Infectious Diseases Associated with Hydrometeorological Hazards in Europe: Disaster Risk Reduction in the Context of the Climate Crisis and the Ongoing COVID-19 Pandemic
Abstract
1. Introduction
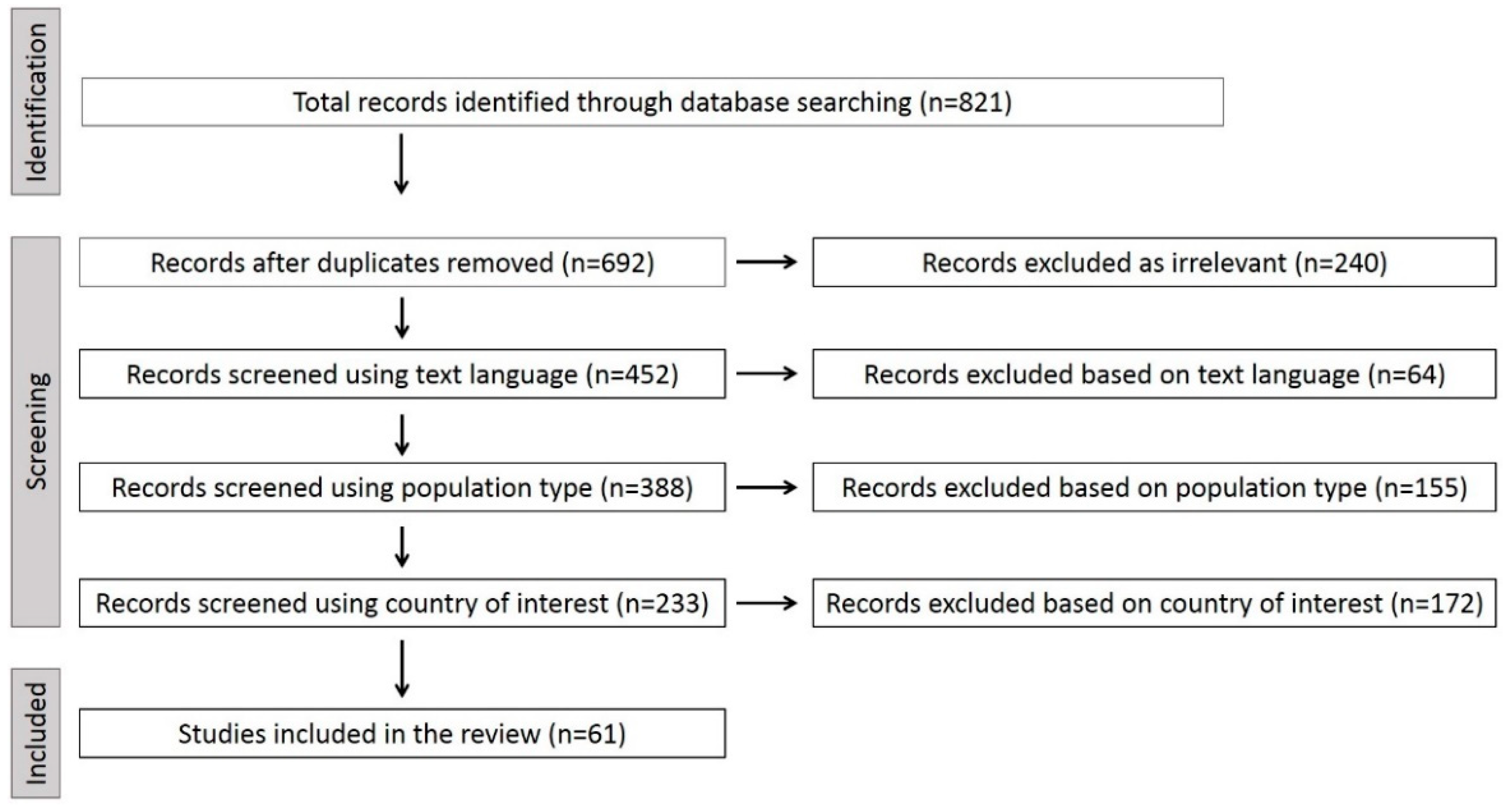
2. Materials and Methods
3. Results
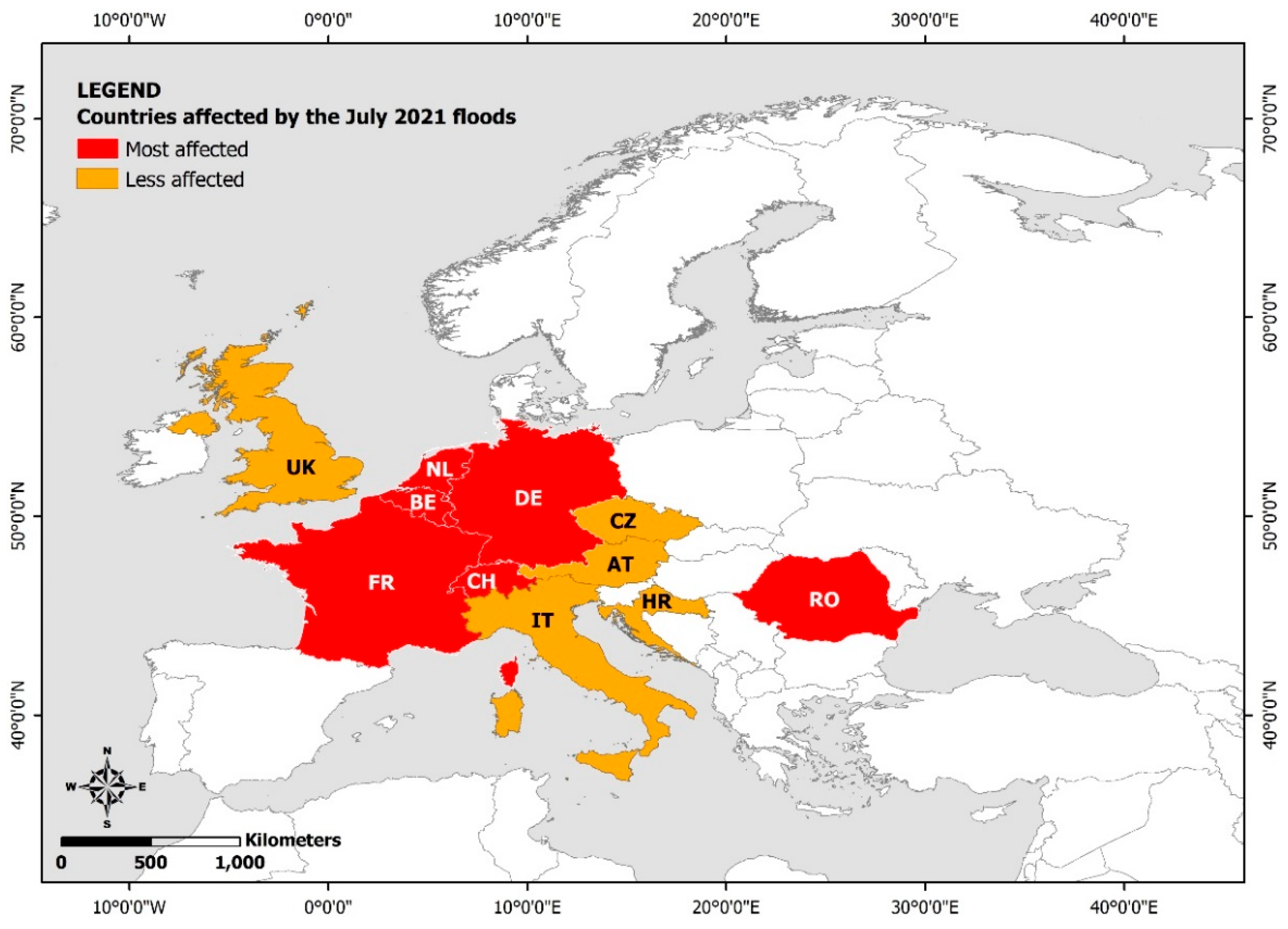
3.1. Study Selection
3.2. Waterborne Diseases
3.2.1. Parasites: Cryptosporidium
3.2.2. Viruses: Norovirus, Hepatitis A Virus
3.2.3. Bacteria: Escherichia coli, Campylobacter jejuni, Shigella sonnei
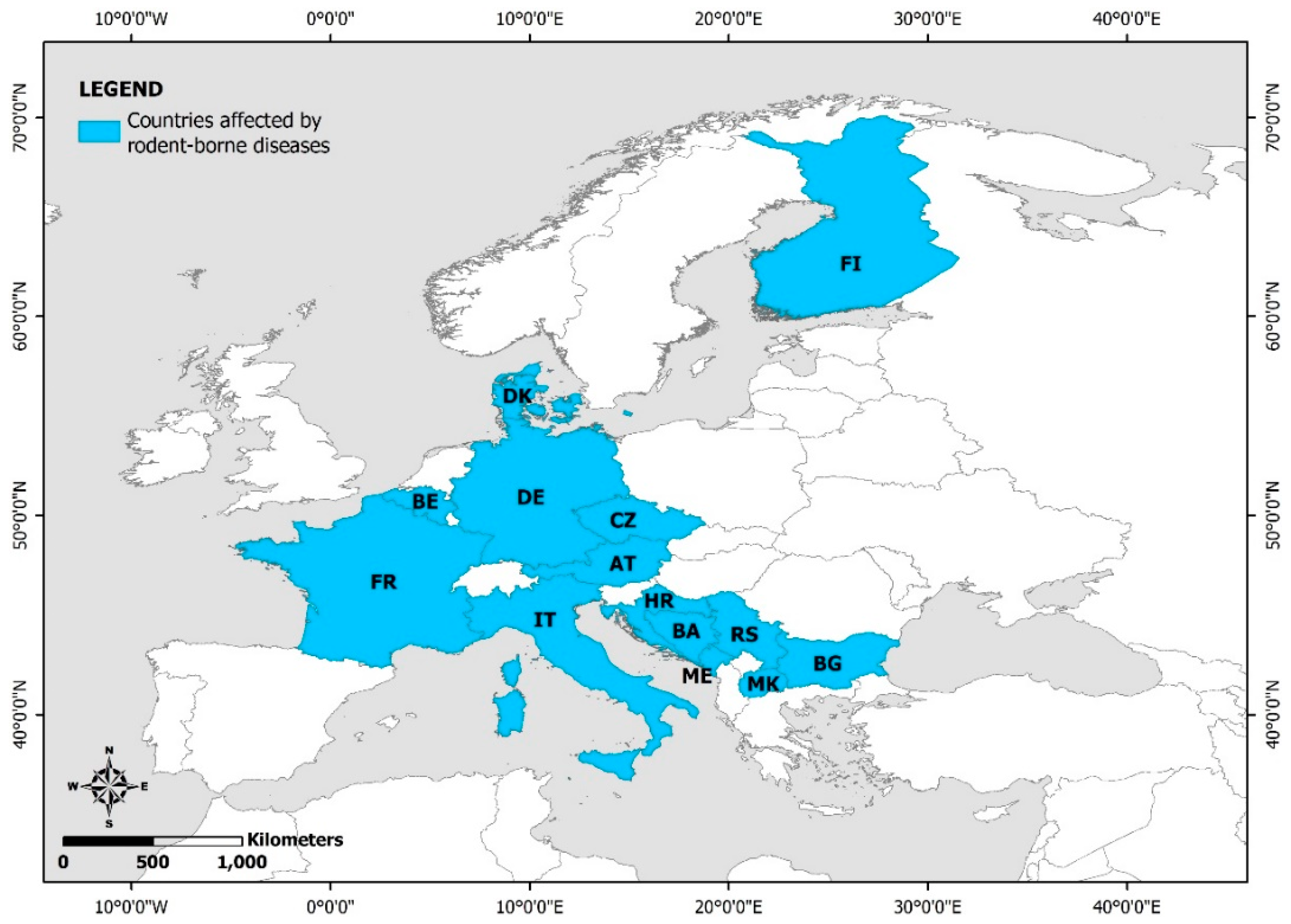
3.3. Rodent-Borne Diseases
3.3.1. Leptospira
3.3.2. Hantavirus
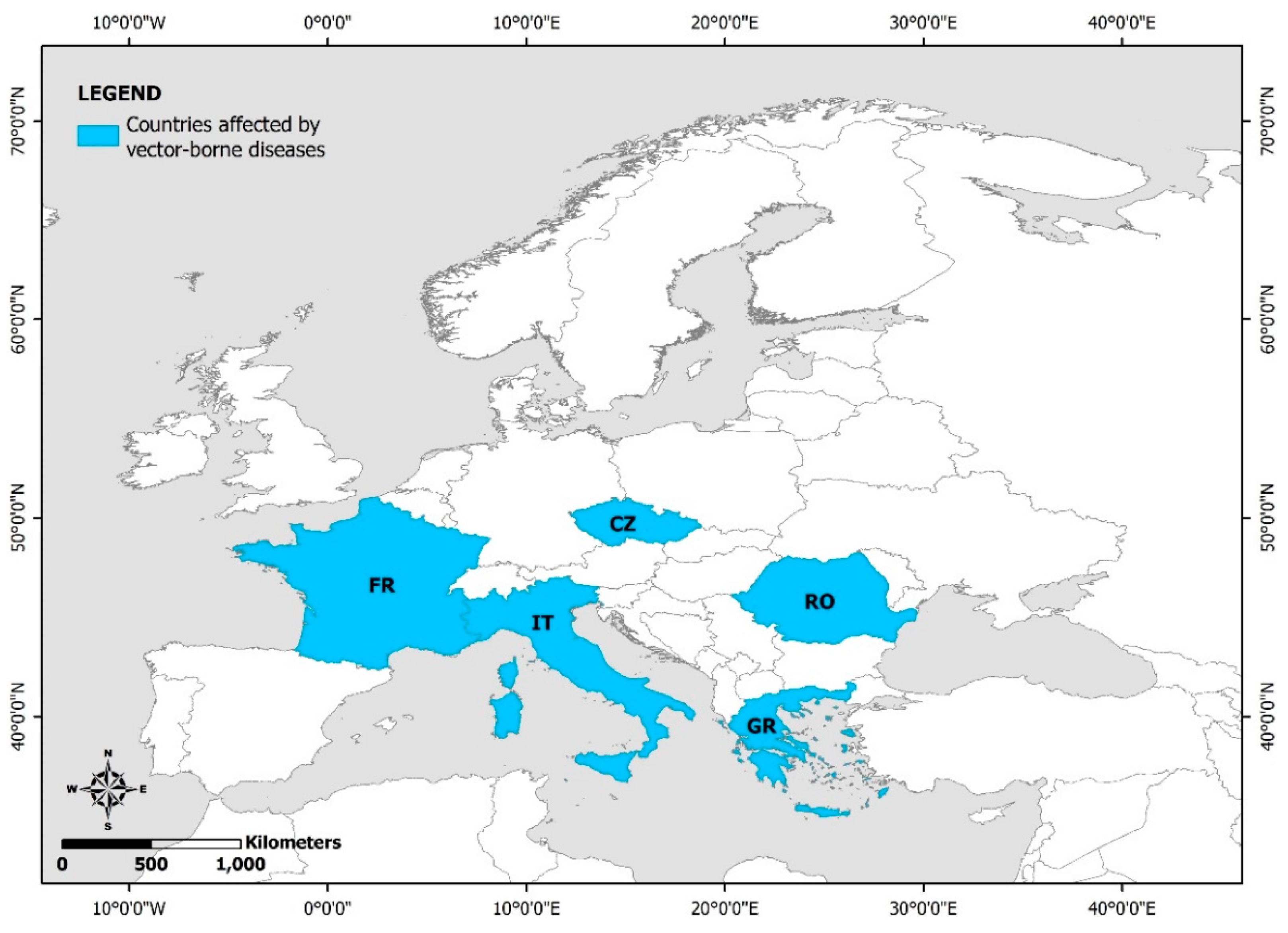
3.4. Vector-Borne Diseases
4. Strategies and Measures for Prevention and Management of Infectious Disease Outbreaks in Flood-Prone and Flood-Affected Areas Respectively
4.1. Preventing an Infectious Disease Outbreak
- regular hand washing with soap and clean, safe running water, or alternatively disinfecting hands with alcohol-based wipes or hand sanitizers that contain at least 60% alcohol in case of lack of soap and water, especially after contact with floodwater and before eating; drinking; touching eyes, nose, and mouth; and treating a cut or wound;
- wearing PPE comprising disposable gloves, rubber boots, and waders for protecting body and limbs from contact with floodwater, related sediments, and debris; and
- wearing face protection gear including mask and safety goggles and glasses for protecting against splashes and droplets in eyes, nose, and mouth.
4.2. Post-Flood Measures for Preventing an Infectious Disease Outbreak
4.3. Mitigation Actions When an Infectious Disease Outbreak Occurs
4.4. When Flooding, Flood-Related Infectious Disease Outbreaks and COVID-19 Collide
- the pre-existing community prevalence of COVID-19 in the disaster affected area;
- the number of people involved in assessing disaster impact and, in response, actions in the disaster affected area;
- the measures applied by responders for personal protection and for the safety of the disaster-affected population especially during the first crucial days of the emergency response;
- the intensity of the generated events and their effects on population (fatalities and injuries), on nature (environmental effects) and on the building stock (structural damage to buildings, infrastructures, and lifelines);
- the immediate housing measures adapted to the unprecedented conditions;
- the need for immediate evacuation without the contribution and support of the Civil Protection staff, responders, and volunteers;
- the accessibility to the affected area during the pre- and post-disaster periods;
- the demographic properties of the affected area with emphasis on the population density and the geographical distribution of residential areas; and
- the level of education, organization, and preparedness of the authorities, agencies, and services involved in the management of the simultaneous impacts of natural hazards and related disasters, as well as biological hazards, such as pandemics and outbreaks.
- Improving whole-of-society coordination mechanisms to aid preparedness, including health, transportation, travel, security, and other crisis first responders.
- Setting up emergency camps or makeshift settlements for displaced people forced to flee their homes.
- Increasing the number of emergency shelters of the same type or using other facilities as emergency shelters in order to avoid overcrowding and maintain physical distancing.
- Configuring individual rooms, separate areas, large facilities, and guidelines for maintaining physical distancing within the emergency shelters.
- Modifying food preparation and distribution practices and adapting to the unprecedented conditions. For instance, packaged meals could be prepared and served on individual and disposable serving utensils by staff wearing masks and disposable gloves with working surfaces being cleaned and disinfected on a regular basis.
- Establishing a surveillance system in emergency shelters and temporary settlements to detect COVID-19 patients early in the displaced population and isolate sick individuals including temporary isolation facilities equipped with the appropriate medical-technological equipment for health assessment, medical care, and counseling.
- Establishing health-care centers or field hospitals for the control of critical points for managing COVID-19-infected waste.
- Meeting the flood-affected people’s immediate health needs, with emphasis on vulnerable people, such as the elderly, women, and children.
- Intensive training and reminders about public health measures such as hand hygiene, respiratory etiquette, and social distancing to health-care providers in provinces that have received flood warnings. Medicine supply, distribution location determination, and patient access to outpatient drugs, as well as delivery and drug distribution personnel training.
- Weekly distribution of sufficient quantities of hygiene items comprising masks, soap, and waterless antiseptic agents including alcohol-based solutions for hands and surfaces disinfection among flood victims.
- Construction of control shared bathrooms, use of clean surfaces with water and detergents, and access to appropriately designed toilets to prevent contamination of groundwater resources.
- Access to psychological consultations and support for flood-affected people, especially if they have lost a family member due to COVID-19.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centre for Research on the Epidemiology of Disasters (CRED). EM-DAT—The International Disaster Database. 2022. Available online: https://public.emdat.be/ (accessed on 10 January 2022).
- Centre for Research on the Epidemiology of Disasters; UN Office for Disaster Risk Reduction (CRED-UNDRR). The Non-COVID Year in Disasters: Global Trends and Perspectives. Available online: http://dl.handle.net/2078.1/245181 (accessed on 10 January 2022).
- Nied, M.; Pardowitz, T.; Nissen, K.; Ulbrich, U.; Hundecha, Y.; Merz, B. On the relationship between hydro-meteorological patterns and flood types. J. Hydrol. 2014, 519, 3249–3262. [Google Scholar] [CrossRef]
- Leal, M.; Ramos, C.; Pereira, S. Different types of flooding lead to different human and material damages: The case of the Lisbon Metropolitan Area. Nat. Hazards 2018, 91, 735–758. [Google Scholar] [CrossRef]
- Li, D.; Lettenmaier, D.P.; Margulis, S.A.; Andreadis, K. The role of rain-on-snow in flooding over the conterminous United States. Water Resour. Res. 2019, 55, 8492–8513. [Google Scholar] [CrossRef]
- Turcotte, B.; Morse, B.; Pelchat, G. Impact of Climate Change on the Frequency of Dynamic Breakup Events and on the Risk of Ice-Jam Floods in Quebec, Canada. Water 2020, 12, 2891. [Google Scholar] [CrossRef]
- Brown, L.; Murray, V. Examining the relationship between infectious diseases and flooding in Europe: A systematic literature review and summary of possible public health interventions. Disaster Health 2013, 1, 117–127. [Google Scholar] [CrossRef]
- Du, W.; FitzGerald, G.J.; Clark, M.; Hou, X.Y. Health impacts of floods. Prehosp. Disaster Med. 2010, 25, 265–272. [Google Scholar] [CrossRef]
- Epstein, P.R. Climate change and emerging infectious diseases. Microbes Infect. 2001, 3, 747–754. [Google Scholar] [CrossRef]
- Egger, G.; Swinburn, B.; Rossner, S. Dusting off the epidemiological triad: Could it work with obesity? Obes Rev. 2003, 4, 115–119. [Google Scholar] [CrossRef]
- Patz, J.A.; Olson, S.H.; Uejio, C.K.; Gibbs, H.K. Disease emergence from global climate and land use change. Med. Clin. N. Am. 2008, 92, 1473–1491. [Google Scholar] [CrossRef]
- Okaka, F.O.; Odhiambo, B.D.O. Relationship between Flooding and Out Break of Infectious Diseasesin Kenya: A Review of the Literature. J. Environ. Public Health 2018, 5452938. [Google Scholar] [CrossRef]
- Centre for Research on the Epidemiology of Disasters (CRED); United Nations Office for Disaster Risk Reduction. 2020: The Non-COVID Year in Disasters; CRED: Brussels, Belgium, 2021; Available online: https://emdat.be/sites/default/files/adsr_2020.pdf (accessed on 10 January 2022).
- Centre for Research on the Epidemiology of Disasters (CRED). 2021 Disasters in Numbers; CRED: Brussels, Belgium, 2022; Available online: https://cred.be/sites/default/files/2021_EMDAT_report.pdf (accessed on 10 January 2022).
- Okura, Y.; Dutta, S.; Begum, A.; Naznin, Z. Monsoon, Floods and COVID-19: Building Community Resilience in Bangladesh. Findings from Union Disaster Management Committees—June 2020; Zurich Flood Resilience Alliance: Zurich, Germany, 2000; 11p. [Google Scholar]
- Walton, D.; Arrighi, J.; van Aalst, M.K.; Claudet, M. The Compound Impact of Extreme Weather Events and COVID-19. An Update of the Number of People Affected and a Look at the Humanitarian Implications in Selected Contexts; The International Federation of Red Cross and Red Crescent Societies: Geneva, Switzerland, 2021; 34p. [Google Scholar]
- Pan American Health Organization/World Health Organization (PAHO/WHO). Epidemiological Alert: Postflood Events in the Context of COVID-19 Pandemic; PAHO/WHO: Washington, DC, USA, 2022; 8p. [Google Scholar]
- World Health Organization (WHO). Communicable Disease Control in Emergencies: A Field Manual; Connolly, M., Ed.; WHO: Geneva, Switzerland, 2005; Available online: http://www.who.int/infectious-disease-news/IDdocs/whocds200527/ISBN_9241546166.pdf (accessed on 8 August 2021).
- Nichols, G.; Lane, C.; Asgari, N.; Verlander, N.Q.; Charlett, A. Rainfall and outbreaks of drinking water related disease and in England and Wales. J. Water Health 2009, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Man, H.; van den Berg, H.H.; Leenen, E.J.; Schijven, J.F.; Schets, F.M.; van der Vliet, J.C.; van Knapen, F.; de RodaHusman, A.M. Quantitative assessment of infection risk from exposure to waterborne pathogens in urban floodwater. Water Res. 2014, 48, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.C.; Pijnacker, R.; de Man, H.; van de Kassteele, J.; van Pelt, W.; Mughini-Gras, L.; Franz, E. “Sickenin’ in the rain”—Increased risk of gastrointestinal and respiratory infections after urban pluvial flooding in a population-based cross-sectional study in the Netherlands. BMC Infect Dis. 2019, 19, 377. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.; O’Dwyer, J.; O’Neill, E.; Hynds, P. Surface water flooding, groundwater contamination, and enteric disease in developed countries: A scoping review of connections and consequences. Environ. Pollut. 2018, 236, 540–549. [Google Scholar] [CrossRef]
- Reacher, M.; McKenzie, K.; Lane, C.; Nichols, T.; Kedge, I.; Iversen, A.; Hepple, P.; Walter, T.; Laxton, C.; Simpson, J. Lewes Flood Action Recovery Team. Health impacts of flooding in Lewes: A comparison of reported gastrointestinal and other illness and mental health in flooded and non-flooded households. Commun. Dis. Public Health 2004, 7, 39–46. [Google Scholar]
- Carroll, B.; Balogh, R.; Morbey, H.; Araoz, G. Health and social impacts of a flood disaster: Responding to needs and implications for practice. Disasters 2010, 34, 1045–1063. [Google Scholar] [CrossRef]
- Schnitzler, J.; Benzler, J.; Altmann, D.; Mücke, I.; Krause, G. Survey on the population’s needs and the public health response during floods in Germany 2002. J. Public Health Manag. Pract. 2007, 13, 461–464. [Google Scholar] [CrossRef]
- Wójcik, O.P.; Holt, J.; Kjerulf, A.; Müller, L.; Ethelberg, S.; Mølbak, K. Personal protective equipment, hygiene behaviours and occupational risk of illness after July 2011 flood in Copenhagen, Denmark. Epidemiol. Infect. 2013, 141, 1756–1763. [Google Scholar] [CrossRef]
- De Man, H.; Mughini Gras, L.; Schimmer, B.; Friesema, I.H.; De Roda Husman, A.M.; van Pelt, W. Gastrointestinal, influenza-like illness and dermatological complaints following exposure to floodwater: A cross-sectional survey in The Netherlands. Epidemiol. Infect. 2016, 144, 1445–1454. [Google Scholar] [CrossRef]
- Harder-Lauridsen, N.M.; Kuhn, K.G.; Erichsen, A.C.; Mølbak, K.; Ethelberg, S. Gastrointestinal illness among triathletes swimming in non-polluted versus polluted seawater affected by heavy rainfall, Denmark, 2010-2011. PLoS ONE 2013, 8, e78371. [Google Scholar] [CrossRef]
- Mellou, K.; Katsioulis, A.; Potamiti-Komi, M.; Pournaras, S.; Kyritsi, M.; Katsiaflaka, A.; Kallimani, A.; Kokkinos, P.; Petinaki, E.; Sideroglou, T.; et al. A large waterborne gastroenteritis outbreak in central Greece, March 2012: Challenges for the investigation and management. Epidemiol. Infect. 2014, 142, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Tornevi, A.; Axelsson, G.; Forsberg, B. Association between precipitation upstream of a drinking water utility and nurse advice calls relating to acute gastrointestinal illnesses. PLoS ONE 2013, 8, e69918. [Google Scholar] [CrossRef] [PubMed]
- Guzman Herrador, B.; de Blasio, B.F.; Carlander, A.; Ethelberg, S.; Hygen, H.O.; Kuusi, M.; Lund, V.; Löfdahl, M.; MacDonald, E.; Martinez-Urtaza, J.; et al. Association between heavy precipitation events and waterborne outbreaks in four Nordic countries, 1992–2012. J. Water Health 2016, 14, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.V.; Girdwood, R.W.; Patterson, W.J.; Hardie, R.; Green, L.A.; Benton, C.; Tulloch, W.; Sharp, J.C.; Forbes, G.I. Waterborne outbreak of cryptosporidiosis. Lancet 1988, 2, 1484. [Google Scholar] [CrossRef]
- Joseph, C.; Hamilton, G.; O’Connor, M.; Nicholas, S.; Marshall, R.; Stanwell-Smith, R.; Sims, R.; Ndawula, E.; Casemore, D.; Gallagher, P.; et al. Cryptosporidiosis in the Isle of Thanet; an outbreak associated with local drinking water. Epidemiol. Infect. 1991, 107, 509–519. [Google Scholar] [CrossRef]
- Atherton, F.; Newman, C.P.; Casemore, D.P. An outbreak of waterborne cryptosporidiosis associated with a public water supply in the UK. Epidemiol. Infect. 1995, 115, 123–131. [Google Scholar] [CrossRef]
- Bridgman, S.A.; Robertson, R.M.; Syed, Q.; Speed, N.; Andrews, N.; Hunter, P.R. Outbreak of cryptosporidiosis associated with a disinfected groundwater supply. Epidemiol. Infect. 1995, 115, 555–566. [Google Scholar] [CrossRef]
- Howe, A.D.; Forster, S.; Morton, S.; Marshall, R.; Osborn, K.S.; Wright, P.; Hunter, P.R. Cryptosporidium oocysts in a water supply associated with a cryptosporidiosis outbreak. Emerg. Infect. Dis. 2002, 8, 619–624. [Google Scholar] [CrossRef]
- Hoek, M.R.; Oliver, I.; Barlow, M.; Heard, L.; Chalmers, R.; Paynter, S. Outbreak of Cryptosporidium parvum among children after a school excursion to an adventure farm, south west England. J. Water Health 2008, 6, 333–338. [Google Scholar] [CrossRef]
- Jennings, P.; Rhatigan, A. Cryptosporidiosis outbreak in Ireland linked to public water supply. Euro Surveill. 2002, 6, 2089. [Google Scholar] [CrossRef]
- Pelly, H.; Cormican, M.; O’Donovan, D.; Chalmers, R.M.; Hanahoe, B.; Cloughley, R.; McKeown, P.; Corbett-Feeney, G. A large outbreak of cryptosporidiosis in western Ireland linked to public water supply: A preliminary report. Euro Surveill. 2007, 12, E070503.3. [Google Scholar] [CrossRef] [PubMed]
- Boudou, M.; Cleary, E.; ÓhAiseadha, C.; Garvey, P.; O’Dwyer, J.; Hynds, P. 04-Impact of the 2015–2016 flood event on the incidence of acute gastrointestinal infection (AGI) in the Republic of Ireland—An epidemiological perspective on a hydrometeorogical problem. In Proceedings of the Irish National Hydrology Conference, Online, 17–18 November 2020. [Google Scholar]
- Boudou, M.; ‘OhAiseadha, C.; Garvey, P.; O’Dwyer, J.; Hynds, P. Flood hydrometeorology and gastroenteric infection: The Winter 2015–2016 flood event in the Republic of Ireland. J. Hydrol. 2021, 599, 126376. [Google Scholar] [CrossRef]
- Gertler, M.; Dürr, M.; Renner, P.; Poppert, S.; Askar, M.; Breidenbach, J.; Frank, C.; Preußel, K.; Schielke, A.; Werber, D.; et al. Outbreak of Cryptosporidium hominis following river flooding in the city of Halle (Saale), Germany, August 2013. BMC Infect. Dis. 2015, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Ryan, U. Cryptosporidium—An update with an emphasis on foodborne and waterborne transmission. Res. Vet. Sci. 2020, 132, 500–512. [Google Scholar] [CrossRef]
- Smith, H.V.; Patterson, W.J.; Hardie, R.; Greene, L.A.; Benton, C.; Tulloch, W.; Gilmour, R.A.; Girdwood, R.W.; Sharp, J.C.; Forbes, G.I. An outbreak of waterborne cryptosporidiosis caused by post-treatment contamination. Epidemiol. Infect. 1989, 103, 703–715. [Google Scholar] [CrossRef]
- Smith, H.V.; Rose, J.B. Waterborne cryptosporidiosis. Parasitol. Today 1990, 6, 8–12. [Google Scholar] [CrossRef]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef]
- Giammanco, G.M.; Di Bartolo, I.; Purpari, G.; Costantino, C.; Rotolo, V.; Spoto, V.; Geraci, G.; Bosco, G.; Petralia, A.; Guercio, A.; et al. Investigation and control of a Norovirus outbreak of probable waterborne transmission through a municipal groundwater system. J. Water Health 2014, 12, 452–464. [Google Scholar] [CrossRef]
- Liang, S.Y.; Messenger, N. Infectious Diseases After Hydrologic Disasters. Emerg. Med. Clin. N. Am. 2018, 36, 835–851. [Google Scholar] [CrossRef]
- Kukkula, M.; Arstila, P.; Klossner, M.L.; Maunula, L.; Bonsdorff, C.H.; Jaatinen, P. Waterborne outbreak of viral gastroenteritis. Scand. J. Infect. Dis. 1997, 29, 415–418. [Google Scholar] [CrossRef]
- Miettinen, I.T.; Zacheus, O.; von Bonsdorff, C.H.; Vartiainen, T. Waterborne epidemics in Finland in 1998–1999. Water Sci. Technol. 2001, 43, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.; Barataud, D.; Gallay, A.; Thiolet, J.M.; Le Guyaguer, S.; Kohli, E.; Vaillant, V. Norovirus foodborne outbreaks associated with the consumption of oysters from the Etang de Thau, France, December 2002. Euro Surveill. 2004, 9, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Beaudeau, P.; de Valk, H.; Vaillant, V.; Mannschott, C.; Tillier, C.; Mouly, D.; Ledrans, M. Lessons learned from ten investigations of waterborne gastroenteritis outbreaks, France, 1998–2006. J. Water Health 2008, 6, 491–503. [Google Scholar] [CrossRef]
- Papadopoulos, V.P.; Vlachos, O.; Isidoriou, E.; Kasmeridis, C.; Pappa, Z.; Goutzouvelidis, A.; Filippou, F. A gastroenteritis outbreak due to Norovirus infection in Xanthi, Northern Greece: Management and public health consequences. J. Gastrointestin. Liver Dis. 2006, 15, 27–30. [Google Scholar] [PubMed]
- Vantarakis, A.; Mellou, K.; Spala, G.; Kokkinos, P.; Alamanos, Y. A gastroenteritis outbreak caused by noroviruses in Greece. Int. J. Environ. Res. Public Health 2011, 8, 3468–3478. [Google Scholar] [CrossRef]
- Schmid, D.; Lederer, I.; Much, P.; Pichler, A.M.; Allerberger, F. Outbreak of norovirus infection associated with contaminated flood water, Salzburg, 2005. Euro Surveill. 2005, 10, E050616.3. [Google Scholar] [CrossRef] [PubMed]
- Riera-Montes, M.; BrusSjölander, K.; Allestam, G.; Hallin, E.; Hedlund, K.O.; Löfdahl, M. Waterborne norovirus outbreak in a municipal drinking-water supply in Sweden. Epidemiol. Infect. 2011, 139, 1928–1935. [Google Scholar] [CrossRef]
- Joosten, R.; Sonder, G.; Parkkali, S.; Brandwagt, D.; Fanoy, E.; Mughini-Gras, L.; Lodder, W.; Ruland, E.; Siedenburg, E.; Kliffen, S.; et al. Risk factors for gastroenteritis associated with canal swimming in two cities in the Netherlands during the summer of 2015: A prospective study. PLoS ONE 2017, 12, e0174732. [Google Scholar] [CrossRef]
- Dura, G.; Pándics, T.; Kádár, M.; Krisztalovics, K.; Kiss, Z.; Bodnár, J.; Asztalos, A.; Papp, E. Environmental health aspects of drinking water-borne outbreak due to karst flooding: Case study. J. Water Health 2010, 8, 513–520. [Google Scholar] [CrossRef]
- Le Guyader, F.S.; Le Saux, J.C.; Ambert-Balay, K.; Krol, J.; Serais, O.; Parnaudeau, S.; Giraudon, H.; Delmas, G.; Pommepuy, M.; Pothier, P.; et al. Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J. Clin. Microbiol. 2008, 46, 4011–4017. [Google Scholar] [CrossRef]
- Gullón, P.; Varela, C.; Martínez, E.V.; Gómez-Barroso, D. Association between meteorological factors and hepatitis A in Spain 2010–2014. Environ. Int. 2017, 102, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Marcheggiani, S.; Puccinelli, C.; Ciadamidaro, S.; Della Bella, V.; Carere, M.; Francesca Blasi, M.; Funari, E.; Mancini, L. Risks of water-borne disease outbreaks after extreme events. Toxicol. Environ. Chem. 2010, 92, 593–599. [Google Scholar] [CrossRef]
- Howie, H.; Mukerjee, A.; Cowden, J.; Leith, J.; Reid, T. Investigation of an outbreak of Escherichia coli O157 infection caused by environmental exposure at a scout camp. Epidemiol. Infect. 2003, 131, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Ihekweazu, C.; Barlow, M.; Roberts, S.; Christensen, H.; Guttridge, B.; Lewis, D.; Painter, S. Outbreak of E. coli O157 infection in the south west of the UK: Risks from streams crossing seaside beaches. Euro Surveill. 2006, 11, 5–6. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.B.; Garvey, P.; O’Riordan, M.; Coughlan, H.; McKeown, P.; Brennan, A.; McNamara, E. Increase in VTEC cases in the south of Ireland: Link to private wells? Euro Surveill. 2008, 13, 18991. [Google Scholar] [CrossRef]
- Hänninen, M.L.; Haajanen, H.; Pummi, T.; Wermundsen, K.; Katila, M.L.; Sarkkinen, H.; Miettinen, I.; Rautelin, H. Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl. Environ. Microbiol. 2003, 69, 1391–1396. [Google Scholar] [CrossRef]
- Kuhn, K.G.; Falkenhorst, G.; Emborg, H.D.; Ceper, T.; Torpdahl, M.; Krogfelt, K.A.; Ethelberg, S.; Mølbak, K. Epidemiological and serological investigation of a waterborne Campylobacter jejuni outbreak in a Danish town. Epidemiol. Infect. 2017, 145, 701–709. [Google Scholar] [CrossRef]
- Richardson, G.; Thomas, D.R.; Smith, R.M.; Nehaul, L.; Ribeiro, C.D.; Brown, A.G.; Salmon, R.L. A community outbreak of Campylobacter jejuni infection from a chlorinated public water supply. Epidemiol. Infect. 2007, 135, 1151–1158. [Google Scholar] [CrossRef]
- Arias, C.; Sala, M.R.; Domínguez, A.; Bartolomé, R.; Benavente, A.; Veciana, P.; Pedrol, A.; Hoyo, G. Outbreak Working Group. Waterborne epidemic outbreak of Shigellasonnei gastroenteritis in Santa Maria de Palautordera, Catalonia, Spain. Epidemiol. Infect. 2006, 134, 598–604. [Google Scholar] [CrossRef]
- Amézquita-López, B.A.; Soto-Beltrán, M.; Lee, B.G.; Yambao, J.C.; Quiñones, B. Isolation, genotyping and antimicrobial resistance of Shiga toxin-producing Escherichia coli. J. Microbiol. Immunol. Infect. 2018, 51, 425–434. [Google Scholar] [CrossRef]
- Kotloff, K.L. Bacterial diarrhoea. Curr. Opin. Pediatr. 2022, 34, 147–155. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, J.; Morris Downes, M.; Adley, C.C. The impact of meteorology on the occurrence of waterborne outbreaks of verocytotoxin-producing Escherichia coli (VTEC): A logistic regression approach. J. Water Health 2016, 14, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.H.; Guth, B.E.; Piazza, R.M.; Leão, S.C.; Ludovico, A.; Ludovico, M.S.; Dahbi, G.; Marzoa, J.; Mora, A.; Blanco, J.; et al. Diversity of Shiga toxin-producing Escherichia coli in sheep flocks of Paraná State, southern Brazil. Vet. Microbiol. 2015, 175, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, R.A.; Maas, J.A.; Blain, A.P.; Gorton, R.; Ward, J.; O’Brien, S.J.; Hunter, P.R.; Rushton, S.P. Spatio-temporal models to determine association between Campylobacter cases and environment. Int. J. Epidemiol. 2018, 47, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Ahern, M.; Kovats, R.S.; Wilkinson, P.; Few, R.; Matthies, F. Global health impacts of floods: Epidemiologic evidence. Epidemiol. Rev. 2005, 27, 36–46. [Google Scholar] [CrossRef]
- Auld, H.E.; MacIver, D.; Klassen, J. Heavy rainfall and waterborne disease outbreaks: The Walkerton example. J. Toxicol. Environ. Health A 2004, 67, 1879–1887. [Google Scholar] [CrossRef]
- Gaynor, K.; Katz, A.R.; Park, S.Y.; Nakata, M.; Clark, T.A.; Effler, P.V. Leptospirosis on Oahu: An outbreak associated with flooding of a university campus. Am. J. Trop. Med. Hyg. 2007, 76, 882–885. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Wang, Y.-C.; Yang, Y.-S.; Chang, F.-Y. Leptospirosis after Typhoon in Taiwan. J. Med. Sci. 2009, 29, 131–134. [Google Scholar]
- Su, H.P.; Chan, T.C.; Chang, C.C. Typhoon-related leptospirosis and melioidosis, Taiwan, 2009. Emerg. Infect. Dis. 2011, 17, 1322–1324. [Google Scholar] [CrossRef]
- Smith, J.K.G.; Young, M.M.; Wilson, K.L.; Craig, S.B. Leptospirosis following a major flood in Central Queensland, Australia. Epidemiol. Infect. 2013, 141, 585–590. [Google Scholar] [CrossRef]
- Dechet, A.M.; Parsons, M.; Rambaran, M.; Mohamed-Rambaran, P.; Florendo-Cumbermack, A.; Persaud, S.; Baboolal, S.; Ari, M.D.; Shadomy, S.V.; Zaki, S.R.; et al. Leptospirosis outbreak following severe flooding: A rapid assessment and mass prophylaxis campaign; Guyana, January-February 2005. PLoS ONE 2012, 7, e39672. [Google Scholar] [CrossRef] [PubMed]
- Bandino, J.P.; Hang, A.; Norton, S.A. The Infectious and Noninfectious Dermatological Consequences of Flooding: A Field Manual for the Responding Provider. Am. J. Clin. Dermatol. 2015, 16, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Naing, C.; Reid, S.A.; Aye, S.N.; Htet, N.H.; Ambu, S. Risk factors for human leptospirosis following flooding: A meta-analysis of observational studies. PLoS ONE 2019, 14, e0217643. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R. Climate change and waterborne and vector-borne disease. J. Appl. Microbiol. 2003, 94, S37–S46. [Google Scholar] [CrossRef]
- Christova, I.; Human, T.E. Human leptospirosis in Bulgaria, 2010–2014. Probl. Infect. Parasit. Dis. 2016, 44, 23–29. [Google Scholar]
- Zitek, K.; Benes, C. Dlouhodobáepidemiologieleptospirózy (1963–2003) v CeskéRepublice [Longitudinal epidemiology of leptospirosis in the Czech Republic (1963–2003)]. Epidemiol. Mikrobiol. Imunol. 2005, 54, 21–26. [Google Scholar]
- Pellizzer, P.; Todescato, A.; Benedetti, P.; Colussi, P.; Conz, P. Leptospirosis following a flood in the Veneto area, north-east Italy. Ann. Ig. Med. Prev. Comunita 2006, 18, 453–456. [Google Scholar]
- Vitale, M.; Agnello, S.; Chetta, M.; Amato, B.; Vitale, G.; Bella, C.D.; Vicari, D.; Presti, V.D.M.L. Human leptospirosis cases in Palermo Italy. The role of rodents and climate. J. Infect. Public Health 2018, 11, 209–214. [Google Scholar] [CrossRef]
- Desai, S.; van Treeck, U.; Lierz, M.; Espelage, W.; Zota, L.; Sarbu, A.; Czerwinski, M.; Sadkowska-Todys, M.; Avdicová, M.; Reetz, J.; et al. Resurgence of field fever in a temperate country: An epidemic of leptospirosis among seasonal strawberry harvesters in Germany in 2007. Clin. Infect. Dis. 2009, 48, 691–697. [Google Scholar] [CrossRef]
- Brockmann, S.; Piechotowski, I.; Bock-Hensley, O.; Winter, C.; Oehme, R.; Zimmermann, S.; Hartelt, K.; Luge, E.; Nöckler, K.; Schneider, T.; et al. Outbreak of leptospirosis among triathlon participants in Germany, 2006. BMC Infect. Dis. 2010, 10, 91. [Google Scholar] [CrossRef]
- Radl, C.; Müller, M.; Revilla-Fernandez, S.; Karner-Zuser, S.; de Martin, A.; Schauer, U.; Karner, F.; Stanek, G.; Balcke, P.; Hallas, A.; et al. Outbreak of leptospirosis among triathlon participants in Langau, Austria, 2010. Wien Klin. Wochenschr. 2011, 123, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Angelakis, E.; Renvoisé, A.; Fournier, P.E.; Marié, J.L.; Davoust, B.; Stein, A.; Raoult, D. Strikes, flooding, rats, and leptospirosis in Marseille, France. Int. J. Infect. Dis. 2011, 15, e710–e715. [Google Scholar] [CrossRef] [PubMed]
- Van Alphen, L.B.; LemckeKunoe, A.; Ceper, T.; Kahler, J.; Kjelso, C.; Ethelberg, S.; Krogfelt, K.A. Trends in human leptospirosis in Denmark, 1980 to 2012. Euro Surveill. 2015, 20, 21019. [Google Scholar] [CrossRef] [PubMed]
- EPI-NEWS. No 5/12. Available online: https://en.ssi.dk/news/epi-news/2012/no-5---2012 (accessed on 10 January 2022).
- EPI-NEWS. No 34b/11. Available online: https://en.ssi.dk/news/epi-news/2011/no-34b---2011 (accessed on 10 January 2022).
- Walker, M.D. Leptospirosis: The possible risk to those participating in water-based sports and activities. Br. J. Gen. Pract. 2018, 68, 394–395. [Google Scholar] [CrossRef]
- Abdul Mutalip, M.H.; Mahmud, M.A.F.; Lodz, N.A.; Yoep, N.; Muhammad, E.N.; Ahmad, A.; Hashim, M.H.; Muhamad, N.A. Environmental risk factors of leptospirosis in urban settings: A systematic review protocol. BMJ Open 2019, 9, e023359. [Google Scholar] [CrossRef]
- Dupouey, J.; Faucher, B.; Edouard, S.; Richet, H.; Kodjo, A.; Drancourt, M.; Davoust, B. Human leptospirosis: An emerging risk in Europe? Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 77–83. [Google Scholar] [CrossRef]
- Mir, S. Hantavirus Induced Kidney Disease. Front. Med. 2022, 8, 795340. [Google Scholar] [CrossRef]
- Vaheri, A.; Henttonen, H.; Voutilainen, L.; Mustonen, J.; Sironen, T.; Vapalahti, O. Hantavirus infections in Europe and their impact on public health. Rev. Med. Virol. 2013, 23, 35–49. [Google Scholar] [CrossRef]
- Stadtherr, L.; Coumou, D.; Petoukhov, V.; Petri, S.; Rahmstorf, S. Record Balkan floods of 2014 linked to planetary wave resonance. Sci. Adv. 2016, 15, e1501428. [Google Scholar] [CrossRef]
- Štrbac, M.; Vuković, V.; Patić, A.; Medić, S.; Pustahija, T.; Petrović, V.; Lendak, D.; Ličina, M.K.; Bakić, M.; Protić, J.; et al. Epidemiological study on the incidence of haemorrhagic fever with renal syndrome in five Western Balkan countries for a 10-year period: 2006–2015. Zoonoses Public Health 2022, 69, 195–206. [Google Scholar] [CrossRef]
- Zeimes, C.B.; Quoilin, S.; Henttonen, H.; Lyytikäinen, O.; Vapalahti, O.; Reynes, J.M.; Reusken, C.; Swart, A.N.; Vainio, K.; Hjertqvist, M.; et al. Landscape and regional environmental analysis of the spatial distribution of hantavirus human cases in europe. Front. Public Health 2015, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Coalson, J.E.; Anderson, E.J.; Santos, E.M.; Madera Garcia, V.; Romine, J.K.; Dominguez, B.; Richard, D.M.; Little, A.C.; Hayden, M.H.; Ernst, K.C. The Complex Epidemiological Relationship between Flooding Events and Human Outbreaks of Mosquito-Borne Diseases: A Scoping Review. Environ. Health Perspect. 2021, 129, 96002, Erratum in Environ. Health Perspect. 2021, 129, 129001. [Google Scholar] [CrossRef] [PubMed]
- Scheuch, D.E.; Schäfer, M.; Eiden, M.; Heym, E.C.; Ziegler, U.; Walther, D.; Schmidt-Chanasit, J.; Keller, M.; Groschup, M.H.; Kampen, H. Detection of Usutu, Sindbis, and Batai Viruses in Mosquitoes (Diptera: Culicidae) Collected in Germany, 2011–2016. Viruses 2018, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Scheday, S.; Boenecke, J.; Gogoi, A.; Maharaj, A.; Korovou, S. Climate Change, Health and Mosquito-Borne Diseases: Trends and Implications to the Pacific Region. Int. J. Environ. Res. Public Health. 2019, 16, 5114. [Google Scholar] [CrossRef] [PubMed]
- Han, L.L.; Popovici, F.; Alexander, J.P., Jr.; Laurentia, V.; Tengelsen, L.A.; Cernescu, C.; Gary, H.E., Jr.; Ion-Nedelcu, N.; Campbell, G.L.; Tsai, T.F. Risk factors for West Nile virus infection and meningoencephalitis, Romania, 1996. J. Infect. Dis. 1999, 179, 230–233. [Google Scholar] [CrossRef]
- Hubálek, Z.; Savage, H.M.; Halouzka, J.; Juricová, Z.; Sanogo, Y.O.; Lusk, S. West Nile virus investigations in South Moravia, Czechland. Viral Immunol. 2000, 13, 427–433. [Google Scholar] [CrossRef]
- Hubálek, Z.; Zeman, P.; Halouzka, J.; Juricová, Z.; Stovícková, E.; Bálková, H.; Sikutová, S.; Rudolf, I. Protilátky k virůmprenosnýmkomáry u stredoceské populace z oblastizasazenépovodní v roce 2002 [Antibodies against mosquito-born viruses in human population of an area of Central Bohemia affected by the flood of 2002]. Epidemiol. Mikrobiol. Imunol. 2004, 53, 112–120. [Google Scholar]
- Hubálek, Z.; Zeman, P.; Halouzka, J.; Juricová, Z.; Stovicková, E.; Bálková, H.; Sikutová, S.; Rudolf, I. Mosquitoborne viruses, Czech Republic, 2002. Emerg. Infect. Dis. 2005, 11, 116–118. [Google Scholar] [CrossRef]
- Danis, K.; Papa, A.; Theocharopoulos, G.; Dougas, G.; Athanasiou, M.; Detsis, M.; Baka, A.; Lytras, T.; Mellou, K.; Bonovas, S.; et al. Outbreak of West Nile virus infection in Greece, 2010. Emerg. Infect. Dis. 2011, 17, 1868–1872. [Google Scholar] [CrossRef]
- Roiz, D.; Boussès, P.; Simard, F.; Paupy, C.; Fontenille, D. Autochthonous Chikungunya Transmission and Extreme Climate Events in Southern France. PLoS Negl. Trop. Dis. 2015, 9, e0003854. [Google Scholar] [CrossRef]
- Moirano, G.; Gasparrini, A.; Acquaotta, F.; Fratianni, S.; Merletti, F.; Maule, M.; Richiardi, L. West Nile Virus infection in Northern Italy: Case-crossover study on the short-term effect of climatic parameters. Environ. Res. 2018, 167, 544–549. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Control and Prevention (ECDC). West Nile Virus Transmission in Europe. Available online: http://ecdc.europa.eu/en/activities/sciadvice/Lists/ECDC%20Reviews/ECDC_DispForm.aspx?List=512ff74f-77d4-4ad8-b6d6-bf0f23083f30&ID=940&RootFolder=%2Fen%2Factivities%2Fsciadvice%2FLists%2FECDC%20Reviews (accessed on 10 April 2022).
- Kouadio, I.K.; Aljunid, S.; Kamigaki, T.; Hammad, K.; Oshitani, H. Infectious diseases following natural disasters: Prevention and control measures. Expert Rev. Anti-Infect. Ther. 2012, 10, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Mor, S.M.; Griffiths, J.K. Water-Related Diseases in the Developing World. In Encyclopedia of Environmental Health, 1st ed.; Jerome, O.N., Ed.; Elsevier Science: Boston, MA, USA, 2011; pp. 741–753. [Google Scholar] [CrossRef]
- Jafari, N.; Shahsanai, A.; Memarzadeh, M.; Loghmani, A. Prevention of communicable diseases after disaster: A review. J. Res. Med. Sci. 2011, 16, 956–962. [Google Scholar] [PubMed]
- Mavrouli, M.; Mavroulis, S.; Lekkas, E.; Tsakris, A. Potential infectious diseases following floods induced by extreme precipitation events. In Proceedings of the 27th ECCMID, Vienna, Austria, 22–25 April 2017. [Google Scholar]
- Mavroulis, S.; Mavrouli, M.; Lekkas, E.; Tsakris, A. Impact of floods induced by extreme precipitation events on public health. Geophys. Res. Abstr. 2017, 19, EGU2017-3886. [Google Scholar]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef]
- Haake, D.A.; Dundoo, M.; Cader, R.; Kubak, B.M.; Hartskeerl, R.A.; Sejvar, J.J.; Ashford, D.A. Leptospirosis, water sports, and chemoprophylaxis. Clin. Infect. Dis. 2002, 34, e40–e43. [Google Scholar] [CrossRef]
- Schneider, M.C.; Velasco-Hernandez, J.; Min, K.D.; Leonel, D.G.; Baca-Carrasco, D.; Gompper, M.E.; Hartskeerl, R.; Munoz-Zanzi., C. The Use of Chemoprophylaxis after Floods to Reduce the Occurrence and Impact of Leptospirosis Outbreaks. Int. J. Environ. Res. Public Health 2017, 14, 594. [Google Scholar] [CrossRef]
- Gilks, C.F.; Lambert, H.P.; Broughton, E.S.; Baker, C.C. Failure ofpenicillin prophylaxis in laboratory acquired leptospirosis. Postgrad. Med. J. 1988, 64, 236–238. [Google Scholar] [CrossRef]
- Glynn, K.; Hartskeel, R.; Ko, A.; Meslin, F. Leptospirosis. In Control of Communicable Diseases Manual, 19th ed.; Heymann, D.L., Ed.; American Public Health Association: Washington, DC, USA, 2008; pp. 351–364. [Google Scholar]
- Linsak, D.T.; Kresic, K.; Coklo, M.; Majanaric, K.; Susnic, V.; Lakoseljac, D.; Linsak, Z. The Impact of the Natural Hazard Flooding in East Part of Croatia—Reducing Possible Consequences. JoRSG 2016, 5, 1000153. [Google Scholar] [CrossRef]
- Yavarian, J.; Shafiei-Jandaghi, N.Z.; Mokhtari-Azad, T. Possible viral infections in flood disasters: A review considering 2019 spring floods in Iran. Iran. J. Microbiol. 2019, 11, 85–89. [Google Scholar] [CrossRef]
- Foltz, S.; Braur, B. Communication, data sharing, and collaboration, at the disaster site. In Proceedings of the International Conference on Computing in Civil Engineering, Cancun, Mexico, 12–15 July 2005; pp. 1–8. [Google Scholar] [CrossRef]
- Babaie, J.; Ardalan, A.; Vatandoost, H.; Goya, M.M.; Akbarisari, A. Performance assessment of communicable disease surveillance in disasters: A systematic review. PLoS Curr. 2015, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johns Hopkins, Red Cross Red Crescent. Public Health Guide in EMERGENCIES. 2008. Available online: http://www.jhsph.edu/research/centers-and-institutes/center-for-refugee-and-disaster-response/publications_tools/publications/_CRDR_ICRC_Public_Health_Guide_Book/Forward.pdf (accessed on 10 April 2022).
- Mavrouli, M.; Mavroulis, S.; Lekkas, E.; Tsakris, A. Respiratory Infections Following Earthquake-Induced Tsunamis: Transmission Risk Factors and Lessons Learned for Disaster Risk Management. Int. J. Environ. Res. Public Health 2021, 18, 4952. [Google Scholar] [CrossRef] [PubMed]
- ECDC Rapid Risk Assessment—Floods in Bosnia and Herzegovina, Croatia, and Serbia: Communicable Disease Risks 18 June 2014; The European Centre for Disease Prevention and Control: Stockholm, Sweden, 2014; Available online: https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/floods-bosnia-croatia-serbia-rapid-risk-assessment-june-2014.pdf (accessed on 10 April 2022).
- Bhardwaj, P.; Kosambiya, J.K.; Desai, V.K. A case control study to explore the risk factors for acquisition of leptospirosis in Surat city, after flood. Indian, J. Med. Sci. 2008, 62, 431–438. [Google Scholar] [CrossRef]
- Salimović-Bešić, I.; Šeremet, M.; Hübschen, J.M.; Hukić, M.; Tihić, N.; Ahmetagić, S.; Delibegović, Z.; Pilav, A.; Mulaomerović, M.; Ravlija, J.; et al. Epidemiologic and laboratory surveillance of the measles outbreak in the Federation of Bosnia and Herzegovina, February 2014–April 2015. Clin. Microbiol. Infect. 2016, 22, 563.e1–563.e7. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Extreme Rainfall and Catastrophic Floods in Western Europe—29 July 2021; ECDC: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/RRA-20210720-1799.pdf (accessed on 10 April 2022).
- Desai, A.N.; Ramatowski, J.W.; Marano, N.; Madoff, L.C.; Lassmann, B. Infectious disease outbreaks among forcibly displaced persons: An analysis of ProMED reports 1996–2016. Confl. Health 2020, 14, 1–10. [Google Scholar] [CrossRef]
- Ejemot-Nwadiaro, R.I.; Ehiri, J.E.; Meremikwu, M.M.; Critchley, J.A. Hand washing for preventing diarrhea. Cochrane Database Syst. Rev. 2015, 9. [Google Scholar] [CrossRef]
- Pérez-Martín, J.J.; Romera Guirado, F.J.; Molina-Salas, Y.; Bernal-González, P.J.; Navarro-Alonso, J.A. Vaccination campaign at a temporary camp for victims of the earthquake in Lorca (Spain). Hum. Vaccin. Immunother. 2017, 13, 1714–1721. [Google Scholar] [CrossRef]
- Suk, J.E.; Vaughan, E.C.; Cook, R.G.; Semenza, J.C. Natural disasters and infectious disease in Europe: A literature review to identify cascading risk pathways. Eur. J. Public Health. 2020, 30, 928–935. [Google Scholar] [CrossRef]
- Hajat, S.; Ebi, K.L.; Kovats, R.S.; Menne, B.; Edwards, S.; Haines, A. The Human Health Consequences of Flooding in Europe: A Review. In Extreme Weather Events and Public Health Responses; Kirch, W., Bertollini, R., Menne, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Peitl, V.; Zatezalo, V.G.; Karlović, D. Mental Health Issues and Psychological Crisis Interventions During the COVID-19 Pandemic and Earthquakes in Croatia. Arch. Psychiatr. Res. 2020, 56, 193–198. [Google Scholar] [CrossRef]
- Čivljak, R.; Markotić, A.; Capak, K. Earthquake in the time of COVID-19: The story from Croatia (CroVID-20). J. Glob. Health 2020, 10, 010349. [Google Scholar] [CrossRef]
- Silva, V.; Paul, N. Potential impact of earthquakes during the 2020 COVID-19 pandemic. Earthq. Spectra 2021, 37, 73–94. [Google Scholar] [CrossRef]
- Ishiwatari, M.; Koike, T.; Hiroki, K.; Toda, T.; Katsube, T. Managing disasters amid COVID-19 pandemic: Approaches of response to flood disasters. Prog. Disaster Sci. 2020, 6, 100096. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Sasaki, D.; Ono, Y.; Makino, Y.; Kodama, E.N. Implementation of evacuation measures during natural disasters under conditions of the novel coronavirus (COVID-19) pandemic based on a review of previous re-sponses to complex disasters in Japan. Prog. Disaster Sci. 2020, 8, 100127. [Google Scholar] [CrossRef] [PubMed]
- Mavroulis, S.; Mavrouli, M.; Lekkas, E. Geological and hydrometeorological hazards and related disasters amid COVID-19 pandemic in Greece: Post-disaster trends and factors affecting the COVID-19 evolution in affected areas. Saf. Sci. 2021, 138, 105236. [Google Scholar] [CrossRef] [PubMed]
- Simonovic, S.P.; Kundzewicz, Z.W.; Wright, N. (2020). Floods and the COVID-19 pandemic—A new double hazard problem. WIREs Water 2021, 8, e1509. [Google Scholar] [CrossRef]
- Mavroulis, S.; Ilgac, M.; Tunçağ, M.; Lekkas, E.; Püskülcü, S.; Kourou, A.; Sextos, A.; Mavrouli, M.; Can, G.; Thoma, T.; et al. Emergency response, intervention, and societal recovery in Greece and Turkey after the 30th October 2020, MW = 7.0, Samos (Aegean Sea) earthquake. Bull. Earthq. Eng. 2022. [Google Scholar] [CrossRef]
- Fatemi, F.; Moslehi, S. Responding simultaneously to flood and COVID-19 in Iran. Disaster Med. Public Health Prep. 2021. [Google Scholar] [CrossRef]
- Knutti, R.; Sedláček, J. Robustness and uncertainties in the new CMIP5 climate model projections. Nat. Clim. Chang. 2013, 3, 369–373. [Google Scholar] [CrossRef]
- International Panel on Climate Change. Climate Change 2013. The Physical Science Basis. Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Summary for Policymakers, Technical Summary and Frequently Asked Questions; World Meteorological Organization and United Nations Environment Programme: Geneva, Switzerland, 2013; ISBN 978-92-9169-138-8. [Google Scholar]
- Rojas, R.; Feyen, L.; Bianchi, A.; Dosio, A. Assessment of future flood hazard in Europe using a large ensemble of bias-corrected regional climate simulations. J. Geophys. Res. Atmos. 2012, 117, D17109. [Google Scholar] [CrossRef]
- Prein, A.F.; Langhans, W.; Fosser, G.; Ferrone, A.; Ban, N.; Goergen, K.; Keller, M.; Tölle, M.; Gutjahr, O.; Feser, F.; et al. A review on regional convecGon-permiKng climate modeling: DemonstraGons, prospects, and challenges. Rev. Geophys. 2015, 53, 323–361. [Google Scholar] [CrossRef]
- Serdeczny, O.; Waters, E.; Chan, S. Non-Economic Loss and Damage in the Context of Climate Change. Understanding the Challenges; Discussion Paper; Deutsches Institut für Entwicklungspolitik: Bonn, Germany, 2016; p. 38. [Google Scholar]
- Hoeppe, P. Trends in weather related disasters–Consequences for insurers and society. Weather Clim. Extrem. 2015, 11, 70–79. [Google Scholar] [CrossRef]
- Arnell, N.W.; Gosling, S.N. The impacts of climate change on river flood risk at the global scale. Clim. Chang. 2016, 134, 387–401. [Google Scholar] [CrossRef]
- Alfieri, L.; Burek, P.; Feyen, L.; Forzieri, G. Global warming increases the frequency of river floods in Europe. Hydrol. Earth Syst. Sci. 2015, 19, 2247–2260. [Google Scholar] [CrossRef]
- Tabari, H. Climate change impact on flood and extreme precipitation increases with water availability. Sci. Rep. 2020, 10, 13768. [Google Scholar] [CrossRef] [PubMed]
- Blenkinsop, S.; Lincoln Muniz Alves, L.M.; Smith, A.J.P. Climate Change Increases Extreme Rainfall and the Chance of Floods. ScienceBrief. Available online: https://zenodo.org/record/4779119 (accessed on 10 May 2022).
- Meresa, H.; Tischbein, B.; Mekonnen, T. Climate change impact on extreme precipitation and peak flood magnitude and frequency: Observations from CMIP6 and hydrological models. Nat. Hazards 2022, 111, 2649–2679. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrouli, M.; Mavroulis, S.; Lekkas, E.; Tsakris, A. Infectious Diseases Associated with Hydrometeorological Hazards in Europe: Disaster Risk Reduction in the Context of the Climate Crisis and the Ongoing COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 10206. https://doi.org/10.3390/ijerph191610206
Mavrouli M, Mavroulis S, Lekkas E, Tsakris A. Infectious Diseases Associated with Hydrometeorological Hazards in Europe: Disaster Risk Reduction in the Context of the Climate Crisis and the Ongoing COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2022; 19(16):10206. https://doi.org/10.3390/ijerph191610206
Chicago/Turabian StyleMavrouli, Maria, Spyridon Mavroulis, Efthymios Lekkas, and Athanassios Tsakris. 2022. "Infectious Diseases Associated with Hydrometeorological Hazards in Europe: Disaster Risk Reduction in the Context of the Climate Crisis and the Ongoing COVID-19 Pandemic" International Journal of Environmental Research and Public Health 19, no. 16: 10206. https://doi.org/10.3390/ijerph191610206
APA StyleMavrouli, M., Mavroulis, S., Lekkas, E., & Tsakris, A. (2022). Infectious Diseases Associated with Hydrometeorological Hazards in Europe: Disaster Risk Reduction in the Context of the Climate Crisis and the Ongoing COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 19(16), 10206. https://doi.org/10.3390/ijerph191610206






