The Rise and Rise of Medicinal Cannabis, What Now? Medicinal Cannabis Prescribing in Australia 2017–2022
Abstract
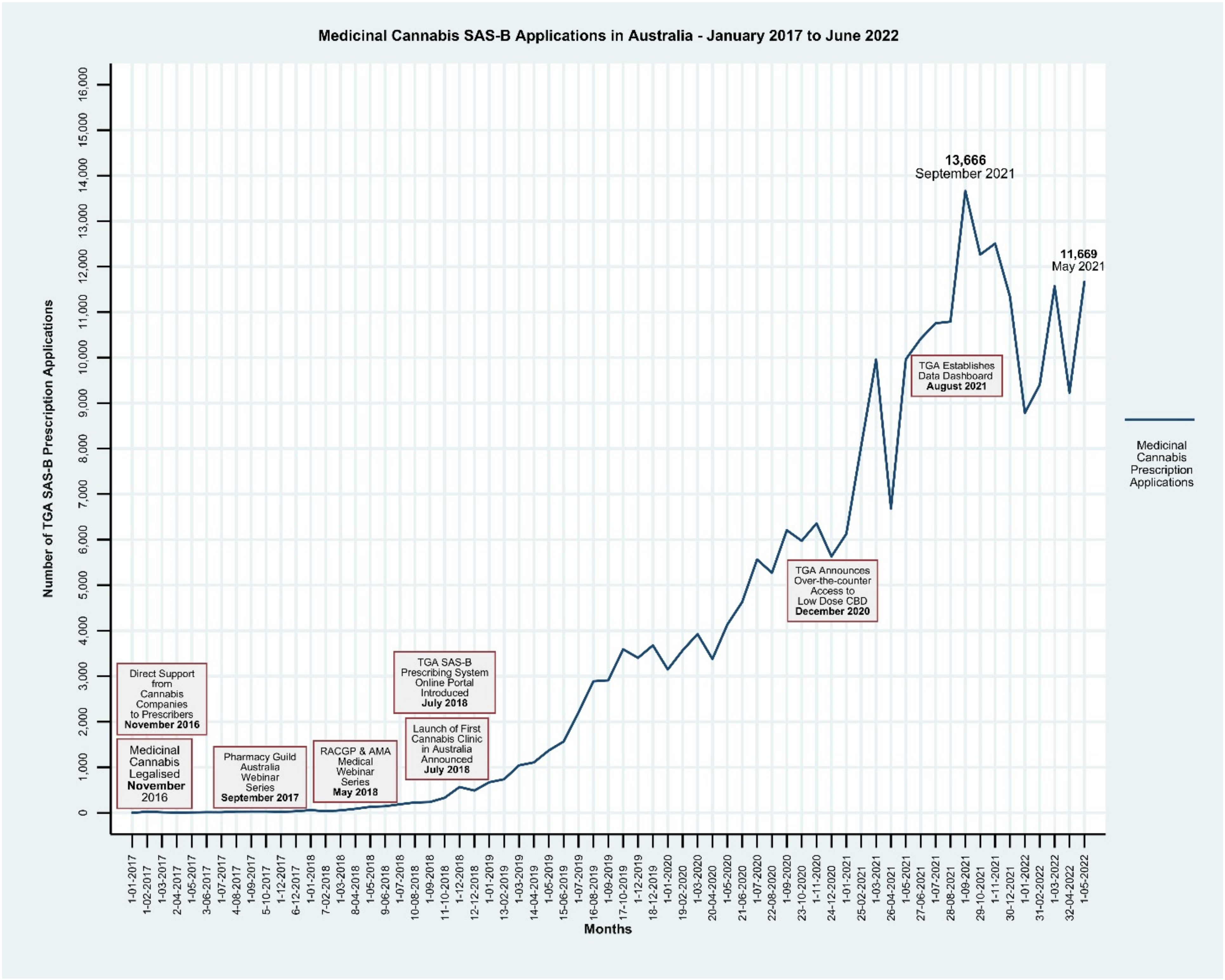
:1. Background and Context
2. Considerations
Monitoring of Access to Medicinal Cannabis in Australia
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- TGA. Medicinal Cannabis: Role of the TGA. Available online: https://www.tga.gov.au/medicinal-cannabis-role-tga (accessed on 1 June 2022).
- TGA. Medicinal Cannabis: Information for Health Professionals. Available online: https://www.tga.gov.au/medicinal-cannabis-information-health-professionals (accessed on 11 January 2020).
- Hallinan, C.M.; Eden, E.; Graham, M.; Greenwood, L.-M.; Mills, J.; Popat, A.; Truong, L.; Bonomo, Y. Over the counter low-dose cannabidiol: A viewpoint from the ACRE Capacity Building Group. J. Psyhopharmacol. 2022, 36, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.; Bazire, S.; Phillips, L.D.; Schlag, A.K. So near yet so far: Why won’t the UK prescribe medical cannabis? BMJ Open 2020, 10, e038687. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I. The therapeutic effects of Cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report. Eur. J. Intern. Med. 2018, 49, 7–11. [Google Scholar] [CrossRef] [PubMed]
- TGA. Access to Medicinal Cannabis Products. Therapeutic Goods Administration. Available online: https://www.tga.gov.au/access-medicinal-cannabis-products-1 (accessed on 1 September 2020).
- Parliment of Australia. Schedule 1-Ammendments Relating to Medicinal Cannabis. Available online: https://www.legislation.gov.au/Details/C2016A00012 (accessed on 1 April 2021).
- Tasmanian Goverment. Pharmaceutical Services Medicinal Cannabis. Available online: https://www.dhhs.tas.gov.au/psbtas/medicinal_cannabis (accessed on 1 August 2022).
- TGA. Medicinal Cannabis Access Data Dashboard. Australian Government Department of Health. Available online: https://www.tga.gov.au/medicinal-cannabis-access-data-dashboard (accessed on 20 August 2021).
- TGA. About the TGA Freedom of Information. Available online: https://www.tga.gov.au/freedom-information (accessed on 15 February 2022).
- Commonwealth of Australia. Official Committee Hansard Current Barriers to Patient Access to Medicinal Cannabis in Australia. Wednesday, 29 January 2020 Melbourne: Australia. 2020. Available online: https://cdaclinics.com.au/wp-content/uploads/2020/10/Current-barriers-to-patient-access-to-medicinal-cannabis-in-Australia-Jan2020.pdf (accessed on 1 September 2020).
- StataCorp. Stata Statistical Software; Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- DJPR. Medicinal Cannabis in Victoria. Victoria State Government. Available online: https://djpr.vic.gov.au/medicinal-cannabis-in-victoria/doctors-and-patients/doctor-access (accessed on 1 April 2021).
- RACGP. Medicinal Cannabis. Available online: https://www.racgp.org.au/education/professional-development/online-learning/webinars/medicinal-cannabis (accessed on 15 February 2022).
- National Health and Medical Research Council. Guidance: Safety Monitoring and Reporting in Clinical Trials Involving Therapeutic Goods; National Health and Medical Research Council: Canberra, Australia, 2016.
- Downing, N.S.; Shah, N.D.; Aminawung, J.A.; Pease, A.M.; Zeitoun, J.-D.; Krumholz, H.M.; Ross, J.S. Postmarket Safety Events among Novel Therapeutics Approved by the US Food and Drug Administration between 2001 and 2010. JAMA 2017, 317, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.M.; Finley, C.R.; Ton, J.; Perry, D.; Ramji, J.; Crawford, K.; Lindblad, A.J.; Korownyk, C.; Kolber, M.R. Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Can. Fam. Physician 2018, 64, e78–e94. (In English) [Google Scholar] [PubMed]
- Chesney, E.; Oliver, D.; Green, A.; Sovi, S.; Wilson, J.; Englund, A.; Freeman, T.; McGuire, P. Adverse effects of cannabidiol: A systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 2020, 45, 1799. [Google Scholar] [CrossRef] [PubMed]
- TGA. Database of Adverse Event Notifications—Medicines. Canberra, Australia: Therapeutic Goods Administration. Available online: https://www.tga.gov.au/database-adverse-event-notifications-daen (accessed on 1 July 2022).
- Doran, T.; Kontopantelis, E.; Valderas, J.M.; Campbell, S.; Roland, M.; Salisbury, C.; Reeves, D. Effect of financial incentives on incentivised and non-incentivised clinical activities: Longitudinal analysis of data from the UK Quality and Outcomes Framework. BMJ 2011, 342, d3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallinan, C.M.; Gunn, J.M.; Bonomo, Y.A. Implementation of medicinal cannabis in Australia: Innovation or upheaval? Perspectives from physicians as key informants, a qualitative analysis. BMJ Open 2021, 11, e054044. [Google Scholar] [PubMed]
- CPASS. Cannabis Patient Advocacy & Support Service: Collaboration. Advocacy. Research. Education. 2021. Available online: https://cannpass.org (accessed on 11 November 2021).
- Project Twenty21. Available online: https://www.drugscience.org.uk/project-twenty21 (accessed on 11 November 2021).
- Innovative Medicines Initiative. Europe’s Partnership for Health; IMI: Brussels, Belgium; Available online: https://www.imi.europa.eu (accessed on 3 February 2021).
- Charles-Smith, L.E.; Reynolds, T.L.; Cameron, M.A.; Conway, M.; Lau, E.H.Y.; Olsen, J.M.; Pavlin, J.A.; Shigematsu, M.; Streichert, L.C.; Suda, K.J.; et al. Using Social Media for Actionable Disease Surveillance and Outbreak Management: A Systematic Literature Review. PLoS ONE 2015, 10, e0139701. (In English) [Google Scholar] [CrossRef] [Green Version]
- Pierce, C.E.; de Vries, S.T.; Bodin-Parssinen, S.; Härmark, L.; Tregunno, P.; Lewis, D.J.; Maskell, S.; van Eemeren, R.; Ptaszynska-Neophytou, A.; Newbould, V.; et al. Recommendations on the Use of Mobile Applications for the Collection and Communication of Pharmaceutical Product Safety Information: Lessons from IMI WEB-RADR. Drug Saf. 2019, 42, 477–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallinan, C.M.; Bonomo, Y.A. The Rise and Rise of Medicinal Cannabis, What Now? Medicinal Cannabis Prescribing in Australia 2017–2022. Int. J. Environ. Res. Public Health 2022, 19, 9853. https://doi.org/10.3390/ijerph19169853
Hallinan CM, Bonomo YA. The Rise and Rise of Medicinal Cannabis, What Now? Medicinal Cannabis Prescribing in Australia 2017–2022. International Journal of Environmental Research and Public Health. 2022; 19(16):9853. https://doi.org/10.3390/ijerph19169853
Chicago/Turabian StyleHallinan, Christine Mary, and Yvonne Ann Bonomo. 2022. "The Rise and Rise of Medicinal Cannabis, What Now? Medicinal Cannabis Prescribing in Australia 2017–2022" International Journal of Environmental Research and Public Health 19, no. 16: 9853. https://doi.org/10.3390/ijerph19169853
APA StyleHallinan, C. M., & Bonomo, Y. A. (2022). The Rise and Rise of Medicinal Cannabis, What Now? Medicinal Cannabis Prescribing in Australia 2017–2022. International Journal of Environmental Research and Public Health, 19(16), 9853. https://doi.org/10.3390/ijerph19169853




