. Mechanistic and thermodynamic aspects of Cs(I) and Sr(II) interactions with smectite-rich natural clay. Environ. Earth Sci. 2017, 76, 274. [Google Scholar] [CrossRef]
- Qi, H.; Liu, H.; Gao, Y. Removal of Sr (II) from aqueous solutions using polyacrylamide modified graphene oxide composites. J. Mol. Liq. 2015, 208, 394–401. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, D.; Wei, X.; Xian, D.; Wang, P.; Hou, J.; Xu, Z.; Liu, C.; Wu, W. Insight into the stability and correlated transport of kaolinite colloid: Effect of pH, electrolytes and humic substances. Environ. Pollut. 2020, 266, 115189. [Google Scholar] [CrossRef]
- Zuo, R.; Chen, M.; Lin, Y.; Yang, J.; Jin, S.; Yue, W.; Wang, J.; Teng, Y. Effect of a humic acid colloid on the sorption behaviour of Sr onto soil in a candidate high-level radioactive waste geological disposal site. Environ. Sci. Pollut. Res. 2019, 26, 25235–25246. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liang, H.; Tan, T.; Wu, W. Adsorption behavior of Th (IV) onto illite: Effect of contact time, pH value, ionic strength, humic acid and temperature. Appl. Clay Sci. 2016, 127, 35–43. [Google Scholar]
- Fan, Q.; Shao, D.; Lu, Y.; Wu, W.; Wang, X. Effect of pH, ionic strength, temperature and humic substances on the sorption of Ni(II) to Na-attapulgite. Chem. Eng. J. 2009, 150, 188–195. [Google Scholar] [CrossRef]
- Du, C.; Zuo, R.; Chen, M.; Wang, J.; Liu, X.; Liu, L.; Lin, Y. Influence of colloidal Fe(OH)3 on the adsorption characteristics of strontium in porous media from a candidate high-level radioactive waste geological disposal site. Environ. Pollut. 2020, 260, 113997. [Google Scholar] [CrossRef]
- Wei, X.; Sun, Y.; Pan, D.; Niu, Z.; Xu, Z.; Jiang, Y.; Wu, W.; Li, Z.; Zhang, L.; Fan, Q. Adsorption properties of Na-palygorskite for Cs sequestration: Effect of pH, ionic strength, humic acid and temperature. Appl. Clay Sci. 2019, 183, 105363. [Google Scholar] [CrossRef]
- Phan, T.T.; Hakala, J.A.; Bain, D.J. Influence of colloids on metal concentrations and radiogenic strontium isotopes in groundwater and oil and gas-produced waters. Appl. Geochem. 2018, 95, 85–96. [Google Scholar] [CrossRef]
- Lead, J.R.; Wilkinson, K. Aquatic Colloids and Nanoparticles: Current Knowledge and Future Trends. Environ. Chem. 2006, 3, 159–171. [Google Scholar] [CrossRef]
- Buffle, J.; Leppard, G.G. Characterization of aquatic colloids and macromolecules. 1. Structure and behavior of colloidal material. Environ. Sci. Technol. 1995, 29, 2169–2175. [Google Scholar] [CrossRef]
- Zuo, R.; Han, K.; Shi, R.; Ding, F.; Liu, L.; Wang, J.; Teng, Y.; Yang, J.; Liu, X. Effect of Colloidal Silicate on the Migration Behaviour of Strontium in Groundwater Environment of Geological Disposal Candidate Site. J. Chem. 2019, 2019, 9606121. [Google Scholar] [CrossRef]
- Luo, X.; Yu, L.; Wang, C.; Yin, X.; Mosa, A.; Lv, J.; Sun, H. Sorption of vanadium (V) onto natural soil colloids under various solution pH and ionic strength conditions. Chemosphere 2017, 169, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Wikiniyadhanee, R.; Chotpantarat, S.; Ong, S.K. Effects of kaolinite colloids on Cd2+ transport through saturated sand under varying ionic strength conditions: Column experiments and modeling approaches. J. Contam. Hydrol. 2015, 182, 146–156. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.F.; Zachara, J.M. Subsurface transport of contaminants—Mobile colloids in the subsurface environment may alter the transport of contaminants. Environ. Sci. Technol. 1989, 23, 496–502. [Google Scholar]
- Li, M.; Liu, H.; Zhu, H.; Gao, H.; Zhang, S.; Chen, T. Kinetics and mechanism of Sr(II) adsorption by Al-Fe2O3: Evidence from XPS analysis. J. Mol. Liq. 2017, 233, 364–369. [Google Scholar] [CrossRef]
- Collins, C.R.; Sherman, D.M.; Ragnarsdöttir, K.V. The Adsorption Mechanism of Sr2+ on the Surface of Goethite. Radiochim. Acta 1998, 81, 201–206. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Cheung, C.; Porter, J.; Mckay, G. Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Res. 2000, 35, 605–612. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Kuleshova, M.L.; Danchenko, N.N.; Kosorukov, V.L.; Sergeev, V.I.; Shimko, T.G. A study of strontium and cesium sorption-desorption on bentonites of different composition. Mosc. Univ. Geol. Bull. 2017, 72, 290–298. [Google Scholar] [CrossRef]
- Murali, M.S.; Mathur, J.N. Sorption characteristics of Am (III), Sr (II) and Cs (I) on bentonite and granite. J. Radioanal. Nucl. Chem. Artic. 2002, 254, 129–136. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Kütahyalı, C.; Çetinkaya, B.; Acar, M.B.; Işık, N.O.; Cireli, I. Investigation of strontium sorption onto Kula volcanics using Central Composite Design. J. Hazard. Mater. 2012, 201–202, 115–124. [Google Scholar] [CrossRef]
- Ivanova, B.; Spiteller, M. Adsorption of uranium composites onto saltrock oxides—Experimental and theoretical study. J. Environ. Radioact. 2014, 135, 75–83. [Google Scholar] [CrossRef]
- Tan, L.; Wang, X.; Tan, X.; Mei, H.; Chen, C.; Hayat, T.; Alsaedi, A.; Wen, T.; Lu, S.; Wang, X. Bonding properties of humic acid with attapulgite and its influence on U(VI) sorption. Chem. Geol. 2017, 464, 91–100. [Google Scholar] [CrossRef]
- Khan, S.A.; Riaz ur, R.; Khan, M.A. Sorption of strontium on bentonite. Waste Manag. (Oxford) 1995, 15, 641–650. [Google Scholar] [CrossRef]
- Ghaemi, A.; Torab-Mostaedi, M.; Ghannadi-Maragheh, M. Characterizations of strontium (II) and barium (II) adsorption from aqueous solutions using dolomite powder. J. Hazard. Mater. 2011, 190, 916–921. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mangwandi, C.; Al-Muhtaseb, A.H.; Walker, G.M.; Allen, S.J.; Ahmad, M.N. Kinetic and thermodynamics of chromium ions adsorption onto low-cost dolomite adsorbent. Chem. Eng. J. 2012, 179, 193–202. [Google Scholar] [CrossRef]
- Zuo, R.; Xu, Z.; Wang, X.; Yang, J.; Du, X.; Du, C.; Cai, W.; Xu, Y.; Wu, Z. Adsorption characteristics of strontium by bentonite colloids acting on claystone of candidate high-level radioactive waste geological disposal sites. Environ. Res. 2022, 213, 113633. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Adsorption of Fe (III), Co (II) and Ni (II) on ZrO-kaolinite and ZrO-montmorillonite surfaces in aqueous medium. Colloid Surf. A 2008, 317, 71–79. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Liu, Y.; Li, M.; Wu, X.; Jiang, T.; Chen, C.; Peng, Y. Mn-substituted goethite for uranium immobilization: A study of adsorption behavior and mechanisms. Environ. Pollut. 2020, 262, 114184. [Google Scholar] [CrossRef]
- Rani, R.D.; Sasidhar, P. Geochemical and thermodynamic aspects of sorption of strontium on kaolinite dominated clay samples at Kalpakkam. Environ. Earth Sci. 2011, 65, 1265–1274. [Google Scholar] [CrossRef]
- Pingitore, N.E.; Lytle, F.W.; Davies, B.M.; Eastman, M.P.; Eller, P.; Larson, E.M. Mode of incorporation of Sr2+ in calcite: Determination by X-ray absorption spectroscopy. Geochim. Cosmochim. Acta 1992, 56, 1531–1538. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Yang, B.; Sun, H.; Wang, D.; Yin, W.; Cao, S.; Wang, Y.; Zhu, Z.; Jiang, K.; Yao, J. Selective adsorption of a new depressant Na2ATP on dolomite: Implications for effective separation of magnesite from dolomite via froth flotation. Sep. Purif. Technol. 2020, 250, 117278. [Google Scholar] [CrossRef]
- Shahwan, T.; Erten, H.N. Characterization of Sr2+ uptake on natural minerals of kaolinite and magnesite using XRPD, SEM/EDS, XPS, and DRIFT. Radiochim. Acta 2005, 93, 225–232. [Google Scholar] [CrossRef]
- Fuller, A.; Shaw, S.; Peacock, C.; Trivedi, D.; Burke, I.T. EXAFS Study of Sr sorption to Illite, Goethite, Chlorite, and Mixed Sediment under Hyperalkaline Conditions. Langmuir 2016, 32, 2937–2946. [Google Scholar] [CrossRef]
- Hodkin, D.J.; Stewart, D.I.; Graham, J.T.; Burke, I.T. Coprecipitation of 14C and Sr with carbonate precipitates: The importance of reaction kinetics and recrystallization pathways. Sci. Total Environ. 2016, 562, 335–343. [Google Scholar] [CrossRef]
- Parkman, R.; Charnock, J.; Livens, F.; Vaughan, D. A study of the interaction of strontium ions in aqueous solution with the surfaces of calcite and kaolinite. Geochim. Cosmochim. Acta 1998, 62, 1481–1492. [Google Scholar] [CrossRef]


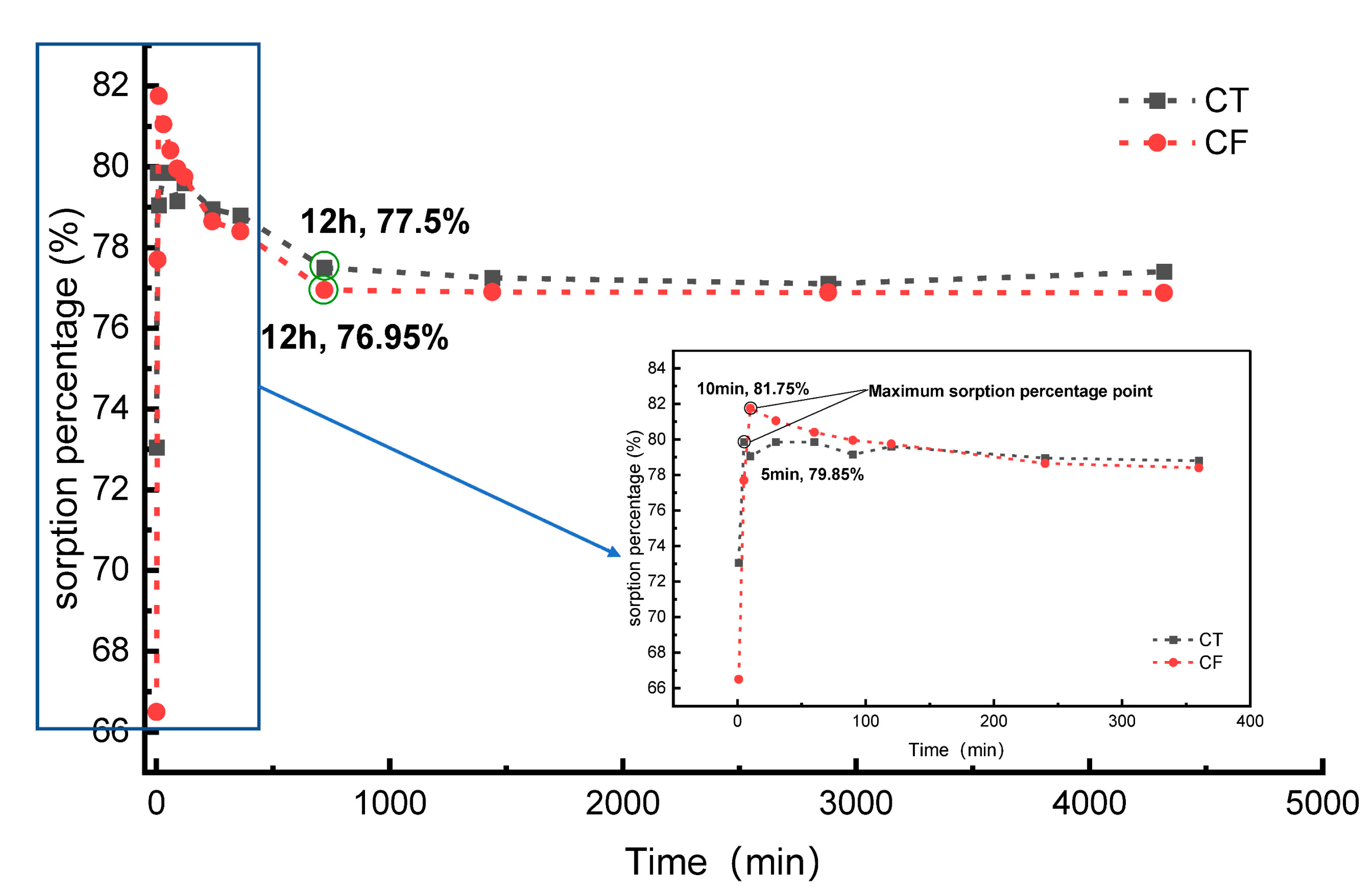



| Sorption System | Sorption Kinetics | Parameter 1 | Parameter 2 | R2 |
|---|---|---|---|---|
| CT | Pseudo-first-order (PFO) | qe (mg·g−1) = 1.5615 | kf (min−1) = 2.7433 | 0.9916 |
| Pseudo-second-order (PSO) | qe (mg·g−1) = 1.5629 | kS (g∙mg−1∙min−1) = 11.1227 | 0.9904 | |
| Elovich | α (g∙mg−1∙min−1) = 8.95 × 104 | β (g·mg-1) = 67.0384 | 0.9752 | |
| Intraparticle Diffusion | Kp (mg∙g−1∙min−1/2) = 0.0039 | C (mg·g-1) = 1.3709 | 0.0366 | |
| CF | Pseudo-first-order (PFO) | qe (mg·g−1) = 1.5494 | kf (min−1) = 1.9547 | 0.9832 |
| Pseudo-second-order (PSO) | qe (mg·g−1) = 1.5543 | kS (g∙mg−1∙min−1) = 4.3983 | 0.9798 | |
| Elovich | α (g∙mg−1∙min−1) = 3.71 × 104 | β (g·mg−1) = 67.3489 | 0.9563 | |
| Intraparticle Diffusion | Kp (mg∙g−1∙min−1/2) = 0.0038 | C (mg·g−1) = 1.3536 | 0.0344 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Cai, W.; Zuo, R.; Du, C. A Study of Sr Sorption Behavior in Claystone from a Candidate High-Level Radioactive Waste Geological Disposal Site under the Action of FeOOH Colloids. Int. J. Environ. Res. Public Health 2022, 19, 9970. https://doi.org/10.3390/ijerph19169970
Wang J, Cai W, Zuo R, Du C. A Study of Sr Sorption Behavior in Claystone from a Candidate High-Level Radioactive Waste Geological Disposal Site under the Action of FeOOH Colloids. International Journal of Environmental Research and Public Health. 2022; 19(16):9970. https://doi.org/10.3390/ijerph19169970
Chicago/Turabian StyleWang, Jinsheng, Weihai Cai, Rui Zuo, and Can Du. 2022. "A Study of Sr Sorption Behavior in Claystone from a Candidate High-Level Radioactive Waste Geological Disposal Site under the Action of FeOOH Colloids" International Journal of Environmental Research and Public Health 19, no. 16: 9970. https://doi.org/10.3390/ijerph19169970
APA StyleWang, J., Cai, W., Zuo, R., & Du, C. (2022). A Study of Sr Sorption Behavior in Claystone from a Candidate High-Level Radioactive Waste Geological Disposal Site under the Action of FeOOH Colloids. International Journal of Environmental Research and Public Health, 19(16), 9970. https://doi.org/10.3390/ijerph19169970






