The Clinical Significance of Urinary Retinol-Binding Protein 4: A Review
Abstract
1. Introduction
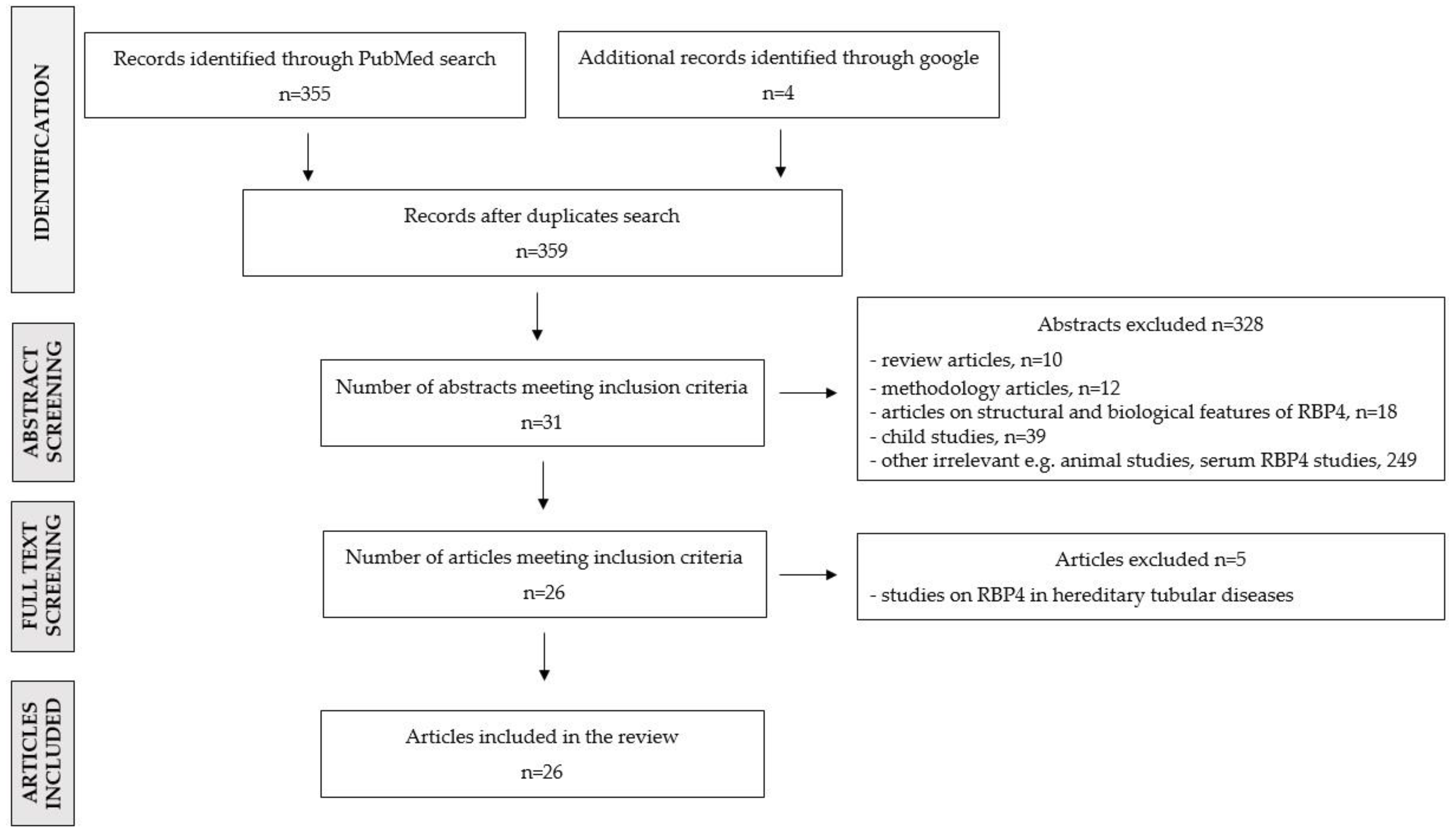
2. Materials and Methods
3. Results
3.1. Urinary RBP4
3.1.1. uRBP4 Levels in Health
3.1.2. uRBP4 Levels in Diseases Involving the Kidneys
3.1.3. Kidney Diseases
Primary Glomerular Diseases
3.1.4. Acute Kidney Injury
3.1.5. Renal Allograft Dysfunction
3.1.6. Chronic Kidney Disease
3.1.7. Renal Cell Carcinoma
3.1.8. Systemic Diseases Affecting Kidneys
Type 2 Diabetes Mellitus
3.1.9. Obesity
3.1.10. Preeclampsia
3.1.11. Coronavirus Disease 2019
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanai, M.; Raz, A.; Goodman, D.S. Retinol-binding protein: The transport protein for vitamin A in human plasma. J. Clin. Investig. 1968, 47, 2025–2044. [Google Scholar] [CrossRef] [PubMed]
- Flower, D.R. The lipocalin protein family: Structure and function. Biochem. J. 1996, 318 Pt 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, C.; Okuno, M.; Tannous, L.; Piantedosi, R.; Allan, M.; Goodman, D.S.; Blaner, W.S. Retinoids and retinoid-binding protein expression in rat adipocytes. J. Biol. Chem. 1992, 267, 1805–1810. [Google Scholar] [CrossRef]
- Zovich, D.C.; Orologa, A.; Okuno, M.; Kong, L.W.; Talmage, D.A.; Piantedosi, R.; Goodman, D.S.; Blaner, W.S. Differentiation-dependent expression of retinoid-binding proteins in BFC-1 beta adipocytes. J. Biol. Chem. 1992, 267, 13884–13889. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Z.; Chen, Y.; Wu, Y.; Liu, Y. RBP4 functions as a hepatokine in the regulation of glucose metabolism by the circadian clock in mice. Diabetologia 2016, 59, 354–362. [Google Scholar] [CrossRef]
- Perduca, M.; Nicolis, S.; Mannucci, B.; Galliano, M.; Monaco, H.L. High resolution crystal structure data of human plasma retinol-binding protein (RBP4) bound to retinol and fatty acids. Data Brief 2018, 18, 1073–1081. [Google Scholar] [CrossRef]
- Goodman, A.B. Retinoid receptors, transporters, and metabolizers as therapeutic targets in late onset Alzheimer disease. J. Cell Physiol. 2006, 209, 598–603. [Google Scholar] [CrossRef]
- Maack, T.; Johnson, V.; Kau, S.T.; Figueiredo, J.; Sigulem, D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: A review. Kidney Int. 1979, 16, 251–270. [Google Scholar] [CrossRef]
- Christensen, E.I.; Moskaug, J.O.; Vorum, H.; Jacobsen, C.; Gundersen, T.E.; Nykjaer, A.; Blomhoff, R.; Willnow, T.E.; Moestrup, S.K. Evidence for an essential role of megalin in transepithelial transport of retinol. J. Am. Soc. Nephrol. 1999, 10, 685–695. [Google Scholar] [CrossRef]
- Raila, J.; Willnow, T.E.; Schweigert, F.J. Megalin-mediated reuptake of retinol in the kidneys of mice is essential for vitamin A homeostasis. J. Nutr. 2005, 135, 2512–2516. [Google Scholar] [CrossRef]
- Bernard, A.; Vyskocyl, A.; Mahieu, P.; Lauwerys, R. Effect of renal insufficiency on the concentration of free retinol-binding protein in urine and serum. Clin. Chim. Acta 1988, 171, 85–93. [Google Scholar] [CrossRef]
- Bernard, A.M.; Vyskocil, A.A.; Mahieu, P.; Lauwerys, R.R. Assessment of urinary retinol-binding protein as an index of proximal tubular injury. Clin. Chem. 1987, 33, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, M.; Patrick, A.W.; McClelland, P.; Stevenson, A.; Mason, H.; White, M.C.; Bell, G.M. Relationship between markers of endothelial dysfunction, oxidant injury and tubular damage in patients with insulin-dependent diabetes mellitus. Clin. Sci. 1993, 85, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.G.; Lapsley, M.; Unwin, R.J. Urine retinol-binding protein 4: A functional biomarker of the proximal renal tubule. Adv. Clin. Chem. 2014, 63, 85–122. [Google Scholar] [CrossRef]
- Li, A.; Yi, B.; Liu, Y.; Wang, J.; Dai, Q.; Huang, Y.; Li, Y.C.; Zhang, H. Urinary NGAL and RBP Are Biomarkers of Normoalbuminuric Renal Insufficiency in Type 2 Diabetes Mellitus. J. Immunol. Res. 2019, 2019, 5063089. [Google Scholar] [CrossRef]
- Rychter, A.M.; Skrzypczak-Zielińska, M.; Zielińska, A.; Eder, P.; Souto, E.B.; Zawada, A.; Ratajczak, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. Is the Retinol-Binding Protein 4 a Possible Risk Factor for Cardiovascular Diseases in Obesity? Int. J. Mol. Sci. 2020, 21, 5229. [Google Scholar] [CrossRef]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front. Physiol. 2021, 12, 659977. [Google Scholar] [CrossRef]
- Norden, A.G.W.; Burling, K.A.; Zeni, L.; Unwin, R.J. A New Estimate of the Glomerular Sieving Coefficient for Retinol-Binding Protein 4 Suggests It Is Not Freely Filtered. Kidney Int. Rep. 2019, 4, 1017–1018. [Google Scholar] [CrossRef]
- Peterson, P.A.; Berggård, I. Isolation and properties of a human retinol-transporting protein. J. Biol. Chem. 1971, 246, 25–33. [Google Scholar] [CrossRef]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef]
- Marek-Bukowiec, K.; Konieczny, A.; Ratajczyk, K.; Macur, K.; Czaplewska, P.; Czyżewska-Buczyńska, A.; Kowal, P.; Witkiewicz, W. The Value of Urinary RBP4 in The Diagnosis of FSGS and other Renal Diseases. Res. Sq. 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Burling, K.A.; Cutillas, P.R.; Church, D.; Lapsley, M.; Norden, A.G. Analysis of molecular forms of urine Retinol-Binding Protein in Fanconi Syndrome and design of an accurate immunoassay. Clin. Chim. Acta 2012, 413, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.M.; Bryant, T.N.; Hall, M.A.; Lowes, J.A.; Rowe, D.J. Neonatal renal function assessment. Arch. Dis. Child. 1989, 64, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.S.; Haycock, G.B.; Dalton, R.N.; Turner, C.; Tomlinson, P.; Stimmler, L.; Scopes, J.W. Prediction of acute renal failure after birth asphyxia. Arch. Dis. Child. 1990, 65, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Winterborn, M.H.; Taylor, C.M.; Lawson, N.; Guy, M. Assessment of retinol-binding protein excretion in normal children. Pediatr. Nephrol. 1994, 8, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Thöny, H.C.; Luethy, C.M.; Zimmermann, A.; Laux-End, R.; Oetliker, O.H.; Bianchetti, M.G. Histological features of glomerular immaturity in infants and small children with normal or altered tubular function. Eur. J. Pediatr. 1995, 154, S65–S68. [Google Scholar] [CrossRef]
- Topping, M.D.; Forster, H.W.; Dolman, C.; Luczynska, C.M.; Bernard, A.M. Measurement of urinary retinol-binding protein by enzyme-linked immunosorbent assay, and its application to detection of tubular proteinuria. Clin. Chem. 1986, 32, 1863–1866. [Google Scholar] [CrossRef]
- Lapsley, M.; Akers, K.; Norden, A.G. Sensitive assays for urinary retinol-binding protein and beta-2-glycoprotein-1 based on commercially available standards. Ann. Clin. Biochem. 1998, 35 Pt 1, 115–119. [Google Scholar] [CrossRef]
- Tomlinson, P.A.; Dalton, R.N.; Turner, C.; Chantler, C. Measurement of beta 2-microglobulin, retinol-binding protein, alpha 1-microglobulin and urine protein 1 in healthy children using enzyme-linked immunosorbent assay. Clin. Chim. Acta 1990, 192, 99–106. [Google Scholar] [CrossRef]
- Bangstad, H.J.; Kierulf, P.; Kjaersgaard, P.; Mevold, K.; Dahl-Jørgensen, K. Urinary excretion of retinol-binding protein in healthy children and adolescents. Pediatr. Nephrol. 1995, 9, 299–302. [Google Scholar] [CrossRef]
- Zahra, N.; Javad, M.A.; Aboalfazl, N. Detection of early stage renal disease by elevation of certain low molecular Weight proteins in urine of diabetes patients. Int. J. Biol. Sci. Appl. 2014, 1, 15–18. [Google Scholar]
- Sarasua, S.M.; Mueller, P.; Kathman, S.; Campagna, D.; Uddin, M.S.; White, M.C. Confirming the utility of four kidney biomarker tests in a longitudinal follow-up study. Ren. Fail. 2003, 25, 797–817. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.A.; Moreira, S.R.; Gomez, L.; Goulart, A.; Lotufo, P.A.; Benseñor, I.; Titan, S. Urinary Retinol-Binding Protein: Relationship to Renal Function and Cardiovascular Risk Factors in Chronic Kidney Disease. PLoS ONE 2016, 11, e0162782. [Google Scholar] [CrossRef] [PubMed]
- Downie, M.L.; Lopez Garcia, S.C.; Kleta, R.; Bockenhauer, D. Inherited Tubulopathies of the Kidney: Insights from Genetics. Clin. J. Am. Soc. Nephrol. 2021, 16, 620–630. [Google Scholar] [CrossRef]
- Ayar, Y.; Ersoy, A.; Isiktas, E.; Ocakoglu, G.; Yildiz, A.; Oruc, A.; Demirayak, D.; Bayrakci, I.; Duger, H.; Bozbudak, T. The analysis of patients with primary and secondary glomerular diseases: A single-center experience. Hong Kong J. Nephrol. 2016, 19, 28–35. [Google Scholar] [CrossRef]
- Mariani, L.H.; Kretzler, M. Pro: ‘The usefulness of biomarkers in glomerular diseases’. The problem: Moving from syndrome to mechanism--individual patient variability in disease presentation, course and response to therapy. Nephrol. Dial. Transpl. 2015, 30, 892–898. [Google Scholar] [CrossRef][Green Version]
- Kirsztajn, G.M.; Nishida, S.K.; Silva, M.S.; Ajzen, H.; Moura, L.A.; Pereira, A.B. Urinary retinol-binding protein as a prognostic marker in glomerulopathies. Nephron 2002, 90, 424–431. [Google Scholar] [CrossRef]
- Kalantari, S.; Rutishauser, D.; Samavat, S.; Nafar, M.; Mahmudieh, L.; Rezaei-Tavirani, M.; Zubarev, R.A. Urinary prognostic biomarkers and classification of IgA nephropathy by high resolution mass spectrometry coupled with liquid chromatography. PLoS ONE 2013, 8, e80830. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, C.; Tang, T.; Wang, H.; Xia, Y.; Shao, Q.; Zhang, M. Clinical significance of urinary biomarkers in patients with primary focal segmental glomerulosclerosis. Am. J. Med. Sci. 2018, 355, 314–321. [Google Scholar] [CrossRef]
- Araumi, A.; Osaka, T.; Ichikawa, K.; Kudo, K.; Suzuki, N.; Watanabe, S.; Watanabe, M.; Konta, T. Urinary and plasma proteomics to discover biomarkers for diagnosing between diabetic nephropathy and minimal change nephrotic syndrome or membranous nephropathy. Biochem. Biophys. Rep. 2021, 27, 101102. [Google Scholar] [CrossRef]
- Varghese, S.A.; Powell, T.B.; Janech, M.G.; Budisavljevic, M.N.; Stanislaus, R.C.; Almeida, J.S.; Arthur, J.M. Identification of diagnostic urinary biomarkers for acute kidney injury. J. Investig. Med. 2010, 58, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Bijol, V.; Sparks, M.A.; Sise, M.E.; Izzedine, H.; Jhaveri, K.D. Pathophysiology and Pathology of Acute Kidney Injury in Patients with COVID-19. Adv. Chronic Kidney Dis. 2020, 27, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Calero, L.; Martin-Lorenzo, M.; Ramos-Barron, A.; Ruiz-Criado, J.; Maroto, A.S.; Ortiz, A.; Gomez-Alamillo, C.; Arias, M.; Vivanco, F.; Alvarez-Llamas, G. Urinary Kininogen-1 and Retinol binding protein-4 respond to Acute Kidney Injury: Predictors of patient prognosis? Sci. Rep. 2016, 6, 19667. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Mohan, S. Managing Patients with Failing Kidney Allograft: Many Questions Remain. Clin. J. Am. Soc. Nephrol. 2022, 17, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, B.; Park, S.I.; Felipe, C.R.; Garcia, R.G.; Machado, P.G.; Pereira, A.B.; Tedesco-Silva, H.; Medina-Pestana, J.O. Predictive value of urinary retinol binding protein for graft dysfunction after kidney transplantation. Transpl. Proc. 2003, 35, 1341–1343. [Google Scholar] [CrossRef]
- Câmara, N.O.; Silva, M.S.; Nishida, S.; Pereira, A.B.; Pacheco-Silva, A. Proximal tubular dysfunction is associated with chronic allograft nephropathy and decreased long-term renal-graft survival. Transplantation 2004, 78, 269–275. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Cheng, D.; Wang, R.; Wang, W.; Zhang, M.; Xu, F.; Wen, J.; Tang, Z. Proteinuria, Estimated Glomerular Filtration Rate and Urinary Retinol-Binding Protein as Clinical Predictors of Long-Term Allograft Outcomes in Transplant Glomerulopathy. Kidney Blood Press. Res. 2018, 43, 1842–1851. [Google Scholar] [CrossRef]
- Jeon, H.J.; Shin, D.H.; Oh, J.; Kee, Y.K.; Park, J.Y.; Ko, K.; Lee, S. Urinary Retinol-Binding Protein 4 is Associated with Renal Function and Rapid Renal Function Decline in Kidney Transplant Recipients. Transpl. Proc. 2022, 54, 362–366. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef]
- Johnson, D.W.; Jones, G.R.; Mathew, T.H.; Ludlow, M.J.; Chadban, S.J.; Usherwood, T.; Polkinghorne, K.; Colagiuri, S.; Jerums, G.; Macisaac, R.; et al. Chronic kidney disease and measurement of albuminuria or proteinuria: A position statement. Med. J. Aust. 2012, 197, 224–225. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Staples, A.; Wong, C. Risk factors for progression of chronic kidney disease. Curr. Opin. Pediatr. 2010, 22, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; O’Donoghue, D.J.; Ritchie, J.; Kanavos, P.G.; Narva, A.S. Early chronic kidney disease: Diagnosis, management and models of care. Nat. Rev. Nephrol. 2015, 11, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Fernando, B.; Alli-Shaik, A.; Hemage, R.K.D.; Badurdeen, Z.; Hettiarachchi, T.W.; Abeysundara, H.T.K.; Abeysekara, T.D.J.; Wazil, A.; Rathnayake, S.; Gunaratne, J.; et al. Pilot Study of Renal Urinary Biomarkers for Diagnosis of CKD of Uncertain Etiology. Kidney Int. Rep. 2019, 4, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Narayanan, S.P.; Mannan, R.; Raskind, G.; Wang, X.; Vats, P.; Su, F.; Hosseini, N.; Cao, X.; Kumar-Sinha, C.; et al. Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proc. Natl. Acad. Sci. USA 2021, 118, e2103240118. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.E.; Harris, G.T. Renal Cell Carcinoma: Diagnosis and Management. Am. Fam. Phys. 2019, 99, 179–184. [Google Scholar]
- Wang, J.; Talmon, G.A.; Feloney, M.; Morris, M.C. Twelve-year survival after multiple recurrences and repeated metastasectomies for renal cell carcinoma. World J. Surg. Oncol. 2011, 9, 155. [Google Scholar] [CrossRef]
- Osawa, T.; Takeuchi, A.; Kojima, T.; Shinohara, N.; Eto, M.; Nishiyama, H. Overview of current and future systemic therapy for metastatic renal cell carcinoma. Jpn. J. Clin. Oncol. 2019, 49, 395–403. [Google Scholar] [CrossRef]
- Kalra, S.; Atkinson, B.J.; Matrana, M.R.; Matin, S.F.; Wood, C.G.; Karam, J.A.; Tamboli, P.; Sircar, K.; Rao, P.; Corn, P.G.; et al. Prognosis of patients with metastatic renal cell carcinoma and pancreatic metastases. BJU Int. 2016, 117, 761–765. [Google Scholar] [CrossRef]
- Shuch, B.; Amin, A.; Armstrong, A.J.; Eble, J.N.; Ficarra, V.; Lopez-Beltran, A.; Martignoni, G.; Rini, B.I.; Kutikov, A. Understanding pathologic variants of renal cell carcinoma: Distilling therapeutic opportunities from biologic complexity. Eur. Urol. 2015, 67, 85–97. [Google Scholar] [CrossRef]
- Santorelli, L.; Stella, M.; Chinello, C.; Capitoli, G.; Piga, I.; Smith, A.; Grasso, A.; Grasso, M.; Bovo, G.; Magni, F. Does the Urinary Proteome Reflect ccRCC Stage and Grade Progression? Diagnostics 2021, 11, 2369. [Google Scholar] [CrossRef] [PubMed]
- Elhefnawy, K.A.; Elsayed, A.M. Prevalence of diabetic kidney disease in patients with type 2 diabetes mellitus. Egypt. J. Intern. Med. 2019, 31, 149–154. [Google Scholar] [CrossRef]
- Xu, J.; Shi, X.; Pan, Y. The Association of Aspartate Aminotransferase/Alanine Aminotransferase Ratio with Diabetic Nephropathy in Patients with Type 2 Diabetes. Diabetes Metab. Syndr. Obes. 2021, 14, 3831–3837. [Google Scholar] [CrossRef]
- Thomas, M.C.; Macisaac, R.J.; Jerums, G.; Weekes, A.; Moran, J.; Shaw, J.E.; Atkins, R.C. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 2009, 32, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Moosaie, F.; Khaloo, P.; Dehghani Firouzabadi, F.; Fatemi Abhari, S.M.; Atainia, B.; Ardeshir, M.; Nakhjavani, M.; Esteghamati, A. Neutrophil Gelatinase-Associated Lipocalin and Retinol-Binding Protein-4 as Biomarkers for Diabetic Kidney Disease. Kidney Blood Press. Res. 2020, 45, 222–232. [Google Scholar] [CrossRef]
- Park, S.E.; Lee, N.S.; Park, J.W.; Rhee, E.J.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Park, C.Y.; Youn, B.S. Association of urinary RBP4 with insulin resistance, inflammation, and microalbuminuria. Eur. J. Endocrinol. 2014, 171, 443–449. [Google Scholar] [CrossRef]
- Bellei, E.; Rossi, E.; Lucchi, L.; Uggeri, S.; Albertazzi, A.; Tomasi, A.; Iannone, A. Proteomic analysis of early urinary biomarkers of renal changes in type 2 diabetic patients. Proteom. Clin. Appl. 2008, 2, 478–491. [Google Scholar] [CrossRef]
- Riaz, S.; Alam, S.S.; Srai, S.K.; Skinner, V.; Riaz, A.; Akhtar, M.W. Proteomic identification of human urinary biomarkers in diabetes mellitus type 2. Diabetes Technol. Ther. 2010, 12, 979–988. [Google Scholar] [CrossRef]
- Titan, S.M.; Vieira, J.M., Jr.; Dominguez, W.V.; Moreira, S.R.; Pereira, A.B.; Barros, R.T.; Zatz, R. Urinary MCP-1 and RBP: Independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J. Diabetes Complicat. 2012, 26, 546–553. [Google Scholar] [CrossRef]
- Phanish, M.K.; Chapman, A.N.; Yates, S.; Price, R.; Hendry, B.M.; Roderick, P.J.; Dockrell, M.E.C. Evaluation of Urinary Biomarkers of Proximal Tubular Injury, Inflammation, and Fibrosis in Patients with Albuminuric and Nonalbuminuric Diabetic Kidney Disease. Kidney Int. Rep. 2021, 6, 1355–1367. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Chen, Y.; Dong, Y. Kidney Damage Caused by Obesity and Its Feasible Treatment Drugs. Int. J. Mol. Sci. 2022, 23, 747. [Google Scholar] [CrossRef] [PubMed]
- Benabdelkamel, H.; Masood, A.; Okla, M.; Al-Naami, M.Y.; Alfadda, A.A. A Proteomics-Based Approach Reveals Differential Regulation of Urine Proteins between Metabolically Healthy and Unhealthy Obese Patients. Int. J. Mol. Sci. 2019, 20, 4905. [Google Scholar] [CrossRef]
- Paternoster, D.M.; Stella, A.; Babbo, G.L.; Pignataro, R.; Mussap, M.; Plebani, M. Markers of tubular damage in pre-eclampsia. Minerva Ginecol. 1999, 51, 373–377. [Google Scholar] [PubMed]
- van der Graaf, A.M.; Toering, T.J.; Faas, M.M.; Lely, A.T. From preeclampsia to renal disease: A role of angiogenic factors and the renin-angiotensin aldosterone system? Nephrol. Dial. Transpl. 2012, 27 (Suppl. S3), iii51–iii57. [Google Scholar] [CrossRef] [PubMed]
- Kattah, A. Preeclampsia and Kidney Disease: Deciphering Cause and Effect. Curr. Hypertens. Rep. 2020, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Gerö, G.; Anthony, F.; Rowe, D.J.; Dennis, K.J. Increased urinary excretion of retinol-binding protein during normal pregnancies. Clin. Chem. 1986, 32, 916–917. [Google Scholar] [CrossRef]
- Facca, T.A.; Kirsztajn, G.M.; Pereira, A.R.; Moreira, S.R.; Teixeira, V.P.; Nishida, S.K.; Sass, N. Renal evaluation in women with preeclampsia. Nephron Extra 2012, 2, 125–132. [Google Scholar] [CrossRef]
- Xiao, J.; Niu, J.; Ye, X.; Yu, Q.; Gu, Y. Combined biomarkers evaluation for diagnosing kidney injury in preeclampsia. Hypertens. Pregnancy 2013, 32, 439–449. [Google Scholar] [CrossRef]
- Available online: https://covid19.who.int (accessed on 26 May 2022).
- Fan, C.; Lu, W.; Li, K.; Ding, Y.; Wang, J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Infection in COVID-19 Patients. Front. Med. 2020, 7, 563893. [Google Scholar] [CrossRef]
- Braun, F.; Lütgehetmann, M.; Pfefferle, S.; Wong, M.N.; Carsten, A.; Lindenmeyer, M.T.; Nörz, D.; Heinrich, F.; Meißner, K.; Wichmann, D.; et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 2020, 396, 597–598. [Google Scholar] [CrossRef]
- Karras, A.; Livrozet, M.; Lazareth, H.; Benichou, N.; Hulot, J.S.; Fayol, A.; Chauvet, S.; Jannot, A.S.; Penet, M.A.; Diehl, J.L.; et al. Proteinuria and Clinical Outcomes in Hospitalized COVID-19 Patients: A Retrospective Single-Center Study. Clin. J. Am. Soc. Nephrol. 2021, 16, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczyk, K.; Konieczny, A.; Czekaj, A.; Piotrów, P.; Fiutowski, M.; Krakowska, K.; Kowal, P.; Witkiewicz, W.; Marek-Bukowiec, K. The Clinical Significance of Urinary Retinol-Binding Protein 4: A Review. Int. J. Environ. Res. Public Health 2022, 19, 9878. https://doi.org/10.3390/ijerph19169878
Ratajczyk K, Konieczny A, Czekaj A, Piotrów P, Fiutowski M, Krakowska K, Kowal P, Witkiewicz W, Marek-Bukowiec K. The Clinical Significance of Urinary Retinol-Binding Protein 4: A Review. International Journal of Environmental Research and Public Health. 2022; 19(16):9878. https://doi.org/10.3390/ijerph19169878
Chicago/Turabian StyleRatajczyk, Krzysztof, Andrzej Konieczny, Adrian Czekaj, Paweł Piotrów, Marek Fiutowski, Kornelia Krakowska, Paweł Kowal, Wojciech Witkiewicz, and Karolina Marek-Bukowiec. 2022. "The Clinical Significance of Urinary Retinol-Binding Protein 4: A Review" International Journal of Environmental Research and Public Health 19, no. 16: 9878. https://doi.org/10.3390/ijerph19169878
APA StyleRatajczyk, K., Konieczny, A., Czekaj, A., Piotrów, P., Fiutowski, M., Krakowska, K., Kowal, P., Witkiewicz, W., & Marek-Bukowiec, K. (2022). The Clinical Significance of Urinary Retinol-Binding Protein 4: A Review. International Journal of Environmental Research and Public Health, 19(16), 9878. https://doi.org/10.3390/ijerph19169878





