A Randomized Clinical Trial to Assess the Efficacy of a Psychological Treatment to Quit Smoking Assisted with an App: Study Protocol
Abstract
:1. Introduction
2. Materials and Methods
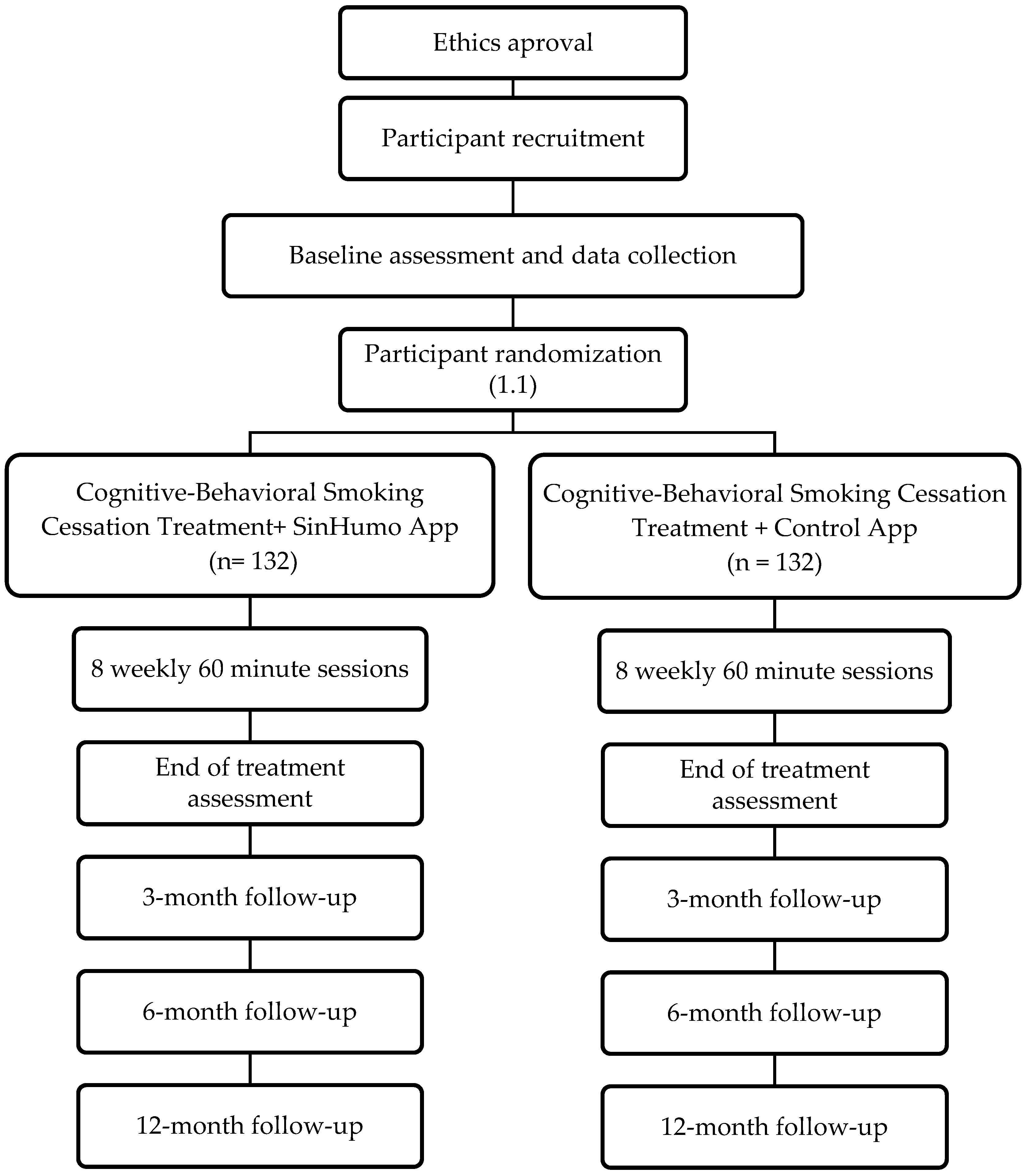
2.1. Research Design
2.2. Recruitment
2.3. Participants
2.4. Treatment Interventions
2.4.1. Cognitive Behavioural Smoking Cessation Intervention and SinHumo App (Experimental Condition)
- -
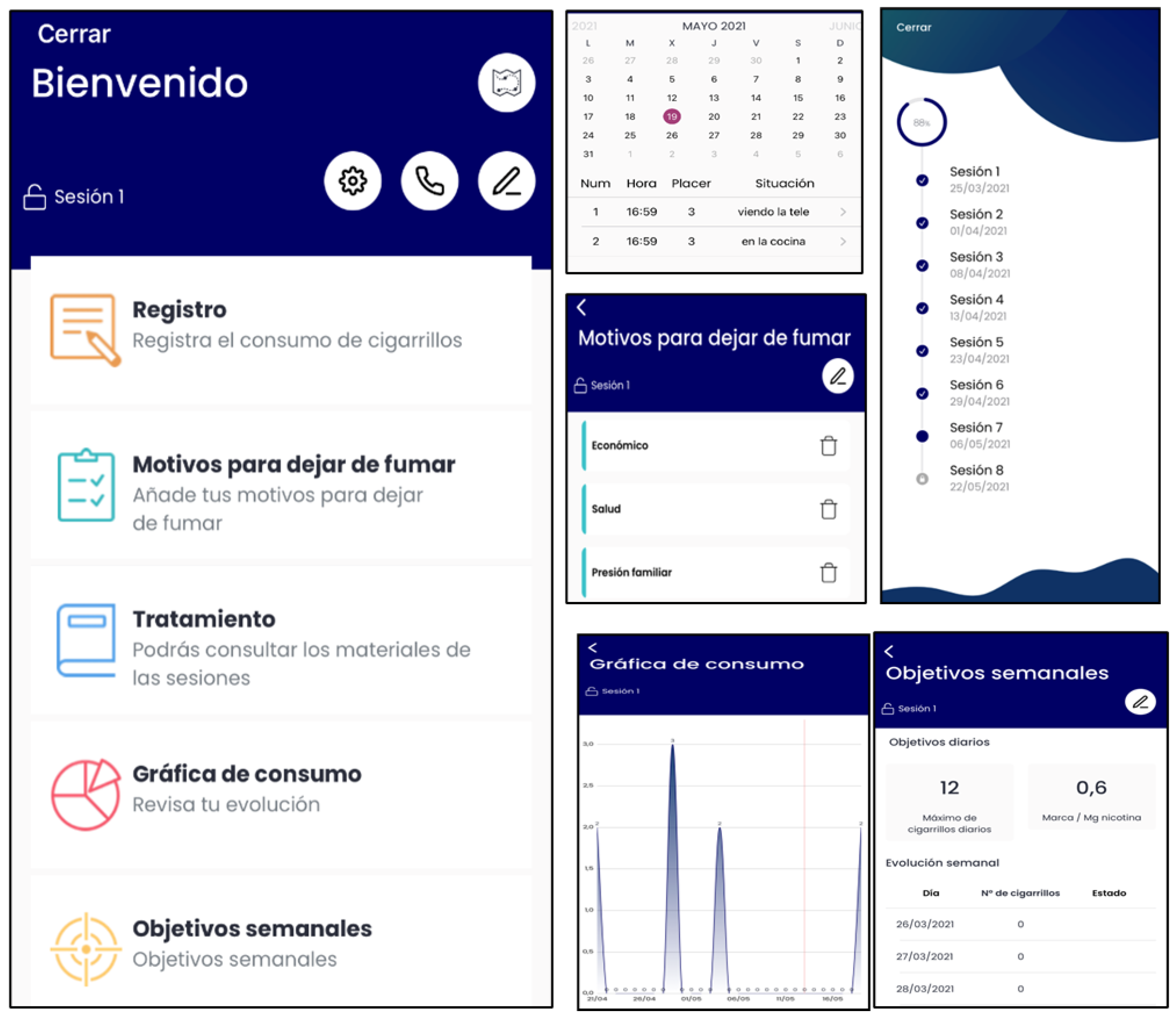
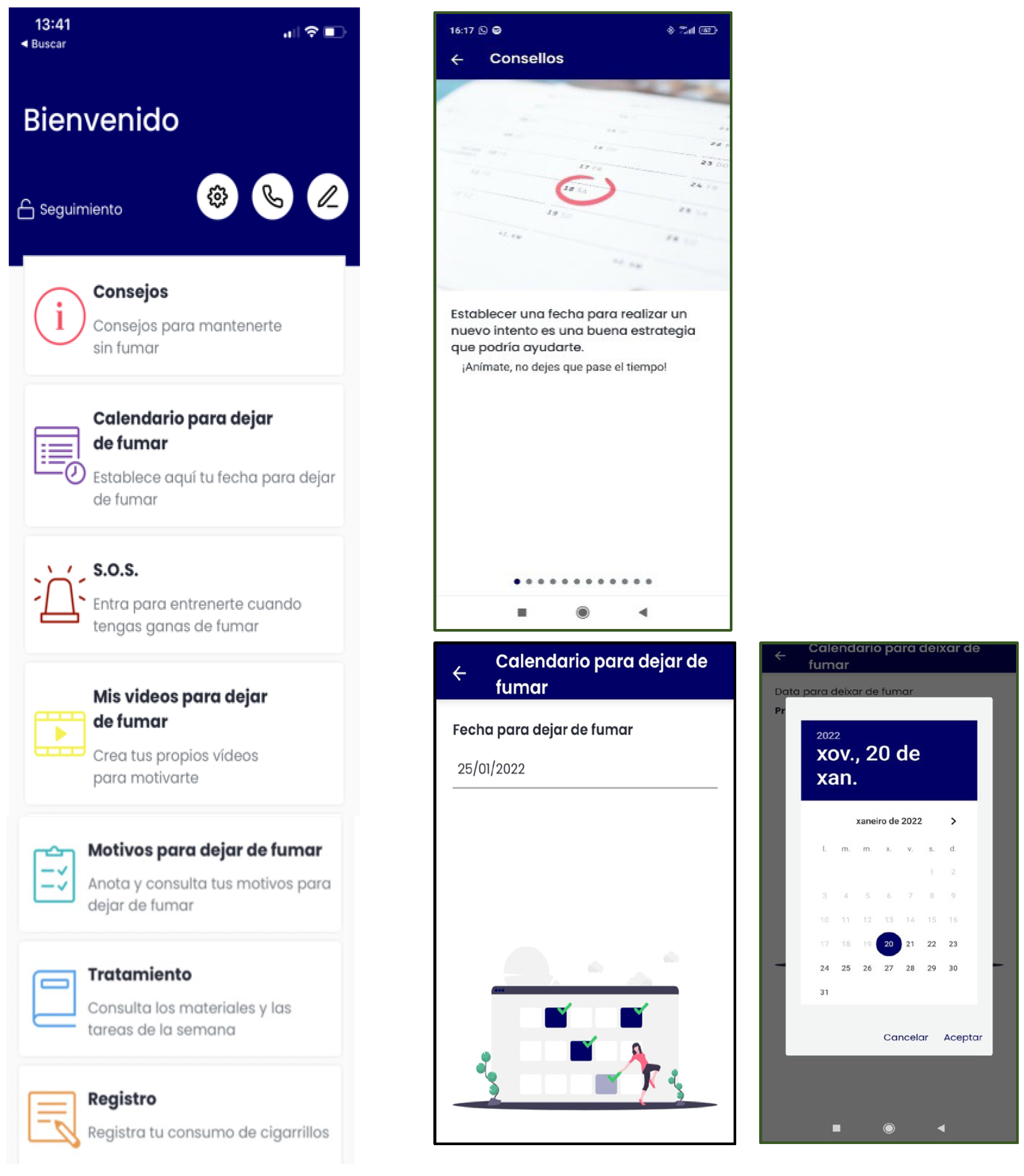
- During Phase 1 (Table 1), the App will have the following content: smoking self-monitoring tool, cigarettes consumption graph, a list of personal reasons to quit smoking, setting weekly cigarette reduction goals tool (to nicotine fading), stimulus control tool (set weekly non-smoking situations), deep breathing videos, self-monitoring of BA activities tool, inter-session motivational and weekly reinforcement notifications of goal achievements, and access to the written materials in pdf format provided during the face-to-face sessions (Figure 2).
- -
- During Phase 2 (Table 2), the App content will differ depending on the participants’ smoking status, which are defined as follows: (a) abstinents, as those participants who report being abstainers; (b) relapsed, as those participants who report abstinence at the end of treatment, but who relapsed during the follow-up period; and (c) smokers, as those participants who never quit smoking during treatment and follow-ups. The specific components are detailed below:
- (a)
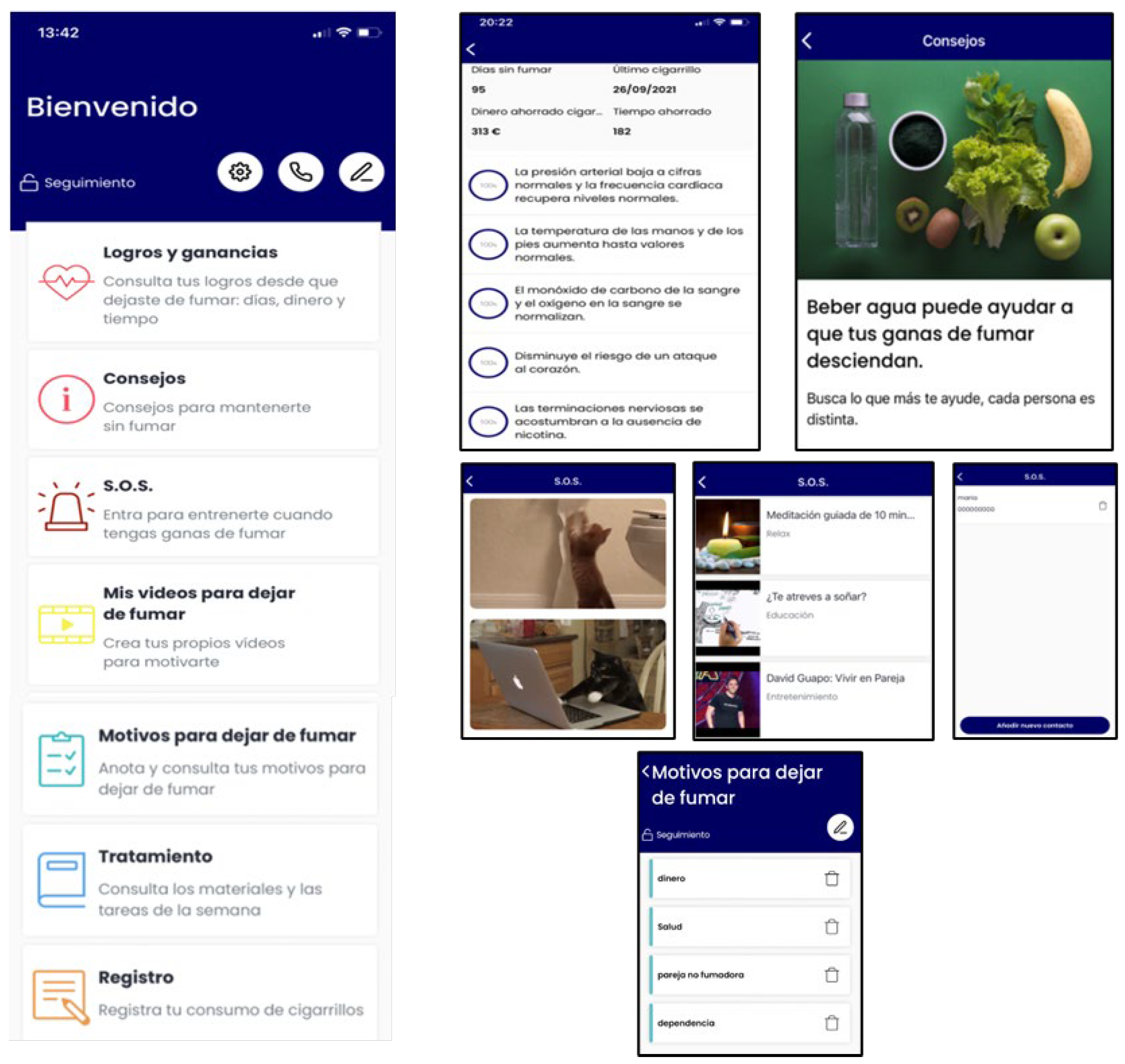
- Abstinent: gains and achievements made since quitting smoking (number of days without smoking, saved money, time gained, and physical improvements), behavioral and cognitive recommendations for abstinence maintenance, strategies for coping with cravings (distraction tools, such as gifs, games, or videos; and social-support-seeking strategies, such as access to an emergency contact list), motivational strategies (list reasons to remain abstinent, personal video recording focused on reasons for quitting, and videos of close friends/relatives motivating them to remain abstinent), access to the materials in pdf format that were provided during the sessions, and the possibility of self-reporting tobacco use if a lapse or relapse occurs (Figure 3). Likewise, they will receive notifications during this follow-up phase according to gains and achievements as they remain abstinent through the one-year follow-up.
- (b)
- Relapsed: behavioral and cognitive advice to quit (tips), the possibility of setting a new quit date in a calendar, strategies to cope with cravings (distraction tools, such as gifs, games, or videos; and social-support-seeking strategies, such as access to an emergency contact list), motivational strategies (list of personal reasons for quitting, personal video with reasons for quitting, and videos of close friends/relatives motivating them to quit), access to the materials in pdf format that were provided during the sessions, and the possibility of self-reporting tobacco use. They will receive notifications to encourage and support a new quit attempt during this follow-up phase (Figure 4).
- (c)
- Smokers: behavioral and cognitive advice to quit (tips), the possibility of setting a quit date in a calendar, notifications consisting of strategies to be prepared for cessation, strategies to cope with cravings (distraction tools, such as gifs, games, or videos; and social-support-seeking strategies, such as access to an emergency contact list), motivational strategies (list of personal reasons for quitting, personal video with reasons for quitting, and videos of close friends/relatives motivating them to quit), access to the materials in pdf format that were provided during the face-to-face sessions, and the possibility of self-reporting tobacco use. Likewise, they will receive notifications to encourage a quit attempt during this follow-up phase (Figure 5).
2.4.2. Cognitive Behavioural Smoking Cessation Intervention and Control App (Control Condition)
2.5. Procedures
2.6. Measures
- -
- Smoking Habit Questionnaire [30]. This questionnaire consists of 56 items gathering information about sociodemographic variables (gender, age, marital status, educational level) and tobacco use (i.e., number of cigarettes smoked per day).
- -
- -
- -
- Structured interview for the assessment of the DSM-5 diagnostic criteria for tobacco use disorder [36].
- -
- Minnesota Nicotine Withdrawal Scale (MNWS) [37]. This 8-item scale measures nicotine withdrawal symptoms (depression, insomnia, irritability/frustration/anger, anxiety, difficulty concentrating, restlessness, increased appetite/weight gain) and craving (smoking urgency). The reliability of this instrument is good (Cronbach’s alpha 0.85).
- -
- Self-Efficacy/Smoking Temptation Scale [38]. This 9-items instrument assesses the temptation to smoke in different situations: positive affect/social situations, positive affect situations, and habit situations.
- -
- Questionnaire of Urgency to Smoke [39]. This questionnaire consists of 10 items evaluating tobacco craving. This questionnaire has two factors: (1) the intention or desire to smoke, and (2) the expectations of negative reinforcement or improvements through smoking.
- -
- -
- EuroQoL-5D Quality of Life Questionnaire [42]. This is a standardized and generic instrument to assess health-related quality of life. It consists of (1) assessment of five dimensions: mobility, self-care, activities of daily living, pain/discomfort, and anxiety/depression; and (2) a visual-analog scale where the individual indicates the score (ranging from 0 to 100) that best represents his/her overall health status on the day of the interview.
- -
- Questionnaire about the use of smartphones and Apps. This is an ad hoc questionnaire collecting information about the use of smartphones (i.e., frequency, time of use, type of use) and Apps (i.e., frequency of use, hours of use, types of Apps).
- -
- End-of-treatment questionnaire. This self-report collects information about quit date, confidence in remaining abstinent, perceived social support, and physical and psychological improvements since the beginning of treatment.
- -
- Client Satisfaction Questionnaire [43]. This 8-item instrument measures overall satisfaction with treatment services.
- -
- Satisfaction with App questionnaire, in which data about satisfaction and usability are collected at the end of treatment and at 3-, 6-, and 12-months follow-up.
- -
- Follow-up questionnaire, in which abstinence and lapse/relapse data are collected at 3-, 6-, and 12-months follow-up.
2.7. Outcomes
2.7.1. Primary Outcome
2.7.2. Secondary Outcomes
2.8. Data Management and Confidentiality
2.9. Ethical Principles
2.10. Trial Status
2.11. Statistical Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Health and Human Services (U.S.D.H.H.S.). Smoking Cessation: A Report of the Surgeon General; U.S. Department of Health and Human Services (U.S.D.H.H.S.): Atlanta, GA, USA, 2020.
- World Health Organization (WHO). WHO Report on the Global Tobacco Epidemic 2021: Addressing New and Emerging Products; World Health Organization (WHO): Geneva, Switzerland, 2021. [Google Scholar]
- U.S. Department of Health and Human Services (U.S.D.H.H.S.). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.: Rockville, MD, USA, 2014.
- Prochaska, J.J.; Das, S.; Young-Wolff, K.C. Smoking, mental illness, and public health. Annu. Rev. Public Health 2017, 38, 165–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodchild, M.; Nargis, N.; Tursan d’Espaignet, E. Global economic cost of smoking-attributable diseases. Tob. Control 2018, 27, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Patnode, C.D.; Henderson, J.T.; Coppola, E.L.; Melnikow, J.; Durbin, S.; Thomas, R.G. Interventions for Tobacco Cessation in Adults, Including Pregnant Persons. JAMA 2021, 325, 280. [Google Scholar] [CrossRef] [PubMed]
- Stead, L.F.; Carroll, A.J.; Lancaster, T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst. Rev. 2017, 3, CD001007. [Google Scholar] [CrossRef]
- Gollust, S.E.; Schroeder, S.A.; Warner, K.E. Helping Smokers Quit: Understanding the Barriers to Utilization of Smoking Cessation Services. Milbank Q. 2008, 86, 601–627. [Google Scholar] [CrossRef] [Green Version]
- Rafful, C.; García-Rodríguez, O.; Wang, S.; Secades-Villa, R.; Martínez-Ortega, J.M.; Blanco, C. Predictors of quit attempts and successful quit attempts in a nationally representative sample of smokers. Addict. Behav. 2013, 38, 1920–1923. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.E.; McGowan, J.A.; Ubhi, H.K.; Proudfoot, H.; Shahab, L.; Brown, J.; West, R. Modelling continuous abstinence rates over time from clinical trials of pharmacological interventions for smoking cessation. Addiction 2019, 114, 787–797. [Google Scholar] [CrossRef]
- Piasecki, T.M. Relapse to smoking. Clin. Psychol. Rev. 2006, 26, 196–215. [Google Scholar] [CrossRef]
- Becoña, E.; López-Durán, A.; Fernández del Río, E.; Martínez, Ú. Changes in the profiles of smokers seeking cessation treatment and in its effectiveness in Galicia (Spain) 2001–10. BMC Public Health 2014, 14, 613. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Lee, H.; Kim, Y.; Kim, J.; Cho, M.; Jang, J.; Jang, H. Mobile App-Based Health Promotion Programs: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2018, 15, 2838. [Google Scholar] [CrossRef] [Green Version]
- Rathbone, A.L.; Prescott, J. The Use of Mobile Apps and SMS Messaging as Physical and Mental Health Interventions: Systematic Review. J. Med. Internet Res. 2017, 19, e295. [Google Scholar] [CrossRef] [Green Version]
- Erbe, D.; Eichert, H.-C.; Riper, H.; Ebert, D.D. Blending Face-to-Face and Internet-Based Interventions for the Treatment of Mental Disorders in Adults: Systematic Review. J. Med. Internet Res. 2017, 19, e306. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Global Diffusion of eHealth: Making Universal Health COVERAGE achievable: Report of the Third Global Survey on eHealth; World Health Organization (WHO): Geneva, Switzerland, 2016. [Google Scholar]
- Marcolino, M.S.; Oliveira, J.A.Q.; D’Agostino, M.; Ribeiro, A.L.; Alkmim, M.B.M.; Novillo-Ortiz, D. The Impact of mHealth Interventions: Systematic Review of Systematic Reviews. JMIR mHealth uHealth 2018, 6, e23. [Google Scholar] [CrossRef] [Green Version]
- Lindhiem, O.; Bennett, C.B.; Rosen, D.; Silk, J. Mobile Technology Boosts the Effectiveness of Psychotherapy and Behavioral Interventions. Behav. Modif. 2015, 39, 785–804. [Google Scholar] [CrossRef]
- Gustafson, D.H.; McTavish, F.M.; Chih, M.-Y.; Atwood, A.K.; Johnson, R.A.; Boyle, M.G.; Levy, M.S.; Driscoll, H.; Chisholm, S.M.; Dillenburg, L.; et al. A Smartphone Application to Support Recovery from Alcoholism. JAMA Psychiatry 2014, 71, 566. [Google Scholar] [CrossRef]
- Shoesmith, E.; Huddlestone, L.; Lorencatto, F.; Shahab, L.; Gilbody, S.; Ratschen, E. Supporting smoking cessation and preventing relapse following a stay in a smoke-free setting: A meta-analysis and investigation of effective behaviour change techniques. Addiction 2021, 116, 2978–2994. [Google Scholar] [CrossRef]
- Barroso-Hurtado, M.; Suárez-Castro, D.; Martínez-Vispo, C.; Becoña, E.; López-Durán, A. Smoking Cessation Apps: A Systematic Review of Format, Outcomes, and Features. Int. J. Environ. Res. Public Health 2021, 18, 11664. [Google Scholar] [CrossRef]
- Regmi, K.; Kassim, N.; Ahmad, N.; Tuah, N. Effectiveness of Mobile Apps for Smoking Cessation: A Review. Tob. Prev. Cessat. 2017, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, R.; McRobbie, H.; Bullen, C.; Rodgers, A.; Gu, Y.; Dobson, R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2019, CD006611. [Google Scholar] [CrossRef]
- Mersha, A.G.; Bovill, M.; Eftekhari, P.; Erku, D.A.; Gould, G.S. The effectiveness of technology-based interventions for smoking cessation: An umbrella review and quality assessment of systematic reviews. Drug Alcohol Rev. 2021, 40, 1294–1307. [Google Scholar] [CrossRef]
- Chu, K.H.; Matheny, S.J.; Escobar-Viera, C.G.; Wessel, C.; Notier, A.E.; Davis, E.M. Smartphone health apps for tobacco Cessation: A systematic review. Addict. Behav. 2021, 112, 106616. [Google Scholar] [CrossRef]
- Wang, X.; Ji, X. Sample Size Estimation in Clinical Research. Chest 2020, 158, S12–S20. [Google Scholar] [CrossRef]
- Becoña, E. Programa para Dejar de Fumar [Smoking Cessation Program]; Nova Galicia Edicións: Vigo, Spain, 2007. [Google Scholar]
- Becoña, E.; Martínez-Vispo, C.; Senra, C.; López-Durán, A.; Rodríguez-Cano, R.; Fernández del Río, E. Cognitive-behavioral treatment with behavioral activation for smokers with depressive symptomatology: Study protocol of a randomized controlled trial. BMC Psychiatry 2017, 17, 134. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Vispo, C.; Rodríguez-Cano, R.; López-Durán, A.; Senra, C.; Del Río, E.F.; Becoña, E. Cognitive-behavioral treatment with behavioral activation for smoking cessation: Randomized controlled trial. PLoS ONE 2019, 14, e0214252. [Google Scholar] [CrossRef] [PubMed]
- Becoña, E. Evaluación de la conducta de fumar [Assessment of smoking behavior]. In Conductas Adictivas: Teoría, Evaluación y Tratamiento; Graña, J.L., Ed.; Debate: Madrid, Spain, 1994; pp. 403–454. [Google Scholar]
- Becoña, E.; Vázquez, F.L. The Fagerström Test for Nicotine Dependence in a Spanish sample. Psychol. Rep. 1998, 83, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Fagerstrom, K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob. Res. 2012, 14, 75–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becoña Iglesias, E.; Lorenzo Pontevedra, M. Evaluación de la conducta de fumar. Adicciones 2004, 16, 201–226. [Google Scholar]
- Becoña, E.; López, A.; Fernández del Río, E.; Míguez, M.C.; Castro, J. Spanish adaptation of the NDSS (Nicotine Dependence Syndrome Scale) and assessment of nicotine-dependent individuals at primary care health centers in Spain. Span. J. Psychol. 2010, 13, 951–960. [Google Scholar] [CrossRef] [Green Version]
- Shiffman, S.; Waters, A.; Hickcox, M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine Tob. Res. 2004, 6, 327–348. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Philadelphia, PA, USA, 2013; ISBN 9780890425572. [Google Scholar]
- Hughes, J.R.; Hatsukami, D. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry 1986, 43, 289–294. [Google Scholar] [CrossRef]
- Velicer, W.F.; Diclemente, C.C.; Rossi, J.S.; Prochaska, J.O. Relapse situations and self-efficacy: An integrative model. Addict. Behav. 1990, 15, 271–283. [Google Scholar] [CrossRef]
- Cepeda-Benito, A.; Reig-Ferrer, A. Development of a Brief Questionnaire of Smoking Urges—Spanish. Psychol. Assess. 2004, 16, 402–407. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.; Steer, R.; Brown, G. Beck Depression Inventory–Second Edition Manual; The Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Sanz, J.; Vazquez, C. Adaptación Española del Inventario para Depresión de Beck-II (BDI-II) [Spanish Adaptation of the Beck Depression Inventory—II (BDI-II)] Manual; Pearson: Madrid, Spain, 2011. [Google Scholar]
- Badia, X.; Roset, M.; Montserrat, S.; Herdman, M.; Segura, A. The Spanish Version of EuroQol: A Description and Its Applications. European Quality of Life Scale; ScienceOpen: Burlington, MA, USA, 1999. [Google Scholar]
- Larsen, D.L.; Attkisson, C.C.; Hargreaves, W.A.; Nguyen, T.D. Assessment of client/patient satisfaction: Development of a general scale. Eval. Prog. Plan. 1979, 2, 197–207. [Google Scholar] [CrossRef]
- Piper, M.E.; Bullen, C.; Krishnan-Sarin, S.; Rigotti, N.A.; Steinberg, M.L.; Streck, J.M.; Joseph, A.M. Defining and Measuring Abstinence in Clinical Trials of Smoking Cessation Interventions: An Updated Review. Nicotine Tob. Res. 2020, 22, 1098–1106. [Google Scholar] [CrossRef]
- White, I.R.; Carpenter, J.; Horton, N.J. Including all individuals is not enough: Lessons for intention-to-treat analysis. Clin. Trials 2012, 9, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Grant, S.; Mayo-Wilson, E.; Montgomery, P.; Macdonald, G.; Michie, S.; Hopewell, S.; Moher, D.; Lawrence Aber, J.; Altman, D.; Bhui, K.; et al. CONSORT-SPI 2018 Explanation and Elaboration: Guidance for reporting social and psychological intervention trials. Trials 2018, 19, 406. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, P.; Grant, S.; Mayo-Wilson, E.; Macdonald, G.; Michie, S.; Hopewell, S.; Moher, D.; Lawrence Aber, J.; Altman, D.; Bhui, K.; et al. Reporting randomised trials of social and psychological interventions: The CONSORT-SPI 2018 Extension. Trials 2018, 19, 407. [Google Scholar] [CrossRef]
- Livingstone-Banks, J.; Norris, E.; Hartmann-Boyce, J.; West, R.; Jarvis, M.; Hajek, P. Relapse prevention interventions for smoking cessation. Cochrane Database Syst. Rev. 2019, 8, CD003999. [Google Scholar] [CrossRef]
- Hendershot, C.S.; Witkiewitz, K.; George, W.H.; Marlatt, G.A. Relapse prevention for addictive behaviors. Subst. Abuse Treat. Prev. Policy 2011, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Benowitz, N.L.; Bernert, J.T.; Foulds, J.; Hecht, S.S.; Jacob, P.; Jarvis, M.J.; Joseph, A.; Oncken, C.; Piper, M.E. Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine Tob. Res. 2020, 22, 1086–1097. [Google Scholar] [CrossRef]
- Mahoney, M.C.; Ashare, R.; Schlienz, N.; Duerr, C.; Hawk, L.W. Making lemonade from SARS coronavirus-2 lemons: Transitioning a smoking cessation trial to a virtual platform. J. Subst. Abuse Treat. 2020, 117, 108100. [Google Scholar] [CrossRef] [PubMed]
- Bol, N.; Helberger, N.; Weert, J.C.M. Differences in mobile health app use: A source of new digital inequalities? Inf. Soc. 2018, 34, 183–193. [Google Scholar] [CrossRef] [Green Version]
| Experimental Condition (SinHumo App) | Control Condition (Control App) | |
|---|---|---|
| Session1 | Overview of treatment and App usage Smoking cessation treatment rationale Review cigarette self-monitoring using the App (tracking cigarettes smoked, pleasure experienced, and smoking antecedents and consequences) Automatically generated cigarettes consumption graph available in the App Discussion of reasons for smoking and for quitting and completion tool available in the App named “reasons to quit” Discussion about smoking history and past quit experiences Explanation of written materials included in the App about tobacco, nicotine dependence, smoking health consequences, and cessation benefits Explain nicotine fading through brand change and set weekly cigarette reduction goals using the App tool Intersession activities:
| Overview of treatment Smoking cessation treatment rationale Review cigarette self-monitoring using the App (tracking cigarettes smoked, pleasure experienced, and smoking antecedents and consequences) Discussion of reasons for smoking and for quitting Discussion about smoking history and past quit experiences Explanation of written materials included in the App about tobacco, nicotine dependence, smoking health consequences, and cessation benefits Explain nicotine fading through brand change Intersession activities:
|
| Session 2 | Check homework and nicotine fading compliance Continue smoking self-monitoring through the App and analyze smoking behavior during the week Automatically generated cigarettes consumption graph available in the App Discuss brand change difficulties New brand change and reduction of number of cigarettes to smoke the next week, setting the cigarette reduction goal using the App tool Review importance of social support Introduce stimulus control technique to remove situations conditioned to smoking using the App Nicotine withdrawal and strategies to avoid it Breathing exercises and relaxation techniques (practice as homework using the videos available in the App) Intersession motivational notification through the App Reinforcement notification of goal achievement through the App | Check homework and nicotine fading compliance Continue smoking self-monitoring through the App and analyze smoking behavior during the week Discuss brand change difficulties New brand change and reduction of number of cigarettes to smoke the next week Review importance of social support Introduce stimulus control technique to remove situations conditioned to smoking Nicotine withdrawal and strategies to avoid it Breathing exercises and relaxation techniques (practice such as homework) |
| Intervention Session 3 | Check nicotine fading, cigarette reduction, and stimulus control compliance Check breathing exercises compliance and strategies to avoid withdrawal symptoms Continue cigarette self-monitoring through the App and analyze smoking behavior Automatically generated cigarettes consumption graph available in the App New brand change and reduce number of cigarettes to smoke the next week setting the cigarette reduction goal using the App tool Continue stimulus control technique using the App Explain written materials for weight control and exercise available in the App Continue with breathing exercises compliance and strategies to avoid withdrawal symptoms Rationale of mood influence in smoking cessation (written materials available in the App) Homework: daily activities self-monitoring using App tool Intersession motivational notification through the App Reinforcement notification of goal achievement through the App | Check nicotine fading, cigarette reduction, and stimulus control compliance Check breathing exercises compliance and strategies to avoid withdrawal symptoms Continue cigarette self-monitoring through the App and analyze smoking behavior New brand change and reduce number of cigarettes to smoke the next week Continue stimulus control technique Explain written materials for weight control and exercise available in the App Continue with breathing exercises compliance and strategies to avoid withdrawal symptoms Rationale of mood influence in smoking cessation (written materials available in the App) Homework: daily activities self-monitoring |
| Session 4 | Check nicotine fading, cigarette reduction, and stimulus control compliance Check breathing exercises compliance and strategies to avoid withdrawal symptoms Continue smoking self-monitoring through the App and analyze smoking behavior Automatically generated cigarettes consumption graph available in the App New brand change and reduction of number of cigarettes to smoke the next week, setting the cigarette reduction goal using the App tool Continue stimulus control technique using the App Stress and anxiety management strategies Check activity scheduling through the App and encourage recognizing patterns of depressed behavior and the way in which engaging in enjoyable and important activities may impact their overall mood Homework: continue activity scheduling through the App, create a pleasant activities list and choose one to do during the week Intersession motivational notification through the App Reinforcement notification of goal achievement through the App | Check nicotine fading, cigarette reduction, and stimulus control compliance Check breathing exercises compliance and strategies to avoid withdrawal symptoms Continue smoking self-monitoring through the App and analyze smoking behavior New brand change and reduction of number of cigarettes to smoke the next week Continue stimulus control technique Stress and anxiety management strategies Check activity scheduling, and encourage recognizing patterns of depressed behavior and the way in which engaging in enjoyable and important activities may impact their overall mood Homework: continue activity scheduling, create a pleasant activities list, and choose one to do during the week |
| Session 5 | Check nicotine fading, cigarette reduction, and stimulus control compliance using the App Check activity scheduling, pleasant activities list elaboration, and pleasant activity compliance using the App tools Continue smoking self-monitoring through the App and analyze smoking behavior Automatically generated cigarettes consumption graph available in the App New brand change and reduction of number of cigarettes to smoke the next week, setting the cigarette reduction goal using the App tool Management of anxiety and anger Self-reinforcing Changing tobacco-related misconceptions Problem-solving training Recognize avoidance behavior and impact on mood Activity scheduling through the App and engagement in two pleasant activities during the week Intersession motivational notification through the App Reinforcement notification of goal achievement through the App | Check nicotine fading, cigarette reduction, and stimulus control compliance Check activity scheduling, pleasant activities list elaboration, and pleasant activity compliance Continue smoking self-monitoring through the App and analyze smoking behavior New brand change and reduction of number of cigarettes to smoke the next week Management of anxiety and anger Self-reinforcing Changing tobacco-related misconceptions Problem-solving training Recognize avoidance behavior and impact on mood Activity scheduling and engagement in two pleasant activities during the week |
| Session 6 | Quitting experience and withdrawal symptoms Discuss and plan for high-risk lapse and relapse situations Motivating factors for maintaining abstinence Benefits of quitting smoking Common barriers for maintaining abstinence Ruminative thoughts, smoking cessation process, and relapse Check activity scheduling and pleasant activity compliance using the App Activity scheduling through the App for the next week and engagement in 2 pleasant activities/week Intersession motivational notification through the App Reinforcement notification of goal achievement through the App | Quitting experience and withdrawal symptoms Discuss and plan for high-risk lapse and relapse situations Motivating factors for maintaining abstinence Benefits of quitting smoking Common barriers for maintaining abstinence Ruminative thoughts, smoking cessation process, and relapse Check activity scheduling and pleasant activity compliance Activity scheduling for the next week and engagement in 2 pleasant activities/week |
| Session 7 | Quitting experience and withdrawal symptoms Discuss and plan for high-risk lapse and relapse situations Motivating factors for maintaining abstinence Benefits of quitting smoking Review how behavioral activation impacts their overall mood Review avoidance behavior and ruminative thoughts’ significance Strategies for relapse prevention Intersession motivational notification through the App Reinforcement notification of goal achievement through the App | Quitting experience and withdrawal symptoms Discuss and plan for high-risk lapse and relapse situations Motivating factors for maintaining abstinence Benefits of quitting smoking Review how behavioral activation impacts their overall mood Review avoidance behavior and ruminative thoughts’ significance Strategies for relapse prevention |
| Session 8 | Managing the future as ex-smokers Encouragement for abstinence maintenance Support for lapses and relapse Review motivating factors, lifestyle changes, physical and cognitive–behavioral health improvement Review behavioral activation strategies Treatment conclusion and management of potential obstacles Intersession motivational notification through the App Reinforcement notification of goal achievement through the App | Managing the future as ex-smokers Encouragement for abstinence maintenance Support for lapses and relapse Review motivating factors, lifestyle changes, physical and cognitive–behavioral health improvement Review behavioral activation strategies Treatment conclusion and management of potential obstacles |
| Smoking Status | Experimental Condition (SinHumo App) | Control Condition (Control App) |
|---|---|---|
| Abstainer | Smoking self-monitoring tool (to use in case a lapse occurs) Access to the intervention written materials in pdf format Gains and achievements according to abstinence duration after the intervention (health benefits, saved money) Abstinence maintenance tips Self-recorded motivational videos List of reasons to quit to consult in case of experiencing tobacco craving Motivational strategies to maintain abstinence Cognitive and behavioral strategies for coping with cravings Motivational notifications during all the follow-up period | Smoking self-monitoring tool Access to the written materials in pdf format |
| Smoker (never quit during phase 1) | Smoking cessation tips Smoking self-monitoring tool Possibility of setting a quit date in a calendar Access to the intervention written materials in pdf format Self-recorded motivational videos List of reasons to quit tool Motivational strategies to quit Cognitive and behavioral strategies for coping with cravings Motivational notifications during the follow-up period promoting the initiation of a quit attempt | Smoking self-monitoring tool Access to the written materials in pdf format |
| Relapser (quit at least 24 h during phase 1) | Smoking cessation tips Smoking self-monitoring tool Possibility of setting a new quit date in a calendar Access to the intervention written materials in pdf format Self-recorded motivational videos List of reasons to quit tool Motivational strategies to initiate a new quit attempt Cognitive and behavioral strategies for coping with cravings Motivational notifications during the follow-up period promoting the initiation of a new quit attempt | Smoking self-monitoring tool Access to the written materials in pdf format |
| Measures | Measurement Time-Point | ||||
|---|---|---|---|---|---|
| Baseline | End of Treatment | 3-Months Follow-Up | 6-Months Follow-Up | 12-Months Follow-Up | |
| Smoking Habit Questionnaire | X | ||||
| TUS Tobacco DSM-5 criteria | X | ||||
| FTCD | X | X | X | X | X |
| NDSS | X | X | X | X | X |
| MNWS | X | X | X | X | X |
| SSE | X | X | X | X | X |
| QSU | X | X | X | X | X |
| BDI-II | X | X | X | X | X |
| EQ-5D | X | X | X | X | X |
| APP-Q | X | ||||
| CSQ-8 | X | ||||
| Satisfaction with App questionnaire | X | X | X | X | |
| Follow-up questionnaire | X | X | X | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Durán, A.; Becoña, E.; Senra, C.; Suárez-Castro, D.; Barroso-Hurtado, M.; Martínez-Vispo, C. A Randomized Clinical Trial to Assess the Efficacy of a Psychological Treatment to Quit Smoking Assisted with an App: Study Protocol. Int. J. Environ. Res. Public Health 2022, 19, 9770. https://doi.org/10.3390/ijerph19159770
López-Durán A, Becoña E, Senra C, Suárez-Castro D, Barroso-Hurtado M, Martínez-Vispo C. A Randomized Clinical Trial to Assess the Efficacy of a Psychological Treatment to Quit Smoking Assisted with an App: Study Protocol. International Journal of Environmental Research and Public Health. 2022; 19(15):9770. https://doi.org/10.3390/ijerph19159770
Chicago/Turabian StyleLópez-Durán, Ana, Elisardo Becoña, Carmen Senra, Daniel Suárez-Castro, María Barroso-Hurtado, and Carmela Martínez-Vispo. 2022. "A Randomized Clinical Trial to Assess the Efficacy of a Psychological Treatment to Quit Smoking Assisted with an App: Study Protocol" International Journal of Environmental Research and Public Health 19, no. 15: 9770. https://doi.org/10.3390/ijerph19159770
APA StyleLópez-Durán, A., Becoña, E., Senra, C., Suárez-Castro, D., Barroso-Hurtado, M., & Martínez-Vispo, C. (2022). A Randomized Clinical Trial to Assess the Efficacy of a Psychological Treatment to Quit Smoking Assisted with an App: Study Protocol. International Journal of Environmental Research and Public Health, 19(15), 9770. https://doi.org/10.3390/ijerph19159770







