Analyzing the Interaction between Clinical, Neurophysiological and Psychological Outcomes Underlying Chronic Plantar Heel Pain: A Network Analysis Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Patients’ Assessment
2.3.1. Pain Intensity
2.3.2. Function
2.3.3. Clinical Presentation
2.3.4. Mechanosensitivity
2.3.5. Psychological Health
2.3.6. Quality of Life
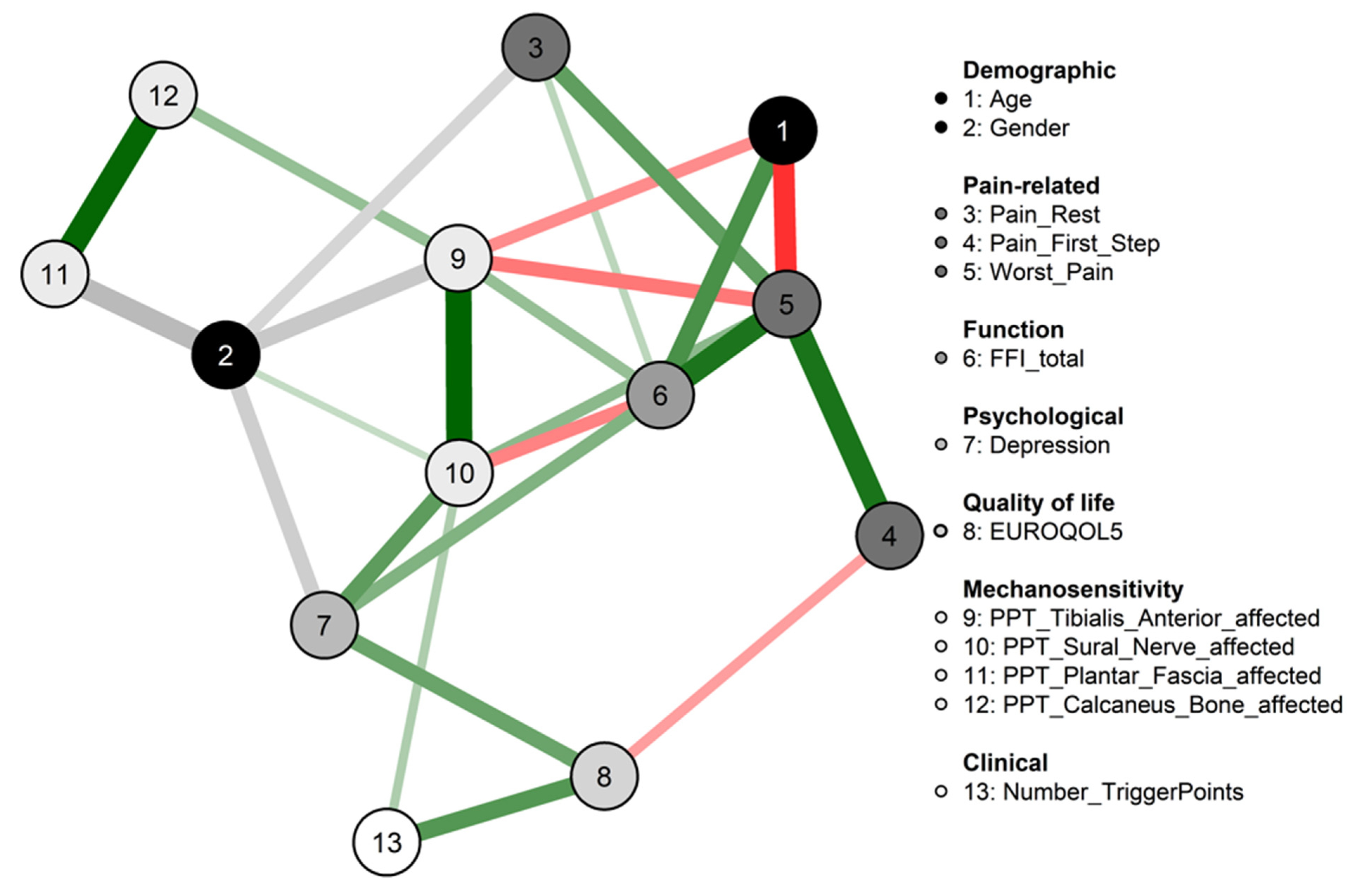
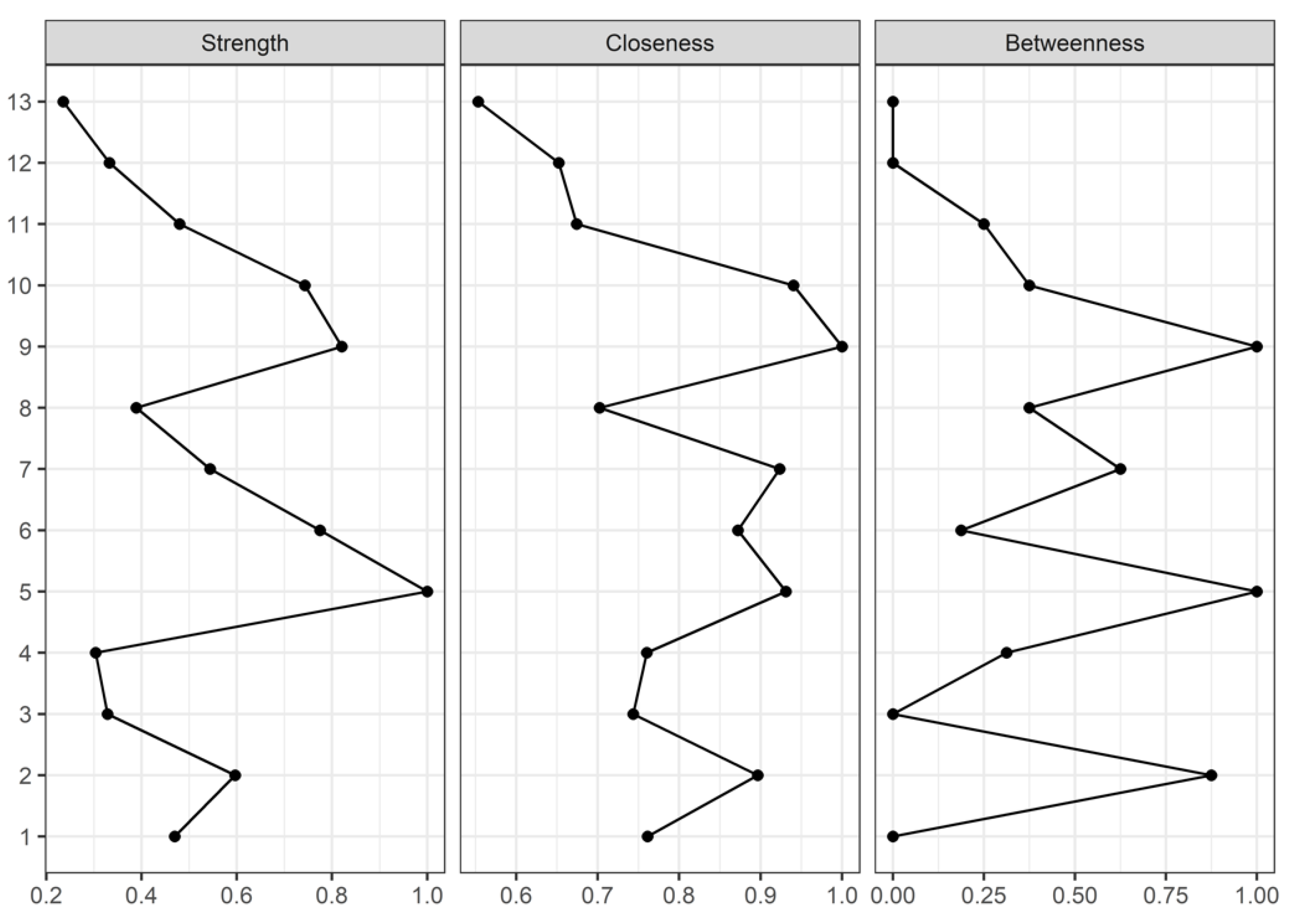
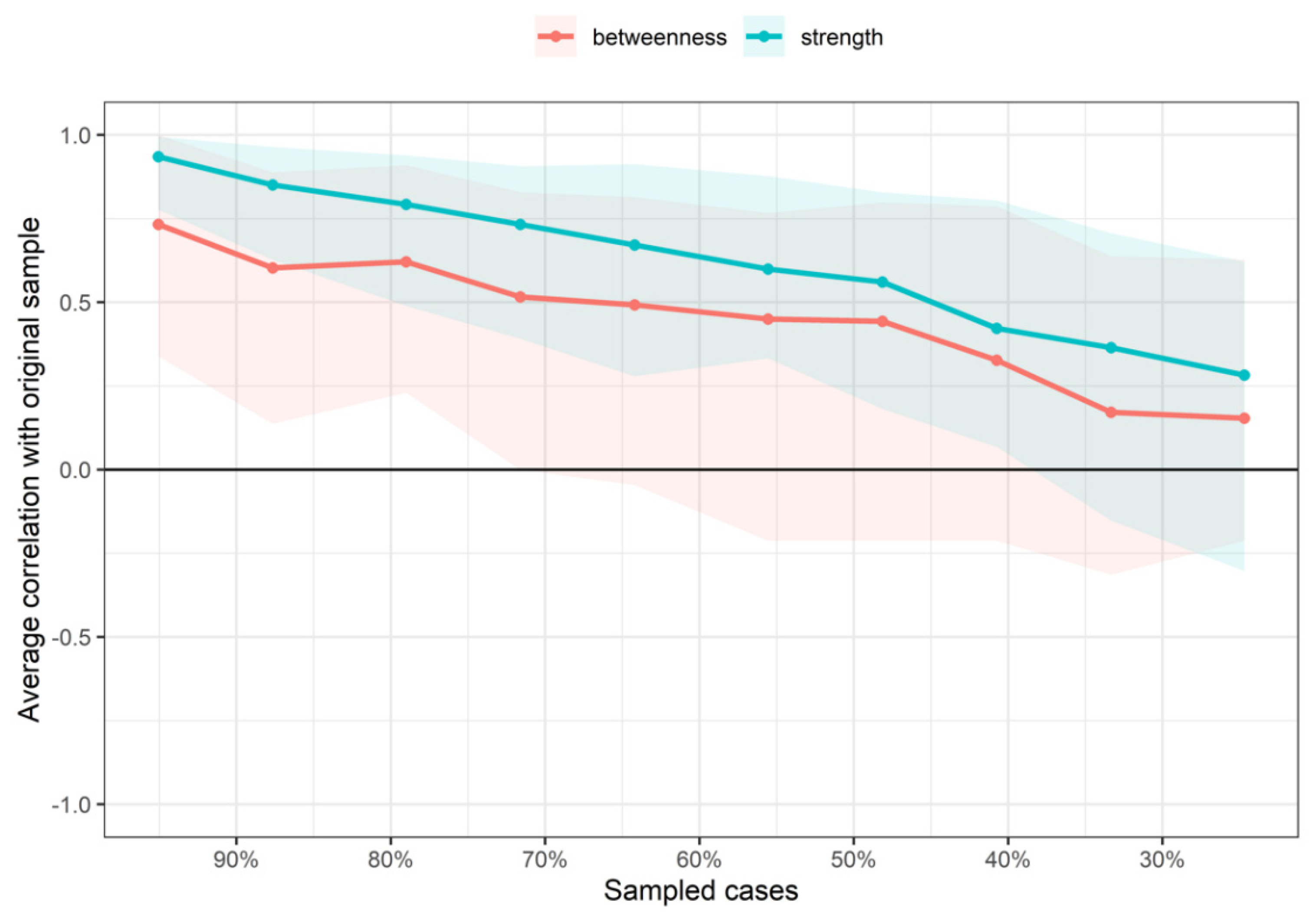
2.4. Network Analysis
- -
- Strength centrality is a blunt measure that takes node’s total level of involvement in the network and not the number of connections with other nodes, being clinically useful to determine which outcomes should be targeted for inducing direct changes in other variables.
- -
- Closeness, which is defined as the inverse sum of the distances of shortest paths of the target node from all other nodes in the network, was interpreted as the expected speed of arrival of something flowing through the network. Therefore, targeting outcomes with high closeness could induce changes to other nodes more quickly than the nodes that are peripheral.
- -
- Betweenness centrality was interpreted as the percentage of shortest paths that must go through the target node. Therefore, a node with a high betweenness centrality would act as an intermediary in the transmission of information or resources between other nodes or even clusters of nodes in the network.
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orchard, J. Plantar fasciitis. BMJ 2012, 345, e6603. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Park, J.; Borg-Stein, J.; Tenforde, A.S. A Systematic Review of Systematic Reviews on the Epidemiology, Evaluation, and Treatment of Plantar Fasciitis. Life 2021, 11, 1287. [Google Scholar] [CrossRef]
- Gutteck, N.; Schilde, S.; Delank, K.S. Pain on the plantar surface of the foot. Dtsch. Ärzteblatt Int. 2019, 116, 83–88. [Google Scholar] [CrossRef]
- Thomas, M.J.; Whittle, R.; Menz, H.B.; Rathod-Mistry, T.; Marshall, M.; Roddy, E. Plantar heel pain in middle-aged and older adults: Population prevalence, associations with health status and lifestyle factors, and frequency of healthcare use. BMC Musculoskelet. Disord. 2019, 20, 337. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.N.; Su, J. Plantar fasciitis: A concise review. Perm. J. 2014, 18, e105–e107. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Schappert, S.M. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: A national study of medical doctors. Foot Ankle Int. 2004, 25, 303–310. [Google Scholar] [CrossRef]
- Martin, R.L.; Davenport, T.E.; Reischl, S.F.; McPoil, T.G.; Matheson, J.W.; Wukich, D.K.; McDonough, C.M.; Altman, R.D.; Beattie, P.; Cornwall, M.; et al. Heel pain-plantar fasciitis: Revision 2014. J. Orthop. Sports Phys. Ther. 2014, 44, A1–A33. [Google Scholar] [CrossRef]
- Sullivan, J.; Pappas, E.; Burns, J. Role of mechanical factors in the clinical presentation of plantar heel pain: Implications for management. Foot 2020, 42, 101636. [Google Scholar] [CrossRef]
- Irving, D.B.; Cook, J.L.; Young, M.A.; Menz, H.B. Impact of chronic plantar heel pain on health-related quality of life. J. Am. Podiatr. Med. Assoc. 2008, 98, 283–289. [Google Scholar] [CrossRef]
- Tong, K.B.; Furia, J. Economic burden of plantar fasciitis treatment in the United States. Am. J. Orthop. 2010, 39, 227–231. [Google Scholar]
- Beeson, P. Plantar fasciopathy: Revisiting the risk factors. Foot Ankle Surg. 2014, 20, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Santiago, R.; Ríos-León, M.; Martín-Casas, P.; Fernández-de-las-Peñas, C.; Plaza-Manzano, G. Active muscle trigger points are associated with pain and related disability in patients with plantar heel pain: A case-control study. Pain Med. 2020, 21, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Manzano, G.; Ríos-León, M.; Martín-Casas, P.; Arendt-Nielsen, L.; Fernández-de-Las-Peñas, C.; Ortega-Santiago, R. Widespread pressure pain hypersensitivity in musculoskeletal and nerve trunk areas as a sign of altered nociceptive processing in unilateral plantar heel pain. J. Pain. 2019, 20, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, D.J.; Fernandez-de-Las-Penas, C.; Blattenberger, T.; Bonneau, D. Altered central pain processing in patients with chronic plantar heel pain: A critically appraised topic. J. Sport Rehabil. 2021, 30, 812–817. [Google Scholar] [CrossRef]
- Ríos-León, M.; Ortega-Santiago, R.; Madeleine, P.; Fernández-de-Las-Peñas, C.; Plaza-Manzano, G. Topographical Pressure Pain Sensitivity Maps of the Feet Reveal Bilateral Pain Sensitivity in Patients With Unilateral Plantar Heel Pain. J. Orthop. Sports Phys. Ther. 2019, 49, 640–646. [Google Scholar] [CrossRef]
- Cotchett, M.P.; Whittaker, G.; Erbas, B. Psychological variables associated with foot function and foot pain in patients with plantar heel pain. Clin. Rheumatol. 2015, 34, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Harutaichun, P.; Pensri, P.; Boonyong, S. Physical and psychological predictors on pain intensity in conscripts with plantar fasciitis. Braz. J. Phys. Ther. 2020, 24, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque-Sendín, F.; Ríos-León, M.; Valera-Calero, J.A.; Plaza-Manzano, G.; Fernández-de-Las-Peñas, C.; Ortega-Santiago, R.; Priscila Rodrigues-de-Souza, D. Clinical, Psychological and Neuro-Physiological Outcomes associated with Pain and Function in Individuals with Unilateral Plantar Heel Pain. Pain Med. 2022, pnac018. [Google Scholar] [CrossRef]
- Epskamp, S.; Fried, E.I. A tutorial on regularized partial correlation networks. Psychol. Methods 2018, 23, 617–634. [Google Scholar] [CrossRef]
- Schmittmann, V.D.; Cramer, A.O.J.; Waldorp, L.J.; Epskamp, S.; Kievit, R.A.; Borsboom, D. Deconstructing the construct: A network perspective on psychological phenomena. New Ideas Psychol. 2013, 31, 43–53. [Google Scholar] [CrossRef]
- Valente, T.W. Network interventions. Science 2012, 337, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Varol, U.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Valera-Calero, J.A. The relevance of headache as an onset symptom in COVID-19: A network analysis of data from the LONG-COVID-EXP-CM multicentre study. Acta Neurol. Belg. 2022, 122, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Palacios-Ceña, M.; Valera-Calero, J.A.; Cuadrado, M.L.; Guerrero-Peral, A.; Pareja, J.A.; Arendt-Nielsen, L.; Varol, U. Understanding the interaction between clinical, emotional and psychophysical outcomes underlying tension-type headache: A network analysis approach. J. Neurol. 2022, 269, 4525–4534. [Google Scholar] [CrossRef] [PubMed]
- Varol, U.; Úbeda-D’Ocasar, E.; Cigarán-Méndez, M.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C.; Gallego-Sendarrubias, G.M.; Valera-Calero, J.A. Understanding the Psychophysiological and Sensitization Mechanisms behind Fibromyalgia Syndrome: A Network Analysis Approach. Pain Med. 2022, pnac121. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Arendt-Nielsen, L.; Cigarán-Méndez, M.; Fernández-de-las-Peñas, C.; Varol, U. Network Analysis for Better Understanding the Complex Psycho-Biological Mechanisms behind Fibromyalgia Syndrome. Diagnostics 2022, 12, 1845. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strobe Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Jensen, M.P.; Turner, J.A.; Romano, J.M.; Fisher, L.D. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999, 83, 157–162. [Google Scholar] [CrossRef]
- Budiman-Mak, E.; Conrad, K.J.; Roach, K.E. The Foot Function Index: A measure of foot pain and disability. J. Clin. Epidemiol. 1991, 44, 561–570. [Google Scholar] [CrossRef]
- Agel, J.; Beskin, J.L.; Brage, M.; Guyton, G.P.; Kadel, N.J.; Saltzman, C.L.; Sands, A.K.; Sangeorzan, B.J.; SooHoo, N.F.; Stroud, C.C.; et al. Reliability of the Foot Function Index: A report of the AOFAS Outcomes Committee. Foot Ankle Int. 2005, 26, 962–967. [Google Scholar] [CrossRef]
- Thummar, R.C.; Rajaseker, S.; Anumasa, R. Association between trigger points in hamstring, posterior leg, foot muscles and plantar fasciopathy: A cross- sectional study. J. Bodyw. Mov. Ther. 2020, 24, 373–378. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Dommerholt, D. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: A Delphi study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Vanderweeën, L.; Oostendorp, R.A.; Vaes, P.; Duquet, W. Pressure algometry in manual therapy. Man. Ther. 1996, 1, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lao, C.; Galiano-Castillo, N.; Cantarero-Villanueva, I.; Martín-Martín, L.; Prados-Olleta, N.; Arroyo-Morales, M. Analysis of Pressure Pain Hypersensitivity, Ultrasound Image, and Quality of Life in Patients with Chronic Plantar Pain: A Preliminary Study. Pain Med. 2016, 17, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Arendt-Nielsen, L.; Andersen, H.; Graven-Nielsen, T. Temporal summation of pain evoked by mechanical stimulation in deep and superficial tissue. J. Pain. 2005, 6, 348–355. [Google Scholar] [CrossRef]
- Xiong, S.; Goonetilleke, R.; Jiang, Z. Pressure thresholds of the human foot: Measurement reliability and effects of stimulus characteristics. Ergonomics 2011, 54, 282–293. [Google Scholar] [CrossRef]
- Fingleton, C.P.; Dempsey, L.; Smart, K.; Doody, C.M. Intraexaminer and interexaminer reliability of manual palpation and pressure algometry of the lower limb nerves in asymptomatic subjects. J. Manip. Physiol. Ther. 2014, 37, 97–104. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gorenstein, C. Assessment of depression in medical patients: A systematic review of the utility of the Beck Depression Inventory-II. Clinics 2013, 68, 1274–1287. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.F.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Keeley, T.; Al-Janabi, H.; Lorgelly, P.; Coast, J. A qualitative assessment of the content validity of the ICECAP-A and EQ-5D-5L and their appropriateness for use in health research. PLoS ONE 2013, 8, e85287. [Google Scholar] [CrossRef]
- Van Hout, B.; Janssen, M.F.; Feng, Y.S.; Kohlmann, T.; Busschbach, J.; Golicki, D.; Lloyd, A.; Scalone, L.; Kind, P.; Pickard, A.S. Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012, 15, 708–715. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Prentice Hall Pearson: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Friedman, J.; Hastie, T.; Tibshirani, R. Glasso: Graphical Lasso Estimation of Gaussian Graphical Models. R Package Version 1.11. 2019. Available online: https://cran.r-project.org/web/packages/glasso/index.html (accessed on 26 July 2022).
- Cook, A.J.; Chastain, D.C. The classification of patients with chronic pain: Age and sex differences. Pain Res. Manag. 2001, 6, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Rustøen, T.; Wahl, A.K.; Hanestad, B.R.; Lerdal, A.; Paul, S.; Miaskowski, C. Age and the experience of chronic pain: Differences in health and quality of life among younger, middle-aged, and older adults. Clin. J. Pain. 2005, 21, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, B.R.; Vieira, E.R. Risk factors for work-related musculoskeletal disorders: A systematic review of recent longitudinal studies. Am. J. Ind. Med. 2010, 53, 285–323. [Google Scholar] [CrossRef] [PubMed]
- Cotchett, M.; Lennecke, A.; Medica, V.G.; Whittaker, G.A.; Bonanno, D.R. The association between pain catastrophising and kinesiophobia with pain and function in people with plantar heel pain. Foot 2017, 32, 8–14. [Google Scholar] [CrossRef]
- Cotchett, M.; Frescos, N.; Whittaker, G.A.; Bonanno, D.R. Psychological factors associated with foot and ankle pain: A mixed methods systematic review. J. Foot Ankle Res. 2022, 15, 10. [Google Scholar] [CrossRef]
- Hidalgo-Lozano, A.; Fernández-de-las-Peñas, C.; Alonso-Blanco, C.; Ge, H.Y.; Arendt-Nielsen, L.; Arroyo-Morales, M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: A blinded, controlled study. Exp. Brain Res. 2010, 202, 915–925. [Google Scholar] [CrossRef]
- Fernández-Carnero, J.; Fernández-de-Las-Peñas, C.; de la Llave-Rincón, A.I.; Ge, H.Y.; Arendt-Nielsen, L. Prevalence of and referred pain from myofascial trigger points in the forearm muscles in patients with lateral epicondylalgia. Clin. J. Pain. 2007, 23, 353–360. [Google Scholar] [CrossRef]
- Wang, C.; Ge, H.Y.; Ibarra, J.M.; Yue, S.W.; Madeleine, P.; Arendt-Nielsen, L. Spatial pain propagation over time following painful glutamate activation of latent myofascial trigger points in humans. J. Pain. 2012, 13, 537–545. [Google Scholar] [CrossRef]
- Ge, H.Y.; Fernández-de-Las-Peñas, C.; Yue, S.W. Myofascial trigger points: Spontaneous electrical activity and its consequences for pain induction and propagation. Chin. Med. 2011, 6, 13. [Google Scholar] [CrossRef]
- Ajimsha, M.S.; Binsu, D.; Chithra, S. Effectiveness of myofascial release in the management of plantar heel pain: A randomized controlled trial. Foot 2014, 24, 66–71. [Google Scholar] [CrossRef]
- Melero-Suárez, R.; Sánchez-Santos, J.A.; Domínguez-Maldonado, G. Evaluation of the Analgesic Effect of Combination Therapy on Chronic Plantar Pain Through the Myofascial Trigger Points Approach. J. Am. Podiatr. Med. Assoc. 2018, 108, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Renan-Ordine, R.; Alburquerque-Sendín, F.; de Souza, D.P.; Cleland, J.A.; Fernández-de-Las-Peñas, C. Effectiveness of myofascial trigger point manual therapy combined with a self-stretching protocol for the management of plantar heel pain: A randomized controlled trial. J. Orthop. Sports Phys. Ther. 2011, 41, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Al-Boloushi, Z.; Gómez-Trullén, E.M.; Arian, M.; Fernández, D.; Herrero, P.; Bellosta-López, P. Comparing two dry needling interventions for plantar heel pain: A randomised controlled trial. BMJ Open 2020, 10, e038033. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Mei, Q.; Li, M.; Gu, M.; Ai, Z.; Tang, K.; Shi, D.; Wu, X.; Wang, X.; Zheng, Y. A comparative efficacy evaluation of ultrasound-guided pulsed radiofrequency treatment in the gastrocnemius in managing plantar heel pain: A randomized and controlled trial. Pain Med. 2015, 16, 782–790. [Google Scholar] [CrossRef]
- Hevey, D. Network analysis: A brief overview and tutorial. Health Psychol. Behav. Med. 2018, 6, 301–328. [Google Scholar] [CrossRef]
| Variable | Sample | Data Distribution |
|---|---|---|
| Age (years) | Mean (SD): 42.2 (13.2) Median (IQR): 43 (20) |  |
| Gender (male/females) | Males: 41 (50.6%) Females: 40 (49.4%) |  |
| Pain Intensity after rest (0–10) | Mean (SD): 5.6 (1.9) Median (IQR): 6 (3) |  |
| Pain Intensity after the first step (0–10) | Mean (SD): 6.2 (2.1) Median (IQR): 7 (3) |  |
| Worst Pain Intensity (0–10) | Mean (SD): 7.4 (1.9) Median (IQR): 8 (2) |  |
| Foot Function Index (0–100) | Mean (SD): 40.7 (18.1) Median (IQR): 40.1 (22.5) |  |
| Beck Depression Inventory (0–63) | Mean (SD): 9.8 (9.4) Median (IQR): 6 (12) | 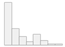 |
| EuroQol-5D-5L (0–1) | Mean (SD): 0.7 (0.2) Median (IQR): 0.7 (0.1) | 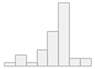 |
| PPT Tibialis Anterior (kg/cm2) | Mean (SD): 3.1 (1.5) Median (IQR): 2.6 (1.9) |  |
| PPT Sural Nerve (kg/cm2) | Mean (SD): 2.0 (1.3) Median (IQR): 1.6 (0.9) |  |
| PPT Plantar Fascia (kg/cm2) | Mean (SD): 2.9 (1.1) Median (IQR): 2.8 (1.1) | 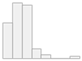 |
| PPT Calcaneus Bone (kg/cm2) | Mean (SD): 4.0 (1.7) Median (IQR): 3.9 (1.9) | 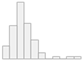 |
| Trigger Points (n) | Mean (SD): 2.7 (2.8) Median (IQR): 2 (3) |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-León, M.; Valera-Calero, J.A.; Ortega-Santiago, R.; Varol, U.; Fernández-de-las-Peñas, C.; Plaza-Manzano, G. Analyzing the Interaction between Clinical, Neurophysiological and Psychological Outcomes Underlying Chronic Plantar Heel Pain: A Network Analysis Study. Int. J. Environ. Res. Public Health 2022, 19, 10301. https://doi.org/10.3390/ijerph191610301
Ríos-León M, Valera-Calero JA, Ortega-Santiago R, Varol U, Fernández-de-las-Peñas C, Plaza-Manzano G. Analyzing the Interaction between Clinical, Neurophysiological and Psychological Outcomes Underlying Chronic Plantar Heel Pain: A Network Analysis Study. International Journal of Environmental Research and Public Health. 2022; 19(16):10301. https://doi.org/10.3390/ijerph191610301
Chicago/Turabian StyleRíos-León, Marta, Juan Antonio Valera-Calero, Ricardo Ortega-Santiago, Umut Varol, César Fernández-de-las-Peñas, and Gustavo Plaza-Manzano. 2022. "Analyzing the Interaction between Clinical, Neurophysiological and Psychological Outcomes Underlying Chronic Plantar Heel Pain: A Network Analysis Study" International Journal of Environmental Research and Public Health 19, no. 16: 10301. https://doi.org/10.3390/ijerph191610301
APA StyleRíos-León, M., Valera-Calero, J. A., Ortega-Santiago, R., Varol, U., Fernández-de-las-Peñas, C., & Plaza-Manzano, G. (2022). Analyzing the Interaction between Clinical, Neurophysiological and Psychological Outcomes Underlying Chronic Plantar Heel Pain: A Network Analysis Study. International Journal of Environmental Research and Public Health, 19(16), 10301. https://doi.org/10.3390/ijerph191610301







