One-Year Real-World Study on Comparison among Different Continuous Subcutaneous Insulin Infusion Devices for the Management of Pediatric Patients with Type 1 Diabetes: The Supremacy of Hybrid Closed-Loop Systems
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
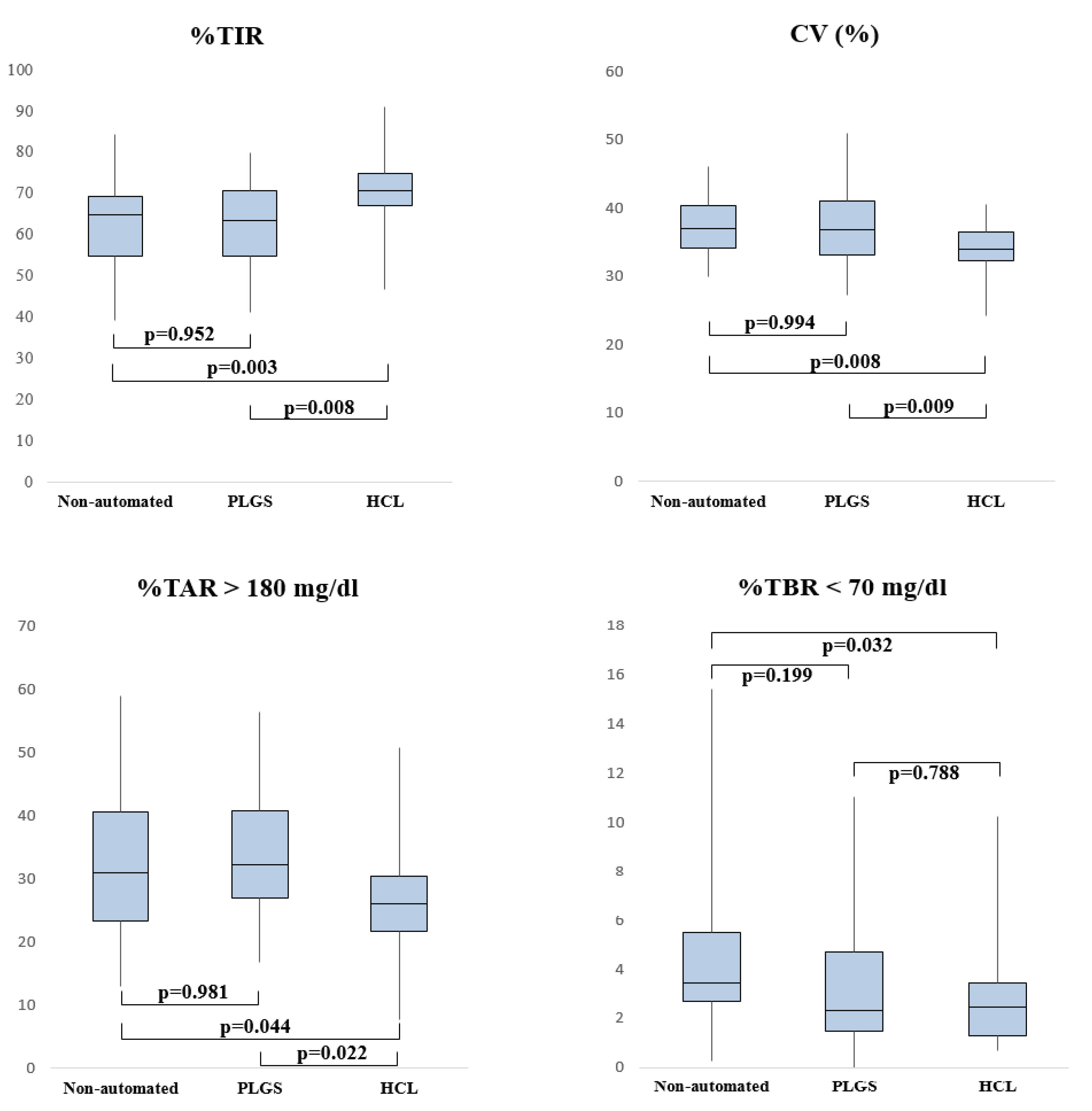
3.1. Comparison of Devices
3.2. Influence of Covariates on Glycemic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Ogle, G.D.; James, S.; Dabelea, D.; Pihoker, C.; Svennson, J.; Maniam, J.; Klatman, E.L.; Patterson, C.C. Global estimates of incidence of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Atlas, 10th edition. Diabetes Res. Clin. Pr. 2021, 183, 109083. [Google Scholar] [CrossRef] [PubMed]
- Pańkowska, E.; Błazik, M.; Dziechciarz, P.; Szypowska, A.; Szajewska, H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: A systematic review and meta-analysis of randomized control trials. Pediatr. Diabetes 2009, 10, 52–58. [Google Scholar] [CrossRef]
- Adolfsson, P.; Ziegler, R.; Hanas, R. Continuous subcutaneous insulin infusion: Special needs for children. Pediatr. Diabetes 2017, 18, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Berget, C.; Messer, L.H.; Forlenza, G.P. A Clinical Overview of Insulin Pump Therapy for the Management of Diabetes: Past, Present, and Future of Intensive Therapy. Diabetes Spectr. 2019, 32, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.R.; Peyrot, M. Treatment satisfaction and quality of life for an integrated continuous glucose monitoring/insulin pump system compared to self-monitoring plus an insulin pump. J. Diabetes Sci. Technol. 2009, 3, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Yin, S.; Chinese Medical Practitioners Association, Endocrinology and Metabolism Branch; Chinese Society of Endocrinology; Chinese Diabetes Society. Insulin pump therapy guidelines for China (July 2010). J. Diabetes 2012, 4, 127–139. [Google Scholar] [CrossRef]
- Papa, G. New horizons for insulin therapy with a pump: The artificial pancreas. J. AMD 2021, 24, 86. [Google Scholar]
- Tumminia, A.; Sciacca, L.; Frittitta, L.; Squatrito, S.; Vigneri, R.; Le Moli, R.; Tomaselli, L. Integrated insulin pump therapy with continuous glucose monitoring for improved adherence: Technology update. Patient Prefer. Adherence 2015, 9, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Messer, L.H.; Berget, C.; Forlenza, G.P. A Clinical Guide to Advanced Diabetes Devices and Closed-Loop Systems Using the CARES Paradigm. Diabetes Technol. Ther. 2019, 21, 462–469. [Google Scholar] [CrossRef]
- Danne, T.; Kordonouri, O.; Holder, M.; Haberland, H.; Golembowski, S.; Remus, K.; Bläsig, S.; Wadien, T.; Zierow, S.; Hartmann, R.; et al. Prevention of hypoglycemia by using low glucose suspend function in sensor-augmented pump therapy. Diabetes Technol. Ther. 2011, 13, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Thomakos, P.; Mitrakou, A.; Kepaptsoglou, O.; Taraoune, I.; Barreto, C.; Zoupas, C.S. The Predictive Low Glucose Management System in Prevention of Clinically Significant Hypoglycemia in Type 1 Diabetes. A Preliminary Study Identifying the Most Common Events Leading Up to Hypoglycemia During Insulin Pump Therapy. Exp. Clin. Endocrinol. Diabetes 2021, 129, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.B.; Nicholas, J.A.; Smith, G.J.; Fairchild, J.M.; King, B.R.; Ambler, G.R.; Cameron, F.J.; Davis, E.A.; Jones, T.W.; on behalf of the PLGM Study Group. Reduction in Hypoglycemia with the Predictive Low-Glucose Management System: A Long-term Randomized Controlled Trial in Adolescents with Type 1 Diabetes. Diabetes Care 2018, 41, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Al Khalaf, F.; Campbell, J.; Umer, F.; Almajaly, D.; Hamdan, M.; Hussain, K. One-year experience of hybrid closed-loop system in children and adolescents with type 1 diabetes previously treated with multiple daily injections: Drivers to successful outcomes. Acta Diabetol. 2021, 58, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.; Teliti, M.; Lezzi, M.; Iosca, A.; Strati, M.F.; Carmisciano, L.; D’Annunzio, G.; Minuto, N.; Maggi, D. A Comparison of Two Hybrid Closed-Loop Systems in Italian Children and Adults with Type 1 Diabetes. Front. Endocrinol. 2021, 12, 802419. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes 2018, 19, 7–19. [Google Scholar] [CrossRef]
- Mortensen, H.B.; Hougaard, P.; Swift, P.; Hansen, L.; Holl, R.W.; Hoey, H.; Bjoerndalen, H.; de Beaufort, C.; Chiarelli, F.; Danne, T.; et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 2009, 32, 1384–1390. [Google Scholar] [CrossRef]
- Breton, M.D.; Kanapka, L.G.; Beck, R.W.; Ekhlaspour, L.; Forlenza, G.P.; Cengiz, E.; Schoelwer, M.; Ruedy, K.J.; Jost, E.; Carria, L.; et al. A Randomized Trial of Closed-Loop Control in Children with Type 1 Diabetes. N. Engl. J. Med. 2020, 383, 836–845. [Google Scholar] [CrossRef]
- Brown, S.A.; Kovatchev, B.P.; Raghinaru, D.; Lum, J.W.; Buckingham, B.A.; Kudva, Y.C.; Laffel, L.M.; Levy, C.J.; Pinsker, J.E.; Wadwa, R.P.; et al. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 1707–1717. [Google Scholar] [CrossRef]
- Collyns, O.J.; Meier, R.A.; Betts, Z.L.; Chan, D.S.; Frampton, C.; Frewen, C.M.; Hewapathirana, N.M.; Jones, S.D.; Roy, A.; Grosman, B.; et al. Improved Glycemic Outcomes with Medtronic MiniMed Advanced Hybrid Closed-Loop Delivery: Results From a Randomized Crossover Trial Comparing Automated Insulin Delivery with Predictive Low Glucose Suspend in People with Type 1 Diabetes. Diabetes Care 2021, 44, 969–975. [Google Scholar] [CrossRef]
- Bekiari, E.; Kitsios, K.; Thabit, H.; Tauschmann, M.; Athanasiadou, E.; Karagiannis, T.; Haidich, A.-B.; Hovorka, R.; Tsapas, A. Artificial pancreas treatment for outpatients with type 1 diabetes: Systematic review and meta-analysis. BMJ 2018, 361, k1310. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Papaioannou, T.G.; Bellos, I.; Alexandraki, K.; Tentolouris, N.; Stefanadis, C.; Chrousos, G.P.; Tousoulis, D. Effectiveness of artificial pancreas in the non-adult population: A systematic review and network meta-analysis. Metabolism 2019, 90, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.; Al Khalifah, R.; McAssey, K. The efficacy and safety of insulin pump therapy with predictive low glucose suspend feature in decreasing hypoglycemia in children with type 1 diabetes mellitus: A systematic review and meta-analysis. Pediatr. Diabetes 2020, 21, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Ferrito, L.; Passanisi, S.; Bonfanti, R.; Cherubini, V.; Minuto, N.; Schiaffini, R.; Scaramuzza, A. Efficacy of advanced hybrid closed loop sys-tems for the management of type 1 diabetes in children. Minerva. Pediatr. 2021, 73, 474–485. [Google Scholar]
- Bally, L.; Thabit, H.; Kojzar, H.; Mader, J.K.; Qerimi-Hyseni, J.; Hartnell, S.; Tauschmann, M.; Allen, J.M.; Wilinska, M.E.; Pieber, T.R.; et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: An open-label, randomised, crossover study. Lancet Diabetes Endocrinol. 2017, 5, 261–270. [Google Scholar] [CrossRef]
- Forlenza, G.P.; Pinhas-Hamiel, O.; Liljenquist, D.R.; Shulman, D.I.; Bailey, T.S.; Bode, B.W.; Wood, M.A.; Buckingham, B.A.; Kaiserman, K.B.; Shin, J.; et al. Safety Evaluation of the MiniMed 670G System in Children 7–13 Years of Age with Type 1 Diabetes. Diabetes Technol. Ther. 2019, 21, 11–19. [Google Scholar] [CrossRef]
- Beato-Víbora, P.I.; Gallego-Gamero, F.; Lázaro-Martín, L.; Romero-Pérez, M.D.M.; Arroyo-Díez, F.J. Prospective Analysis of the Impact of Commercialized Hybrid Closed-Loop System on Glycemic Control, Glycemic Variability, and Patient-Related Outcomes in Children and Adults: A Focus on Superiority Over Predictive Low-Glucose Suspend Technology. Diabetes Technol. Ther. 2020, 22, 912–919. [Google Scholar] [CrossRef]
- Varimo, T.; Pulkkinen, M.A.; Hakonen, E.; Hero, M.; Miettinen, P.J.; Tuomaala, A.K. First year on commercial hybrid closed-loop system-experience on 111 children and adolescents with type 1 diabetes. Pediatr. Diabetes. 2021, 22, 909–915. [Google Scholar] [CrossRef]
- Breton, M.D.; Kovatchev, B.P. One Year Real-World Use of the Control-IQ Advanced Hybrid Closed-Loop Technology. Diabetes Technol. Ther. 2021, 23, 601–608. [Google Scholar] [CrossRef]
- Piona, C.; Ventrici, C.; Marcovecchio, L.; Chiarelli, F.; Maffeis, C.; Bonfanti, R.; Rabbone, I. Long-term complications of type 1 diabetes: What do we know and what do we need to understand? Minerva. Pediatr. 2021, 73, 504–522. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Rama Chandran, S.; Tay, W.L.; Lye, W.K.; Lim, L.L.; Ratnasingam, J.; Tan, A.T.B.; Gardner, D.S.L. Beyond HbA1c: Comparing Glycemic Variability and Glycemic Indices in Predicting Hypoglycemia in Type 1 and Type 2 Diabetes. Diabetes Technol. Ther. 2018, 20, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, E.E.; Abraham, M.B.; Smith, G.J.; Mountain, J.A.; Jones, T.W.; Davis, E.A. Continuous Glucose Monitoring Improves Glycemic Outcomes in Children with Type 1 Diabetes: Real-World Data From a Population-Based Clinic. Diabetes Care 2021, 44, e171–e172. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L.M.; Kanapka, L.G.; Beck, R.W.; Bergamo, K.; Clements, M.A.; Criego, A.; DeSalvo, D.J.; Goland, R.; Hood, K.; Liljenquist, D.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adolescents and Young Adults with Type 1 Diabetes: A Randomized Clinical Trial. JAMA 2020, 323, 2388–2396. [Google Scholar] [CrossRef]
- Duffus, S.H.; Ta’ani, Z.A.; Slaughter, J.C.; Niswender, K.D.; Gregory, J.M. Increased proportion of time in hybrid closed-loop “Auto Mode” is associated with improved glycaemic control for adolescent and young patients with adult type 1 diabetes using the MiniMed 670G insulin pump. Diabetes Obes. Metab. 2020, 22, 688–693. [Google Scholar] [CrossRef]
- Goodwin, G.; Waldman, G.; Lyons, J.; Oladunjoye, A.; Steil, G. OR14-5 Challenges in implementing Hybrid Closed Loop Insulin Pump Therapy (medtronic 670g) In A “Real World” Clinical Setting. J. Endocr. Soc. 2019, 3 (Suppl. 1), OR14-5. [Google Scholar] [CrossRef]
- Passanisi, S.; Salzano, G.; Galletta, F.; Aramnejad, S.; Caminiti, L.; Pajno, G.B.; Lombardo, F. Technologies for Type 1 Diabetes and Contact Dermatitis: Therapeutic Tools and Clinical Outcomes in a Cohort of Pediatric Patients. Front. Endocrinol. 2022, 13, 846137. [Google Scholar] [CrossRef] [PubMed]
- Passanisi, S.; Salzano, G.; Lombardo, F. Skin Involvement in Paediatric Patients with Type 1 Diabetes. Curr. Diabetes Rev. 2022, 18, e030921196145. [Google Scholar] [CrossRef]
- Lombardo, F.; Passanisi, S.; Tinti, D.; Messina, M.F.; Salzano, G.; Rabbone, I. High Frequency of Dermatological Complications in Children and Adolescents with Type 1 Diabetes: A Web-Based Survey. J. Diabetes Sci. Technol. 2021, 15, 1377–1381. [Google Scholar] [CrossRef]
- Bisio, A.; Brown, S.A.; McFadden, R.; Pajewski, M.; Yu, P.L.; DeBoer, M.D.; Schoelwer, M.J.; Bonner, H.G.; Wakeman, C.A.; Cherñavvsky, D.R.; et al. Sleep and diabetes-specific psycho-behavioral outcomes of a new automated insulin delivery system in young children with type 1 diabetes and their parents. Pediatr. Diabetes. 2021, 22, 495–502. [Google Scholar] [CrossRef]
- Roze, S.; Buompensiere, M.I.; Ozdemir, Z.; de Portu, S.; Cohen, O. Cost-effectiveness of a novel hybrid closed-loop system compared with continuous subcutaneous insulin infusion in people with type 1 diabetes in the UK. J. Med. Econ. 2021, 24, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Pease, A.J.; Zomer, E.; Liew, D.; Earnest, A.; Soldatos, G.; Ademi, Z.; Zoungas, S. Cost-Effectiveness Analysis of a Hybrid Closed-Loop System Versus Multiple Daily Injections and Capillary Glucose Testing for Adults with Type 1 Diabetes. Diabetes Technol. Ther. 2020, 22, 812–821. [Google Scholar] [CrossRef] [PubMed]

| Non Automated | PLGS | HCL | p-Value | |
|---|---|---|---|---|
| Gender | 0.621 | |||
| Male | 14 (51.8%) | 17 (58.6%) | 23 (51.1%) | |
| Female | 13 (48.1%) | 12 (41.3%) | 22 (48.8%) | |
| Age (years) | 13.2 ± 3.3 | 12.6 ± 3.8 | 12.1 ± 3.5 | 0.416 |
| Duration of diabetes (years) | 6.3 ± 2.9 | 5.2 ± 2.9 | 5.5 ± 3.3 | 0.389 |
| BMI z-score | 0.48 ± 0.81 | 0.26 ± 0.78 | 0.64 ± 1.01 | 0.226 |
| Duration of use CSII (years) | 2.8 ± 1.7 | 2.8 ± 1.9 | 1.4 ± 1.2 | <0.001 * |
| HbA1c (%) | 6.7 ± 0.5 | 7.1 ± 0.8 | 7.1 ± 0.6 | 0.040 * |
| %TBR < 54 mg/dL | 1.1 ± 1.4 | 0.6 ± 1 | 0.5 ± 0.9 | 0.057 |
| %TBR 54–70 mg/dL | 3.6 ± 2.5 | 2.8 ± 1.9 | 2.4 ± 1.4 | 0.035 * |
| %TIR 70–180 mg/dL | 61.7 ± 11.6 | 62.6 ± 10.4 | 70.2 ± 8.7 | 0.001 * |
| %TAR 180–250 mg/dL | 24.5 ± 7.1 | 24.6 ± 5.9 | 21.8 ± 6.5 | 0.099 |
| %TAR > 250 mg/dL | 8. 9 ± 6.9 | 9.4 ± 6 | 5.3 ± 3.8 | 0.002 * |
| GMI (%) | 7.1 ± 0.4 | 7.1 ± 0.4 | 6.9 ± 0.3 | 0.028 * |
| Use of sensor (%) | 80.9 ± 22.6 | 74.5 ± 19.6 | 82.4 ± 15.1 | 0.204 |
| Mean glucose levels (mg/dL) | 158.2 ± 20.6 | 160.5 ± 16.1 | 150.8 ± 12.6 | 0.028 * |
| SD glucose levels (mg/dL) | 59.7 ± 10.7 | 60.1 ± 10.9 | 51.7 ± 7.6 | <0.001 * |
| CV (%) | 37.5 ± 4.4 | 37.3 ± 5.4 | 34 ± 3.9 | 0.001 * |
| Daily insulin dose (IU/kg) | 0.82 ± 0.16 | 0.81 ± 0.18 | 0.9 ± 0.2 | 0.422 |
| Basal insulin (%) | 63.8 ± 6.8 | 62.9 ± 11.2 | 50.4 ± 10.4 | <0.001 * |
| Bolus (%) | 36.1 ± 6.6 | 37.1 ± 11.2 | 49.6 ± 10.4 | <0.001 * |
| Adjusted OR | 95% C.I. | p Value | |
|---|---|---|---|
| Age (years) | −0.91 | −0.69–0.51 | 0.765 |
| Gender | 2.53 | −1.38–6.44 | 0.203 |
| BMI z-score | −2.09 | −4.30–0.11 | 0.063 |
| Duration of diabetes | −0.38 | −1.11–0.34 | 0.296 |
| Duration of CSII use | 0.69 | −0.66–2.04 | 0.313 |
| Sensor daily use (%) | 0.12 | 0.01–0.23 | 0.031 * |
| HCL use | 8.58 | 4.27–12.90 | <0.001 * |
| Adjusted OR | 95% C.I. | p Value | |
|---|---|---|---|
| Age (years) | −0.28 | −0.60–0.02 | 0.073 |
| Gender | 0.25 | −1.59–2.10 | 0.784 |
| BMI z-score | −0.66 | −1.77–0.43 | 0.231 |
| Duration of diabetes | +0.36 | −0.18–0.74 | 0.061 |
| Duration of CSII use | −0.35 | −0.93–0.21 | 0.213 |
| Sensor daily use (%) | −0.04 | −0.08–0.008 | 0.098 |
| HCL use | −3.45 | −5.58–−1.32 | <0.002 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bombaci, B.; Passanisi, S.; Alibrandi, A.; D’Arrigo, G.; Patroniti, S.; Averna, S.; Salzano, G.; Lombardo, F. One-Year Real-World Study on Comparison among Different Continuous Subcutaneous Insulin Infusion Devices for the Management of Pediatric Patients with Type 1 Diabetes: The Supremacy of Hybrid Closed-Loop Systems. Int. J. Environ. Res. Public Health 2022, 19, 10293. https://doi.org/10.3390/ijerph191610293
Bombaci B, Passanisi S, Alibrandi A, D’Arrigo G, Patroniti S, Averna S, Salzano G, Lombardo F. One-Year Real-World Study on Comparison among Different Continuous Subcutaneous Insulin Infusion Devices for the Management of Pediatric Patients with Type 1 Diabetes: The Supremacy of Hybrid Closed-Loop Systems. International Journal of Environmental Research and Public Health. 2022; 19(16):10293. https://doi.org/10.3390/ijerph191610293
Chicago/Turabian StyleBombaci, Bruno, Stefano Passanisi, Angela Alibrandi, Giulia D’Arrigo, Serena Patroniti, Simona Averna, Giuseppina Salzano, and Fortunato Lombardo. 2022. "One-Year Real-World Study on Comparison among Different Continuous Subcutaneous Insulin Infusion Devices for the Management of Pediatric Patients with Type 1 Diabetes: The Supremacy of Hybrid Closed-Loop Systems" International Journal of Environmental Research and Public Health 19, no. 16: 10293. https://doi.org/10.3390/ijerph191610293
APA StyleBombaci, B., Passanisi, S., Alibrandi, A., D’Arrigo, G., Patroniti, S., Averna, S., Salzano, G., & Lombardo, F. (2022). One-Year Real-World Study on Comparison among Different Continuous Subcutaneous Insulin Infusion Devices for the Management of Pediatric Patients with Type 1 Diabetes: The Supremacy of Hybrid Closed-Loop Systems. International Journal of Environmental Research and Public Health, 19(16), 10293. https://doi.org/10.3390/ijerph191610293









