Hydrogen Peroxide Activated by Biochar-Supported Sulfidated Nano Zerovalent Iron for Removal of Sulfamethazine: Response Surface Method Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analytical Instruments and Methods
2.3. Preparation of Biochar
2.4. Preparation of S-nZVI/BC
2.5. Experimental Procedure
2.6. Response Surface Method Model
3. Results and Discussion
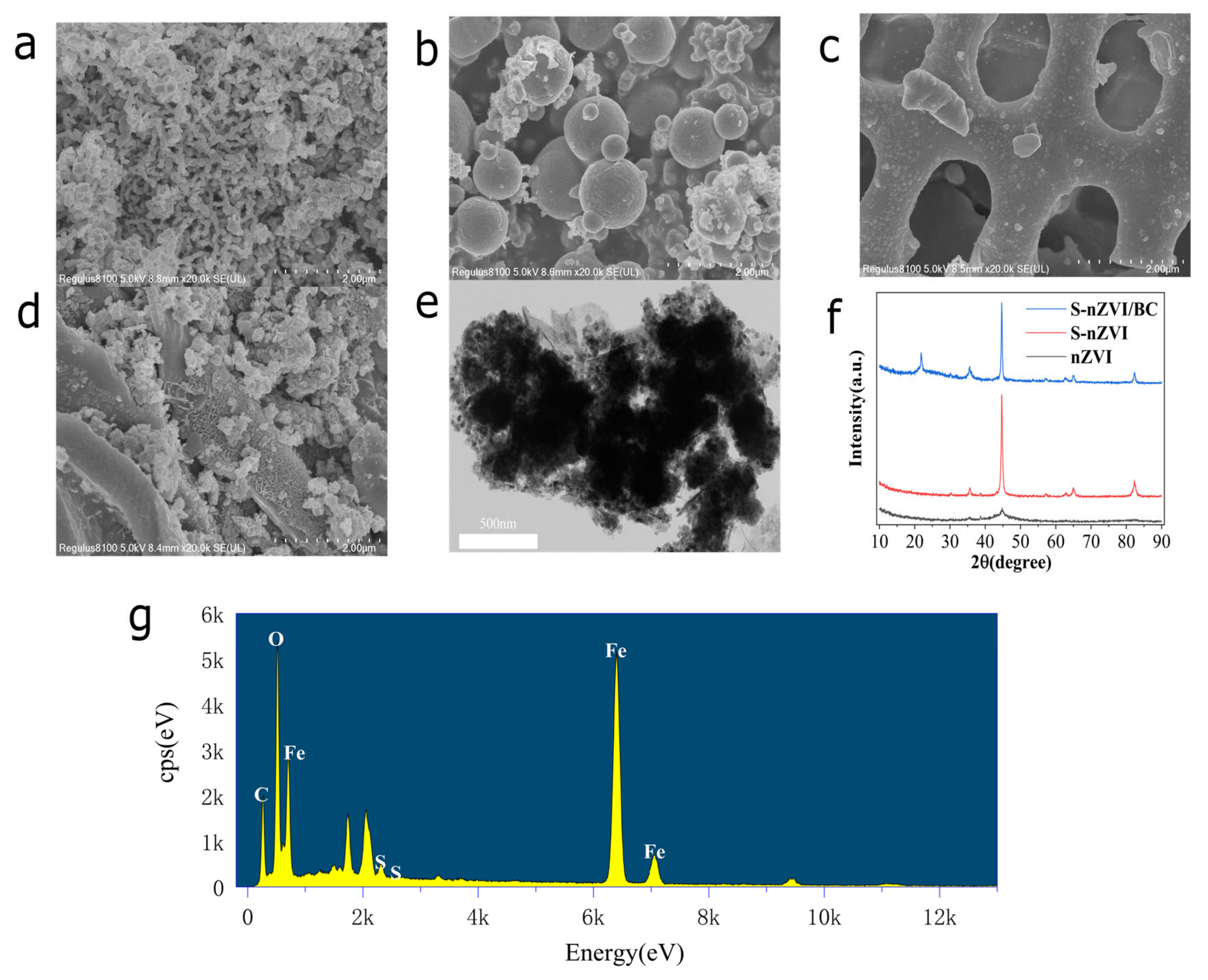
3.1. Characterization
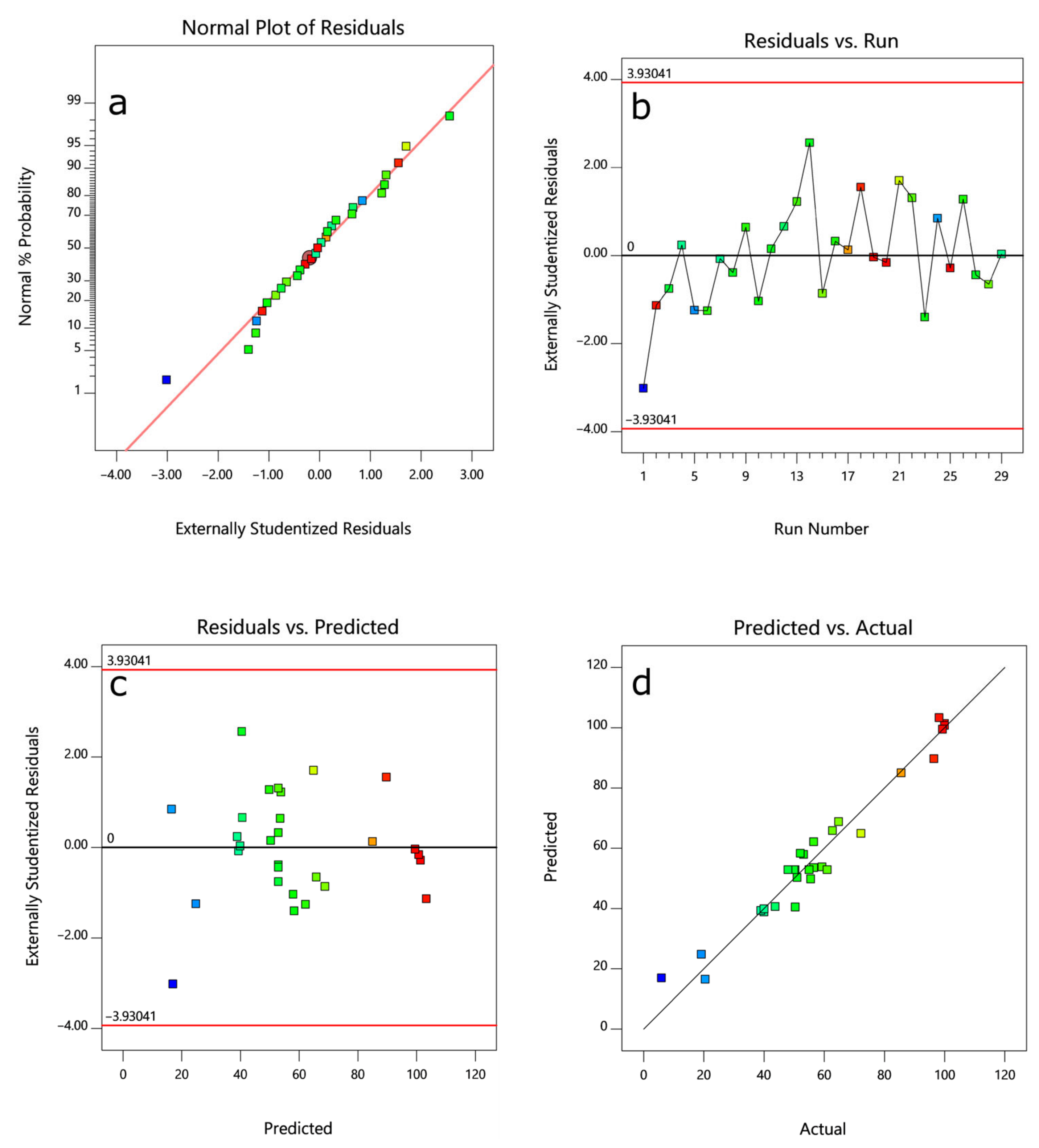
3.2. Model Fitting and Statistical Analysis
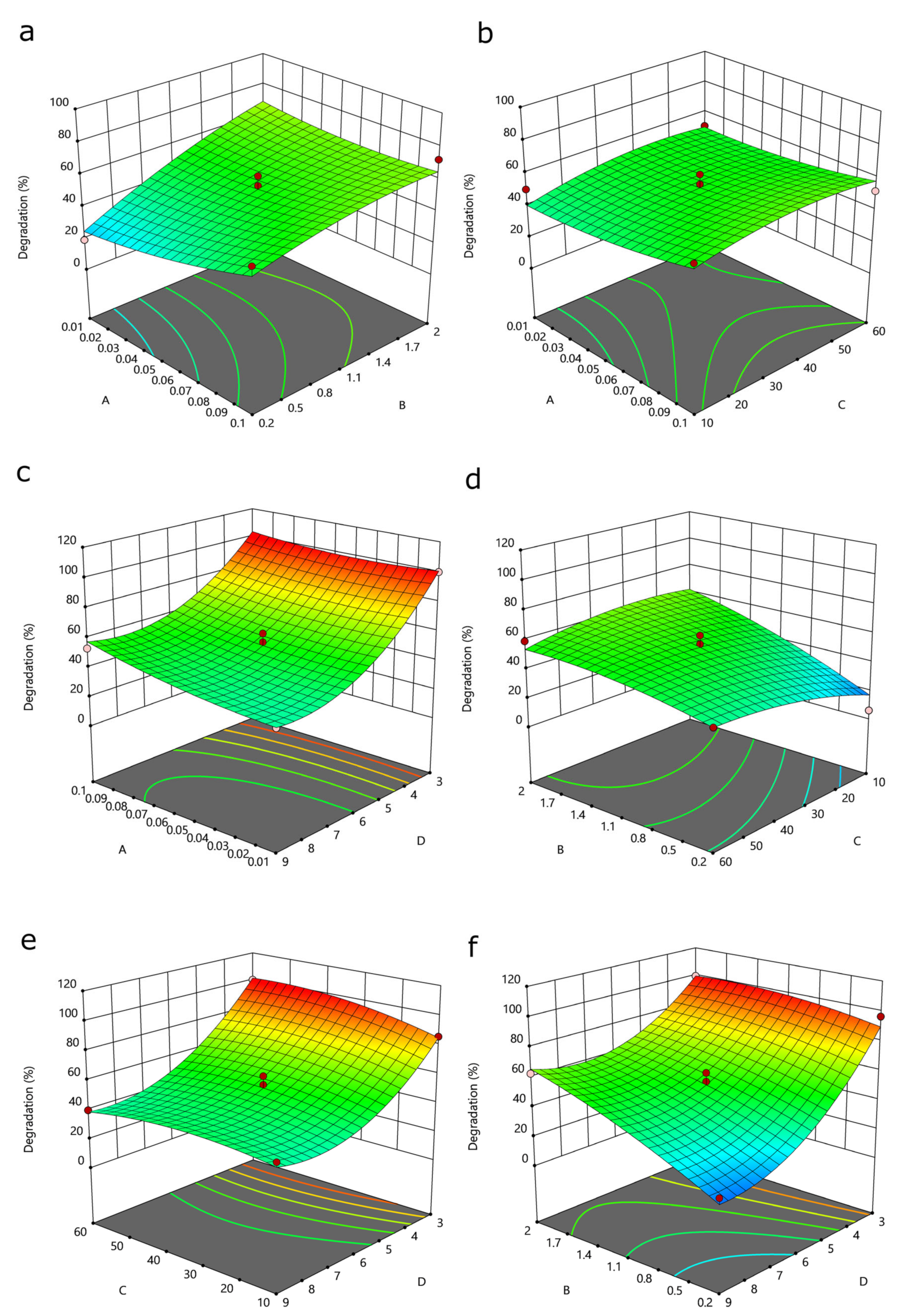
3.3. Analysis of Response Surfaces
3.4. Model Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, Z.; Wang, J. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J. Hazard. Mater. 2017, 324, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, N.; Hashmi, I. Assessment of immunohematological, hematological and biochemical responses in cultivable fish Cyprinus carpio exposed to an antibiotic sulfamethoxazole (SMX). J. Water Health 2021, 19, 108–119. [Google Scholar] [CrossRef]
- Xu, D.; Pan, H.; Yao, J.; Feng, Y.; Wu, P.; Shao, K. Stress responses and biological residues of sulfanilamide antibiotics in Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2020, 199, 110727. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, J.; Wang, J.; Chi, W.; Zhou, W.; Tian, Q.; Hong, Y.; Zhou, X.; Ye, H.; Tian, X.; et al. Assessing the drug resistance profiles of oral probiotic lozenges. J. Oral Microbiol. 2022, 14, 2019992. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Giannika, V. Comparative techno-economic and environmental analysis of minimal liquid discharge (MLD) and zero liquid discharge (ZLD) desalination systems for seawater brine treatment and valorization. Sustain. Energy Technol. Assess. 2022, 53, 102477. [Google Scholar] [CrossRef]
- Ingerslev, F.; Halling-Sørensen, B. Biodegradability properties of sulfonamides in activated sludge. Environ. Toxicol. Chem. 2000, 19, 2467–2473. [Google Scholar] [CrossRef]
- Kosutic, K.; Dolar, D.; Asperger, D.; Kunst, B. Removal of antibiotics from a model wastewater by RO/NF membranes. Sep. Purif. Technol. 2007, 53, 244–249. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Yang, Y.; Sun, P. Fenton-Like Oxidation of Antibiotic Ornidazole Using Biochar-Supported Nanoscale Zero-Valent Iron as Heterogeneous Hydrogen Peroxide Activator. Int. J. Environ. Res. Public Health 2020, 17, 1324. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Jiang, L.; Li, X.; Zhang, J.; Wang, H. Reutilization of cathode material from spent batteries as a heterogeneous catalyst to remove antibiotics in wastewater via peroxymonosulfate activation. Chem. Eng. J. 2020, 400, 125903. [Google Scholar] [CrossRef]
- Milh, H.; Yu, X.; Cabooter, D.; Dewil, R. Degradation of ciprofloxacin using UV-based advanced removal processes: Comparison of persulfate-based advanced oxidation and sulfite-based advanced reduction processes. Sci. Total Environ. 2021, 764, 144510. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Cheng, Y.-J. Effectiveness of using nanoscale zero-valent iron and hydrogen peroxide in degrading sulfamethazine in water. J. Taiwan Inst. Chem. Eng. 2021, 118, 179–186. [Google Scholar] [CrossRef]
- Luo, K.; Yang, Q.; Pang, Y.; Wang, D.; Li, X.; Lei, M.; Huang, Q. Unveiling the mechanism of biochar-activated hydrogen peroxide on the degradation of ciprofloxacin. Chem. Eng. J. 2019, 374, 520–530. [Google Scholar] [CrossRef]
- Mondal, S.K.; Saha, A.K.; Sinha, A. Removal of ciprofloxacin using modified advanced oxidation processes: Kinetics, pathways and process optimization. J. Clean. Prod. 2018, 171, 1203–1214. [Google Scholar] [CrossRef]
- Dong, H.; He, Q.; Zeng, G.; Tang, L.; Zhang, L.; Xie, Y.; Zeng, Y.; Zhao, F. Degradation of trichloroethene by nanoscale zero-valent iron (nZVI) and nZVI activated persulfate in the absence and presence of EDTA. Chem. Eng. J. 2017, 316, 410–418. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Tong, S.; Jiang, X.; Wang, L.; Sun, X.; Li, J.; Liu, X.; Shen, J. Biochar supported sulfide-modified nanoscale zero-valent iron for the reduction of nitrobenzene. RSC Adv. 2018, 8, 22161–22168. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Han, D.; Xu, Y.; Liu, Y.; Shang, J. Persulfate activation by sulfide-modified nanoscale iron supported by biochar (S-nZVI/BC) for degradation of ciprofloxacin. Sep. Purif. Technol. 2020, 235, 116202. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, E.; Zhang, D.; Wang, X.; Zhao, B.; Liu, L.; Ma, X.; Song, G.; Yu, S. Precursor impact and mechanism analysis of uranium elimination by biochar supported sulfurized nanoscale zero-valent iron. J. Environ. Chem. Eng. 2022, 10, 107288. [Google Scholar] [CrossRef]
- Mao, Q.; Zhou, Y.; Yang, Y.; Zhang, J.; Liang, L.; Wang, H.; Luo, S.; Luo, L.; Jeyakumar, P.; Ok, Y.S.; et al. Experimental and theoretical aspects of biochar-supported nanoscale zero-valent iron activating H2O2 for ciprofloxacin removal from aqueous solution. J. Hazard. Mater. 2019, 380, 120848. [Google Scholar] [CrossRef]
- Khataee, A.; Bozorg, S.; Vahid, B. Response surface optimization of heterogeneous Fenton-like degradation of sulfasalazine using Fe-impregnated clinoptilolite nanorods prepared by Ar-plasma. Res. Chem. Intermed. 2017, 43, 3989–4005. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Xiang, L.; Huang, Q.; Qiu, J.; Zhang, H.; Sivaiah, M.V.; Baron, F.; Barrault, J.; Petit, S.; et al. Heterogeneous photo-Fenton decolorization of Orange II over Al-pillared Fe-smectite: Response surface approach, degradation pathway, and toxicity evaluation. J. Hazard. Mater. 2015, 287, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Y.; An, Y.; Yang, L.; Sun, Q.; Ma, J.; Zheng, H. Chitin-biocalcium as a novel superior composite for ciprofloxacin removal: Synergism of adsorption and flocculation. J. Hazard. Mater. 2022, 423, 126917. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Kim, J.H.; Choi, J.W. Synthesis of magnetic porous carbon composite derived from metal-organic framework using recovered terephthalic acid from polyethylene terephthalate (PET) waste bottles as organic ligand and its potential as adsorbent for antibiotic tetracycline hydrochloride. Compos. B Eng. 2020, 187, 107867. [Google Scholar] [CrossRef]
- Li, M.; Rong, L.; Zhou, S.; Xiao, X.; Wu, L.; Fan, Y.; Lu, C.; Zou, X. Dissipation of sulfonamides in soil emphasizing taxonomy and function of microbiomes by metagenomic analysis. J. Agric. Food Chem. 2020, 68, 13594–13607. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.x.; Xu, Q.y.; Guo, R.t.; Wang, Z.y.; Liu, X.y.; Shi, X.; Qiu, Z.z.; Qin, H.; Jia, P.y.; Qin, Y.; et al. Removal of NO by using sodium persulfate/limestone slurry: Modeling by response surface methodology. Fuel 2019, 254, 115612. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Xu, J.; Zhang, J.; Yang, Y.; Zhou, J.; Xu, X.; Lowry, G.V. Removal of antibiotic florfenicol by sulfide-modified nanoscale zero-valent iron. Environ. Sci. Technol. 2017, 51, 11269–11277. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, H.; Lowry, G.V.; Shi, X.; Pan, X.; Xu, X.; Henkelman, G.; Xu, J. Unveiling the role of sulfur in rapid defluorination of florfenicol by sulfidized nanoscale zero-valent iron in water under ambient conditions. Environ. Sci. Technol. 2021, 55, 2628–2638. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate chemical analysis of oxygenated graphene-based materials using X-ray photoelectron spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Y.; Jiang, S.; Wang, Y.; Li, H.; Han, W.; Qu, J.; Wang, L.; Hu, Y. Graphene-like carbon sheet-supported nZVI for efficient atrazine oxidation degradation by persulfate activation. Chem. Eng. J. 2021, 403, 126309. [Google Scholar] [CrossRef]
- Zhang, S.; Lyu, H.; Tang, J.; Song, B.; Zhen, M.; Liu, X. A novel biochar supported CMC stabilized nano zero-valent iron composite for hexavalent chromium removal from water. Chemosphere 2019, 217, 686–694. [Google Scholar] [CrossRef]
- Han, Y.; Yan, W. Reductive dechlorination of trichloroethene by zero-valent iron nanoparticles: Reactivity enhancement through sulfidation treatment. Environ. Sci. Technol. 2016, 50, 12992–13001. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Chen, B.; Qiu, X.; Li, J.; Tratnyek, P.G.; He, F. Sulfidation of zero-valent iron by direct reaction with elemental sulfur in water: Efficiencies, mechanism, and dechlorination of trichloroethylene. Environ. Sci. Technol. 2021, 55, 645–654. [Google Scholar] [CrossRef]
- Song, S.; Su, Y.; Adeleye, A.S.; Zhang, Y.; Zhou, X. Optimal design and characterization of sulfide-modified nanoscale zerovalent iron for diclofenac removal. Appl. Catal. B Environ. 2017, 201, 211–220. [Google Scholar] [CrossRef]
- Nam, S.-N.; Cho, H.; Han, J.; Her, N.; Yoon, J. Photocatalytic degradation of acesulfame K: Optimization using the Box–Behnken design (BBD). Process Saf. Environ. Prot. 2018, 113, 10–21. [Google Scholar] [CrossRef]
- Mansouriieh, N.; Sohrabi, M.R.; Khosravi, M. Optimization of profenofos organophosphorus pesticide degradation by zero-valent bimetallic nanoparticles using response surface methodology. Arab. J. Chem. 2019, 12, 2524–2532. [Google Scholar] [CrossRef]
- Junmin Deng, H.D. Nanoscale zero-valent iron/biochar composite as an activator for Fenton- like removal of sulfamethazine. Sep. Purif. Technol. 2018, 202, 130–137. [Google Scholar] [CrossRef]
- Tao, X.; Yuan, X.; Huang, L. Effects of Fe(II)/Fe(III) of Fe-MOFs on catalytic performance in plasma/Fenton-like system. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125745. [Google Scholar] [CrossRef]
- Li, B.; Zhu, J. Removal of p-chloronitrobenzene from groundwater: Effectiveness and degradation mechanism of a heterogeneous nanoparticulate zero-valent iron (NZVI)-induced Fenton process. Chem. Eng. J. 2014, 255, 225–232. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsu, S.T. Performance of nZVI/H2O2 process in degrading polyvinyl alcohol in aqueous solutions. Sep. Purif. Technol. 2018, 203, 111–116. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, C.; Deng, J.; Jiang, Z.; Zhang, L.; Cheng, Y.; Hou, K.; Tang, L.; Zeng, G. Factors influencing degradation of trichloroethylene by sulfide-modified nanoscale zero-valent iron in aqueous solution. Water Res. 2018, 135, 1–10. [Google Scholar] [CrossRef]

| Variable | Coding | Scope and Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| H2O2 (mol/L) | A | 0.01 | 0.055 | 0.1 |
| C/Fe | B | 0.2 | 1.1 | 2 |
| Fe/S | C | 10 | 35 | 60 |
| Initial pH | D | 3 | 6 | 9 |
| nZVI | S-nZVI | S-nZVI/BC | |
|---|---|---|---|
| BET surface area (m2/g) | 19.06 | 13.84 | 344.09 |
| Total pore volume (cm3/g) | 0.0806 | 0.0425 | 0.2611 |
| Serial Number | Variable Value | Removal Rate (%) | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 0 | −1 | −1 | 0 | 5.96 |
| 2 | 1 | 0 | 0 | −1 | 98.17 |
| 3 | 0 | 0 | 0 | 0 | 48.00 |
| 4 | 0 | 0 | 1 | 1 | 40.00 |
| 5 | −1 | −1 | 0 | 0 | 19.19 |
| 6 | 0 | 1 | −1 | 0 | 56.50 |
| 7 | −1 | 0 | 0 | 1 | 38.98 |
| 8 | 0 | 0 | 0 | 0 | 50.35 |
| 9 | 1 | 0 | −1 | 0 | 56.54 |
| 10 | 1 | 0 | 0 | 1 | 53.18 |
| 11 | −1 | 0 | 1 | 0 | 50.99 |
| 12 | 0 | 0 | −1 | 1 | 43.73 |
| 13 | 0 | 1 | 1 | 0 | 59.32 |
| 14 | −1 | 0 | −1 | 0 | 50.40 |
| 15 | −1 | 1 | 0 | 0 | 64.80 |
| 16 | 0 | 0 | 0 | 0 | 55.00 |
| 17 | 0 | 0 | −1 | −1 | 85.60 |
| 18 | 0 | −1 | 0 | −1 | 96.48 |
| 19 | 0 | 1 | 0 | −1 | 99.30 |
| 20 | −1 | 0 | 0 | −1 | 100.00 |
| 21 | 1 | 1 | 0 | 0 | 72.22 |
| 22 | 0 | 0 | 0 | 0 | 61.00 |
| 23 | 1 | 0 | 1 | 0 | 52.10 |
| 24 | 0 | −1 | 0 | 1 | 20.44 |
| 25 | 0 | 0 | 1 | −1 | 100.00 |
| 26 | 1 | −1 | 0 | 0 | 55.54 |
| 27 | 0 | 0 | 0 | 0 | 50.00 |
| 28 | 0 | 1 | 0 | 1 | 62.77 |
| 29 | 0 | −1 | 1 | 0 | 40.04 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value | |
|---|---|---|---|---|---|---|
| Model | 15,908.66 | 14 | 1136.33 | 22.52 | <0.0001 | Significant |
| A | 334.86 | 1 | 334.86 | 6.64 | 0.022 | |
| B | 2618.43 | 1 | 2618.43 | 51.9 | <0.0001 | |
| C | 159.29 | 1 | 159.29 | 3.16 | 0.0973 | |
| D | 8557.35 | 1 | 8557.35 | 169.61 | <0.0001 | |
| AB | 209.24 | 1 | 209.24 | 4.15 | 0.0611 | |
| AC | 6.33 | 1 | 6.33 | 0.1254 | 0.7286 | |
| AD | 64.24 | 1 | 64.24 | 1.27 | 0.2781 | |
| BC | 244.3 | 1 | 244.3 | 4.84 | 0.0451 | |
| BD | 390.26 | 1 | 390.26 | 7.74 | 0.0147 | |
| CD | 82.17 | 1 | 82.17 | 1.63 | 0.2226 | |
| A2 | 71.42 | 1 | 71.42 | 1.42 | 0.2539 | |
| B2 | 110.39 | 1 | 110.39 | 2.19 | 0.1612 | |
| C2 | 199.65 | 1 | 199.65 | 3.96 | 0.0666 | |
| D2 | 2375.22 | 1 | 2375.22 | 47.08 | <0.0001 | |
| Residual | 706.33 | 14 | 50.45 | |||
| Lack of Fit | 597.39 | 10 | 59.74 | 2.19 | 0.2335 | Not significant |
| Pure Error | 108.94 | 4 | 27.23 | |||
| Cor Total | 16,614.99 | 28 | ||||
| R2 | 0.9575 | |||||
| Adjusted R2 | 0.915 | |||||
| Std. Dev. | 7.1 | Predicted R2 | 0.7827 | |||
| Mean | 58.16 | Adeq Precision | 16.9869 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Hu, C.; Li, Q.; Chen, C.; Hu, J.; Xiao, X.; Li, M.; Zou, X.; Huang, L. Hydrogen Peroxide Activated by Biochar-Supported Sulfidated Nano Zerovalent Iron for Removal of Sulfamethazine: Response Surface Method Approach. Int. J. Environ. Res. Public Health 2022, 19, 9923. https://doi.org/10.3390/ijerph19169923
Zhang T, Hu C, Li Q, Chen C, Hu J, Xiao X, Li M, Zou X, Huang L. Hydrogen Peroxide Activated by Biochar-Supported Sulfidated Nano Zerovalent Iron for Removal of Sulfamethazine: Response Surface Method Approach. International Journal of Environmental Research and Public Health. 2022; 19(16):9923. https://doi.org/10.3390/ijerph19169923
Chicago/Turabian StyleZhang, Tiao, Cui Hu, Qian Li, Chuxin Chen, Jianhui Hu, Xiaoyu Xiao, Mi Li, Xiaoming Zou, and Liangliang Huang. 2022. "Hydrogen Peroxide Activated by Biochar-Supported Sulfidated Nano Zerovalent Iron for Removal of Sulfamethazine: Response Surface Method Approach" International Journal of Environmental Research and Public Health 19, no. 16: 9923. https://doi.org/10.3390/ijerph19169923
APA StyleZhang, T., Hu, C., Li, Q., Chen, C., Hu, J., Xiao, X., Li, M., Zou, X., & Huang, L. (2022). Hydrogen Peroxide Activated by Biochar-Supported Sulfidated Nano Zerovalent Iron for Removal of Sulfamethazine: Response Surface Method Approach. International Journal of Environmental Research and Public Health, 19(16), 9923. https://doi.org/10.3390/ijerph19169923






