Potential Effects on Mental Health Status Associated with Occupational Exposure to Pesticides among Thai Farmers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Data Collection
2.3. Data Analysis
3. Results
4. Discussion
4.1. Principal Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Kittirattanapaiboon, P.; Tantirangsee, N.; Chutha, W.; Tanaree, A.; Kwansanit, P.; Assanangkornchai, S.; Supanya, S. Prevalence of Mental Disorders and Mental Health Problems: Results from Thai National Mental Health Survey 2013. Available online: https://www.dmh-elibrary.org/items/show/177 (accessed on 7 May 2021).
- Suicide Report. Available online: https://www.dmh.go.th/report/suicide/ (accessed on 7 May 2021).
- Malekirad, A.A.; Faghih, M.; Mirabdollahi, M.; Kiani, M.; Fathi, A.; Abdollahi, M. Neurocognitive, mental health, and glucose disorders in farmers exposed to organophosphorus pesticides. Arh. Hig. Rada Toksikol. 2013, 64, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavanja, M.C.; Hoppin, J.A.; Kamel, F. Health effects of chronic pesticide exposure: Cancer and neurotoxicity. Annu. Rev. Public Health 2004, 25, 155–197. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie Ross, S.J.; Brewin, C.R.; Curran, H.V.; Furlong, C.E.; Abraham-Smith, K.M.; Harrison, V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol. Teratol. 2010, 32, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Medina, A.; Ugalde-Lizárraga, A.; Bojorquez-Cuevas, M.S.; Garnica-Ruiz, J.; González-Corral, M.A.; García-Ledezma, A.; Pineda-García, G.; Cornejo-Bravo, J.M. Neuropsychiatric Disorders in Farmers Associated with Organophosphorus Pesticide Exposure in a Rural Village of Northwest México. Int. J. Environ. Res. Public Health 2019, 16, 689. [Google Scholar] [CrossRef] [Green Version]
- Beseler, C.L.; Stallones, L. A cohort study of pesticide poisoning and depression in Colorado farm residents. Ann. Epidemiol. 2008, 18, 768–774. [Google Scholar] [CrossRef]
- Campos, Ÿ.; Dos Santos Pinto da Silva, V.; Sarpa Campos de Mello, M.; Barros Otero, U. Exposure to pesticides and mental disorders in a rural population of Southern Brazil. Neurotoxicology 2016, 56, 7–16. [Google Scholar] [CrossRef]
- Harrison, V.; Mackenzie Ross, S. Anxiety and depression following cumulative low-level exposure to organophosphate pesticides. Environ. Res. 2016, 151, 528–536. [Google Scholar] [CrossRef]
- Kim, J.; Ko, Y.; Lee, W.J. Depressive symptoms and severity of acute occupational pesticide poisoning among male farmers. Occup. Environ. Med. 2013, 70, 303–309. [Google Scholar] [CrossRef]
- Koh, S.B.; Kim, T.H.; Min, S.; Lee, K.; Kang, D.R.; Choi, J.R. Exposure to pesticide as a risk factor for depression: A population-based longitudinal study in Korea. Neurotoxicology 2017, 62, 181–185. [Google Scholar] [CrossRef]
- Beard, J.D.; Umbach, D.M.; Hoppin, J.A.; Richards, M.; Alavanja, M.C.; Blair, A.; Sandler, D.P.; Kamel, F. Pesticide exposure and depression among male private pesticide applicators in the Agricultural Health Study. Environ. Health Perspect. 2014, 122, 984–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beseler, C.L.; Stallones, L.; Hoppin, J.A.; Alavanja, M.C.; Blair, A.; Keefe, T.; Kamel, F. Depression and pesticide exposures among private pesticide applicators enrolled in the Agricultural Health Study. Environ. Health Perspect. 2008, 116, 1713–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onwuameze, O.E.; Paradiso, S.; Peek-Asa, C.; Donham, K.J.; Rautiainen, R.H. Modifiable risk factors for depressed mood among farmers. Ann. Clin. Psychiatry 2013, 25, 83–90. [Google Scholar] [PubMed]
- Weisskopf, M.G.; Moisan, F.; Tzourio, C.; Rathouz, P.J.; Elbaz, A. Pesticide exposure and depression among agricultural workers in France. Am. J. Epidemiol. 2013, 178, 1051–1058. [Google Scholar] [CrossRef] [Green Version]
- Basic Information on Agriculture in Chiang Mai. Available online: http://www.chiangmai.doae.go.th/web2020/ (accessed on 10 July 2021).
- Questionnaires & Study Data. 2013. Available online: http://aghealth.nih.gov/collaboration/questionnaires.html (accessed on 11 November 2019).
- Summary Report on Import of Hazardous Substances in 2019. Available online: https://www.doa.go.th/ard/wp-content/uploads/2020/02/HASTAT62_03.pdf (accessed on 11 March 2020).
- Blair, A.; Tarone, R.; Sandler, D.; Lynch, C.F.; Rowland, A.; Wintersteen, W.; Steen, W.C.; Samanic, C.; Dosemeci, M.; Alavanja, M.C. Reliability of reporting on life-style and agricultural factors by a sample of participants in the Agricultural Health Study from Iowa. Epidemiology 2002, 3, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Fuhrimann, S.; Farnham, A.; Staudacher, P.; Atuhaire, A.; Manfioletti, T.; Niwagaba, C.B.; Namirembe, S.; Mugweri, J.; Winkler, M.S.; Portengen, L.; et al. Exposure to multiple pesticides and neurobehavioral outcomes among smallholder farmers in Uganda. Environ. Int. 2021, 152, 106477. [Google Scholar] [CrossRef]
- Fuhrimann, S.; Staudacher, P.; Lindh, C.; van Wendel de Joode, B.; Mora, A.M.; Winkler, M.S.; Kromhout, H. Variability and predictors of weekly pesticide exposure in applicators from organic, sustainable and conventional smallholder farms in Costa Rica. Occup. Environ. Med. 2020, 77, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Beusenberg, M.; Orley, J.H.; World Health Organization. A User’s Guide to the Self Reporting Questionnaire (SRQ); No. WHO/MNH/PSF/94.8; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Buralli, R.J.; Ribeiro, H.; Iglesias, V.; Muñoz-Quezada, M.T.; Leão, R.S.; Marques, R.C.; Almeida, M.M.C.; Guimarães, J.R.D. Occupational exposure to pesticides and health symptoms among family farmers in Brazil. Rev. Saude Publica 2020, 54, 133. [Google Scholar] [CrossRef]
- van der Westhuizen, C.; Wyatt, G.; Williams, J.K.; Stein, D.J.; Sorsdahl, K. Validation of the Self Reporting Questionnaire 20-Item (SRQ-20) for Use in a Low- and Middle-Income Country Emergency Centre Setting. Int. J. Ment. Health Addict. 2016, 14, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Cherry, N.; Burstyn, I.; Beach, J.; Senthilselvan, A. Mental health in Alberta grain farmers using pesticides over many years. Occup. Med. 2012, 62, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Alavanja, M.C.; Hoppin, J.A.; Rusiecki, J.A.; Kamel, F.; Blair, A.; Sandler, D.P. Mortality among pesticide applicators exposed to chlorpyrifos in the Agricultural Health Study. Environ. Health Perspect. 2007, 115, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Mundhe, S.A.; Birajdar, S.V.; Chavan, S.S.; Pawar, N.R. Imidacloprid Poisoning: An Emerging Cause of Potentially Fatal Poisoning. Indian J. Crit. Care Med. 2017, 21, 786–788. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.A.M.; Guimarães, A.T.B.; Montalvão, M.F.; Mendes, B.O.; Rodrigues, A.S.L.; Malafaia, G. The chronic exposure to abamectin causes spatial memory deficit and depressive behavior in mice. Chemosphere 2018, 194, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albretsen, J.C. Rodenticides and Avicides. In Clinical Veterinary Toxicology; Plumlee, K.H., Ed.; Mosby: Maryland Heights, MI, USA, 2004; pp. 443–459. [Google Scholar]
- Amr, M.M.; Abbas, E.Z.; El-Samra, M.; El Batanuoni, M.; Osman, A.M. Neuropsychiatric syndromes and occupational exposure to zinc phosphide in Egypt. Environ. Res. 1997, 73, 200–206. [Google Scholar] [CrossRef]
- Talcott, P.A. Insecticides and Molluscicides. In Clinical Veterinary Toxicology; Plumlee, K.H., Ed.; Mosby: Maryland Heights, MI, USA, 2004; pp. 177–192. [Google Scholar]
- Chen, G. Metaldehyde. In Encyclopedia of Toxicology; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 46–47. [Google Scholar]
- Wesseling, C.; van Wendel de Joode, B.; Keifer, M.; London, L.; Mergler, D.; Stallones, L. Symptoms of psychological distress and suicidal ideation among banana workers with a history of poisoning by organophosphate or n-methyl carbamate pesticides. Occup. Environ. Med. 2010, 67, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hao, Y.; Tian, D.; He, S.; Sun, X.; Yang, H. Relationship between cumulative exposure to pesticides and sleep disorders among greenhouse vegetable farmers. BMC Public Health 2019, 19, 373. [Google Scholar] [CrossRef] [Green Version]
- Baumert, B.O.; Carnes, M.U.; Hoppin, J.A.; Jackson, C.L.; Sandler, D.P.; Freeman, L.B.; Henneberger, P.K.; Umbach, D.M.; Shrestha, S.; Long, S.; et al. Sleep apnea and pesticide exposure in a study of US farmers. Sleep Health 2018, 4, 20–26. [Google Scholar] [CrossRef]
- Ahmed, F. Headache disorders: Differentiating and managing the common subtypes. Br. J. Pain 2012, 6, 124–132. [Google Scholar] [CrossRef]
- Ford, K.; Jampaklay, A.; Chamratrithirong, A. Mental health in a conflict area: Migration, economic stress and religiosity in the three southernmost provinces of Thailand. Int. J. Soc. Psychiatry 2017, 63, 91–98. [Google Scholar] [CrossRef]
- Jampaklay, A.; Vapattanawong, P. The Subjective Well-Being of Children in Transnational and Non-Migrant Households: Evidence from Thailand. Asian Pac. Migr. J. 2013, 22, 377–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Socio-Demographic Factors | n (%) | Work-Related Factors | n (%) |
|---|---|---|---|
| Sex | Work experience (years) [Mean ± SD] | 23.3 ± 13.0 | |
| Male | 3752 (53.8%) | Rice farm | 3624 (52.0%) |
| Female | 3222 (46.2%) | Vegetable farm | 2047 (29.4%) |
| Age (years) [Mean ± SD] | 55.2 ± 11.7 | Fruit farm | 4508 (64.6%) |
| ≤40 years | 768 (11.0%) | Flower farm | 495 (7.1%) |
| 41–50 years | 1222 (17.5%) | Agriculture land | |
| 51–60 years | 2257 (32.4%) | ≤8000 m2 | 3753 (53.8%) |
| >60 years | 2727 (39.1%) | 8001–16,000 m2 | 1843 (26.4%) |
| Marital status | >16,000 m2 | 1378 (19.8%) | |
| Married | 5314 (76.2%) | Entry into farmland | |
| Single/Divorced | 1660 (23.8%) | <1 time/month | 498 (7.1%) |
| Education level | Every month | 703 (10.1%) | |
| No | 984 (14.1%) | Every week | 3490 (50.1%) |
| Primary school | 4603 (66.0%) | Everyday | 2283 (32.7%) |
| Secondary school or higher | 1387 (19.9%) | History of pesticide use in agriculture | |
| Monthly family income | Never | 929 (13.3%) | |
| ≤5000 Baht | 3216 (46.1%) | Ever used | 6045 (86.7%) |
| 5001–10,000 Baht | 2770 (39.7%) | Mixer (n = 6045) | 2715 (44.9%) |
| >10,000 Baht | 988 (14.2%) | Sprayer (n = 6045) | 4540 (75.1%) |
| Currently smoking | 1273 (18.3%) | PPE score (n = 6045) [Mean ± SD] | 0.33 ± 0.20 |
| Currently drinking alcohol | 2233 (32.0%) | Bathing after application (n = 6045) | |
| Distance from home to nearest farm | Always | 5557 (91.9%) | |
| <100 m | 928 (13.3%) | Sometimes | 353 (5.9%) |
| 100–300 m | 1174 (16.8%) | Never | 135 (2.2%) |
| 300 m–1 km | 2353 (33.8%) | Pesticide poisoning (n = 6045) | |
| 2–5 km | 1778 (25.5%) | No | 5000 (82.7%) |
| >5 km | 741 (10.6%) | Yes | 1045 (17.3%) |
| Pesticide | WHO Class | n (%) | Lifetime Application Days: Percentile (P) | |||||
|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 90th | 95th | ||||
| Pesticide | 6045 (100%) | 25 | 73 | 219 | 510 | 786 | ||
| 1. | Herbicide | 5439 (90.0%) | 9 | 22 | 73 | 170 | 262 | |
| 1.1 | Glycine: Glyphosate | III | 4489 (74.3%) | 40 | 103 | 290 | 590 | 990 |
| 1.2 | Bipyridylium: Paraquat | II | 3953 (65.4%) | 40 | 103 | 368 | 590 | 1200 |
| 1.3 | Phenoxy: 2,4-D | II | 1019 (16.9%) | 25 | 100 | 225 | 590 | 840 |
| 1.4 | Chloroacetamide/Anilide: | 714 (10.2%) | 51 | 109 | 396 | 990 | 1980 | |
| 1.4.1 | Alachlor | II | 463 (7.7%) | 51 | 109 | 368 | 767 | 1628 |
| 1.4.2 | Butachlor/Propanil | III/II | 380 (6.3%) | 51 | 116 | 396 | 990 | 1628 |
| 2. | Insecticide | 5519 (91.3%) | 12 | 33 | 84 | 191 | 262 | |
| 2.1 | Organochlorine (OC): | 797 (13.2%) | 40 | 100 | 236 | 749 | 1110 | |
| 2.1.1 | Endosulfan | II | 555 (9.2%) | 40 | 100 | 225 | 457 | 767 |
| 2.1.2 | DDT | II | 246 (4.1%) | 40 | 109 | 368 | 767 | 990 |
| 2.1.3 | Chlordane | II | 66 (1.1%) | 25 | 128 | 375 | 789 | 1478 |
| 2.1.4 | Heptachlor | O | 43 (0.7%) | 51 | 116 | 368 | 643 | 767 |
| 2.1.5 | Dieldrin/Aldrin | O/O | 41 (0.7%) | 49 | 116 | 663 | 1500 | 1628 |
| 2.2 | Organophosphate (OP): | 1367 (22.6%) | 40 | 103 | 290 | 792 | 1628 | |
| 2.2.1 | Chlorpyrifos | II | 524 (8.7%) | 56 | 116 | 290 | 679 | 1200 |
| 2.2.2 | Methyl parathion | Ia | 521 (8.6%) | 40 | 100 | 290 | 590 | 840 |
| 2.2.3 | Methamidophos | Ib | 252 (4.2%) | 51 | 116 | 236 | 590 | 893 |
| 2.2.4 | Dichlorvos | Ib | 202 (3.3%) | 36 | 40 | 40 | 78 | 282 |
| 2.2.5 | Monocrotophos | Ib | 85 (1.4%) | 56 | 173 | 590 | 661 | 990 |
| 2.2.6 | EPN | Ia | 80 (1.3%) | 19 | 100 | 389 | 840 | 1628 |
| 2.2.7 | Mevinphos | Ia | 49 (0.8%) | 25 | 109 | 302 | 840 | 1234 |
| 2.2.8 | Dicrotophos | Ib | 42 (0.7%) | 40 | 110 | 390 | 767 | 1509 |
| 2.2.9 | Profenofos | II | 38 (0.6%) | 51 | 116 | 590 | 2325 | 3000 |
| 2.3 | Carbamate (CM): | 1672 (27.7%) | 40 | 100 | 282 | 780 | 1535 | |
| 2.3.1 | Methomyl | Ib | 811 (13.4%) | 40 | 100 | 236 | 590 | 990 |
| 2.3.2 | Carbofuran | Ib | 607 (10.0%) | 25 | 56 | 116 | 590 | 930 |
| 2.3.3 | Carbaryl | II | 556 (9.2%) | 40 | 78 | 225 | 457 | 840 |
| 2.3.4 | Carbosulfan | II | 389 (6.4%) | 51 | 109 | 368 | 590 | 840 |
| 2.4 | PY: Permethrin/Cypermethrin | II/II | 318 (5.3%) | 51 | 225 | 525 | 2100 | 2100 |
| 2.5 | NN: Imidacloprid | II | 359 (5.9%) | 51 | 100 | 236 | 840 | 2100 |
| 2.6 | AV: Abamectin/Emamectin | Ib | 2900 (48.0%) | 56 | 116 | 368 | 590 | 767 |
| 3. | Fungicide | 3917 (64.8%) | 17 | 38 | 94 | 203 | 313 | |
| 3.1 | Phenylamide: Metalaxyl | II | 345 (5.7%) | 56 | 116 | 396 | 767 | 990 |
| 3.2 | Inorganic: Bordeaux mixture/CuSO4 | II | 246 (4.1%) | 78 | 212 | 457 | 1053 | 1200 |
| 3.3 | Dithiocarbamate (DT): | 728 (12.0%) | 51 | 109 | 236 | 668 | 990 | |
| 3.3.1 | Mancozeb | U | 547 (9.0%) | 51 | 100 | 225 | 457 | 767 |
| 3.3.2 | Maneb/Zineb | U/U | 161 (2.7%) | 51 | 100 | 230 | 825 | 990 |
| 3.3.3 | Propineb | U | 151 (2.5%) | 51 | 100 | 236 | 840 | 990 |
| 4. | Rodenticide | 667 (11.0%) | 40 | 100 | 236 | 590 | 930 | |
| 5. | Molluscicide | 1219 (20.2%) | 40 | 100 | 173 | 396 | 840 | |
| No. | Neurotic Symptoms | All(n = 6974) | No Pesticide Group(n = 929) | Pesticide Group(n = 6045) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| 1. | Sleeping problems | 560 (8.0%) | 70 (7.5%) | 490 (8.1%) |
| 2. | Headache | 427 (6.1%) | 47 (5.1%) | 380 (6.3%) |
| 3. | Lack of appetite | 292 (4.2%) | 30 (3.2%) | 262 (4.3%) |
| 4. | Feeling nervous | 242 (3.5%) | 30 (3.2%) | 212 (3.5%) |
| 5. | Easily tiring | 154 (2.2%) | 20 (2.2%) | 134 (2.2%) |
| 6. | Poor digestion | 142 (2.0%) | 17 (1.8%) | 125 (2.1%) |
| 7. | Shaking hands | 142 (2.0%) | 13 (1.4%) | 129 (2.1%) |
| 8. | Being frightened | 94 (1.3%) | 11 (1.2%) | 83 (1.4%) |
| 9. | Not thinking clearly | 92 (1.3%) | 6 (0.6%) | 86 (1.4%) |
| 10. | Being unhappy | 68 (1.0%) | 12 (1.3%) | 56 (0.9%) |
| 11. | Always feeling tried | 62 (0.9%) | 9 (1.0%) | 53 (0.9%) |
| 12. | Work suffering | 53 (0.8%) | 8 (0.9%) | 45 (0.7%) |
| 13. | Difficulty with decision-making | 46 (0.7%) | 8 (0.9%) | 38 (0.6%) |
| 14. | Not enjoying activities | 39 (0.6%) | 4 (0.4%) | 35 (0.6%) |
| 15. | Stomach problems | 34 (0.5%) | 2 (0.2%) | 32 (0.5%) |
| 16. | Loss of interest in life | 28 (0.4%) | 2 (0.2%) | 26 (0.4%) |
| 17. | Crying more than normally | 25 (0.4%) | 3 (0.3%) | 22 (0.4%) |
| 18. | Feeling worthless | 24 (0.3%) | 3 (0.3%) | 21 (0.3%) |
| 19. | Thinking of ending life | 24 (0.3%) | 3 (0.3%) | 21 (0.3%) |
| 20. | Not feeling life is useful | 21 (0.3%) | 4 (0.4%) | 17 (0.3%) |
| Percentile 90 (≥2 symptoms) | 537 (7.7%) | 68 (7.3%) | 469 (7.8%) | |
| Percentile 95 (≥3 symptoms) | 303 (4.3%) | 36 (3.9%) | 267 (4.4%) | |
| Probable mental disorder | 101 (1.4%) | 8 (0.9%) | 93 (1.5%) |
| Variable | ≥2 Symptoms | ≥3 Symptoms | ≥6 Symptoms |
|---|---|---|---|
| AOR (95%CI) | AOR (95%CI) | AOR (95%CI) | |
| Pesticide poisoning (n = 6045) | |||
| No (n = 5000) | 1 | 1 | 1 |
| Yes (n = 1045) | 8.38 (6.84, 10.27) * | 8.41 (6.48, 10.92) * | 7.97 (5.16, 12.31) * |
| Cumulative exposure to any pesticide | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Low exposure (<P50) (n = 3026) | 0.95 (0.71, 1.27) | 0.91 (0.61, 1.34) | 1.05 (0.47, 2.34) |
| Medium exposure (P50–P90) (n = 2424) | 1.33 (0.99, 1.77) | 1.31 (0.88, 1.94) | 1.97 (0.90, 4.28) |
| High exposure (>P90) (n = 595) | 1.98 (1.39, 2.82) * | 3.07 (1.98, 4.75) * | 6.24 (2.80, 13.89) * |
| Cumulative exposure: functional group | |||
| Herbicide | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 606) | 0.89 (0.59, 1.34) | 0.99 (0.58, 1.69) | 1.30 (0.47, 3.62) |
| Low exposure (<P50) (n = 2824) | 0.89 (0.66, 1.19) | 0.82 (0.55, 1.23) | 0.93 (0.41, 2.12) |
| Medium exposure (P50–P90) (n = 2078) | 1.59 (1.19, 2.13) * | 1.65 (1.12, 2.43) * | 2.78 (1.29, 5.99) * |
| High exposure (>P90) (n = 537) | 1.81 (1.25, 2.61) * | 2.64 (1.67, 4.17) * | 4.62 (2.00, 10.67) * |
| Insecticide | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 526) | 0.77 (0.49, 1.20) | 0.63 (0.33, 1.19) | 0.55 (0.14, 2.09) |
| Low exposure (<P50) (n = 2777) | 0.97 (0.72, 1.30) | 0.98 (0.66, 1.45) | 1.58 (0.72, 3.45) |
| Medium exposure (P50–P90) (n = 2200) | 1.51 (1.13, 2.02) * | 1.58 (1.07, 2.33) * | 2.40 (1.11, 5.18) * |
| High exposure (>P90) (n = 542) | 1.61 (1.10, 2.34) * | 2.23 (1.39, 3.57) * | 3.28 (1.36, 7.94) * |
| Fungicide | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 2128) | 1.45 (1.08, 1.94) * | 1.29 (0.87, 1.91) | 1.30 (0.58, 2.92) |
| Low exposure (<P50) (n = 2082) | 0.90 (0.65, 1.22) | 1.00 (0.66, 1.52) | 1.68 (0.75, 3.76) |
| Medium exposure (P50–P90) (n = 1449) | 1.22 (0.89, 1.68) | 1.48 (0.98, 2.25) | 3.06 (1.39, 6.70) * |
| High exposure (>P90) (n = 386) | 1.23 (0.79, 1.93) | 1.66 (0.95, 2.91) | 2.80 (1.03, 7.59) * |
| Rodenticide | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 5378) | 1.05 (0.80, 1.38) | 1.01 (0.70, 1.46) | 1.32 (0.6 2.79) |
| Low exposure (<P50) (n = 334) | 1.36 (0.85, 2.16) | 1.65 (0.92, 2.98) | 3.56 (1.34, 9.45) * |
| High exposure (>P50) (n = 333) | 3.95 (2.73, 5.71) * | 5.99 (3.82, 9.39) * | 10.89 (4.83, 24.56) * |
| Molluscicide | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 4826) | 0.94 (0.71, 1.24) | 0.85 (0.58, 1.24) | 0.94 (0.43, 2.03) |
| Low exposure (<P50) (n = 629) | 1.58 (1.09, 2.28) * | 1.85 (1.14, 2.99) * | 4.08 (1.73, 9.63) * |
| High exposure (>P50) (n = 590) | 3.15 (2.26, 4.40) * | 4.80 (3.15, 7.31) * | 9.63 (4.38, 21.18) * |
| Cumulative exposure: chemical class | |||
| Glycine: Glyphosate | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 1695) | 0.89 (0.64, 1.22) | 0.81 (0.52, 1.26) | 1.06 (0.44, 2.53) |
| Low exposure (<P50) (n = 2129) | 0.86 (0.63, 1.17) | 0.85 (0.56, 1.30) | 1.09 (0.47, 2.52) |
| Medium exposure (P50–P90) (n = 1782) | 1.75 (1.30, 2.35) * | 1.90 (1.28, 2.81) * | 3.09 (1.43, 6.67) * |
| High exposure (>P90) (n = 439) | 1.97 (1.35, 2.87) * | 2.63 (1.64, 4.22) * | 4.51 (1.91, 10.65) * |
| Bipyridylium: Paraquat | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 2092) | 1.04 (.77, 1.41) | 0.95 (0.63, 1.44) | 1.33 (0.59, 3.04) |
| Low exposure (<P50) (n = 1978) | 0.85 (0.61, 1.16) | 0.87 (0.57, 1.33) | 0.97 (0.41, 2.29) |
| Medium exposure (P50–P90) (n = 1595) | 1.80 (1.33, 2.43) * | 2.03 (1.37, 3.01) * | 3.35 (1.55, 7.25) * |
| High exposure (>P90) (n = 380) | 1.58 (1.04, 2.40) * | 2.14 (1.28, 3.59) * | 4.15 (1.69, 10.20) * |
| Phenoxy: 2,4-D | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 5026) | 1.06 (0.81, 1.40) | 1.07 (0.74, 1.54) | 1.58 (0.75, 3.34) |
| Low exposure (<P50) (n = 527) | 1.20 (0.79, 1.81) | 0.97 (0.54, 1.76) | 1.29 (0.41, 3.99) |
| High exposure (>P50) (n = 492) | 2.58 (1.81, 3.67) * | 3.74 (2.41, 5.82) * | 5.92 (2.59, 13.51) * |
| Chloroacetamide/Anilide | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 5331) | 1.06 (0.81, 1.40) | 1.10 (0.76, 1.58) | 1.55 (0.73, 3.26) |
| Low exposure (<P50) (n = 370) | 1.67 (1.09, 2.57) * | 1.65 (0.93, 2.94) | 2.40 (0.82, 7.05) |
| High exposure (>P50) (n = 344) | 2.93 (2.00, 4.29) * | 3.70 (2.29, 5.99) * | 7.41 (3.17, 17.31) * |
| Organochlorine | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 5248) | 1.02 (0.78, 1.34) | 0.99 (0.68, 1.43) | 1.36 (0.64, 2.88) |
| Low exposure (<P50) (n = 401) | 1.77 (1.17, 2.66) * | 1.95 (1.14, 3.34) * | 2.89 (1.06, 7.86) * |
| High exposure (>P50) (n = 396) | 3.05 (2.12, 4.39) * | 4.63 (2.95, 7.25) * | 9.26 (4.08, 20.99) * |
| Organophosphate | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 4678) | 1.09 (0.83, 1.44) | 1.07 (0.74, 1.55) | 1.50 (0.71, 3.17) |
| Low exposure (<P50) (n = 695) | 0.85 (0.56, 1.28) | 1.07 (0.63, 1.81) | 1.46 (0.54, 3.96) |
| High exposure (>P50) (n = 672) | 2.27 (1.62, 3.18) * | 2.88 (1.87, 4.42) * | 5.35 (2.40, 11.95) * |
| Carbamate | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 4373) | 0.96 (0.72, 1.26) | 0.97 (0.66, 1.41) | 1.36 (0.64, 2.91) |
| Low exposure (<P50) (n = 868) | 1.19 (0.83, 1.70) | 1.00 (0.61, 1.66) | 1.38 (0.56, 3.64) |
| High exposure (>P50) (n = 804) | 2.60 (1.89, 3.58) * | 3.37 (2.23, 5.09) * | 5.97 (2.71, 13.19) * |
| Pyrethroid: Permethrin/Cypermethrin | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 5727) | 1.18 (0.90, 1.54) | 1.22 (0.85, 1.75) | 1.87 (0.90, 3.91) |
| Ever used pyrethroid (n = 318) | 1.48 (0.93, 2.36) | 2.11 (1.20, 3.69) * | 2.60 (0.89, 7.63) |
| Neonicotinoid: Imidacloprid | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 5686) | 1.16 (0.88, 1.52) | 1.23 (0.86, 1.77) | 1.87 (0.90, 3.91) |
| Ever used imidacloprid (n = 359) | 1.79 (1.16, 2.75) * | 1.72 (0.96, 3.10) | 2.65 (0.90, 7.80) |
| Avermectin: Abamectin/Emamectin | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 3145) | 1.23 (0.93, 1.63) | 1.31 (0.90, 1.91) | 2.05 (0.96, 4.35) |
| Low exposure (<P50) (n = 1521) | 0.79 (0.56, 1.11) | 0.82 (0.52, 1.29) | 1.29 (0.54, 3.12) |
| High exposure (>P50) (n = 1379) | 1.57 (1.15, 2.15) * | 1.64 (1.09, 2.48) * | 2.20 (0.97, 4.99) |
| Dithiocarbamate | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 5317) | 1.16 (0.88, 1.52) | 1.21 (0.84, 1.74) | 1.78 (0.85, 3.73) |
| Low exposure (<P50) (n = 364) | 0.81 (0.48, 1.37) | 1.05 (0.55, 2.02) | 1.78 (0.57, 5.55) |
| High exposure (>P50) (n = 364) | 2.05 (1.36, 3.07) * | 2.24 (1.32, 3.82) * | 4.14 (1.60, 10.68) * |
| Phenylamide: Metalaxyl | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 5700) | 1.15 (0.88, 1.51) | 1.22 (0.85, 1.75) | 1.86 (0.89, 3.89) |
| Ever used metalaxyl (n = 345) | 1.93 (1.27, 2.94) * | 1.98 (1.14, 3.45) * | 2.69 (0.96, 7.56) |
| Inorganic: Bordeaux mixture/CuSO4 | |||
| No history of pesticide use (n = 929) | 1 | 1 | 1 |
| Ever used other pesticides (n = 5799) | 1.15 (0.87, 1.50) | 1.19 (0.83, 1.71) | 1.75 (0.84, 3.66) |
| Ever used CuSO4 (n = 246) | 2.35 (1.50, 3.68) * | 2.94 (1.68, 5.15) * | 5.82 (2.24, 15.10) * |
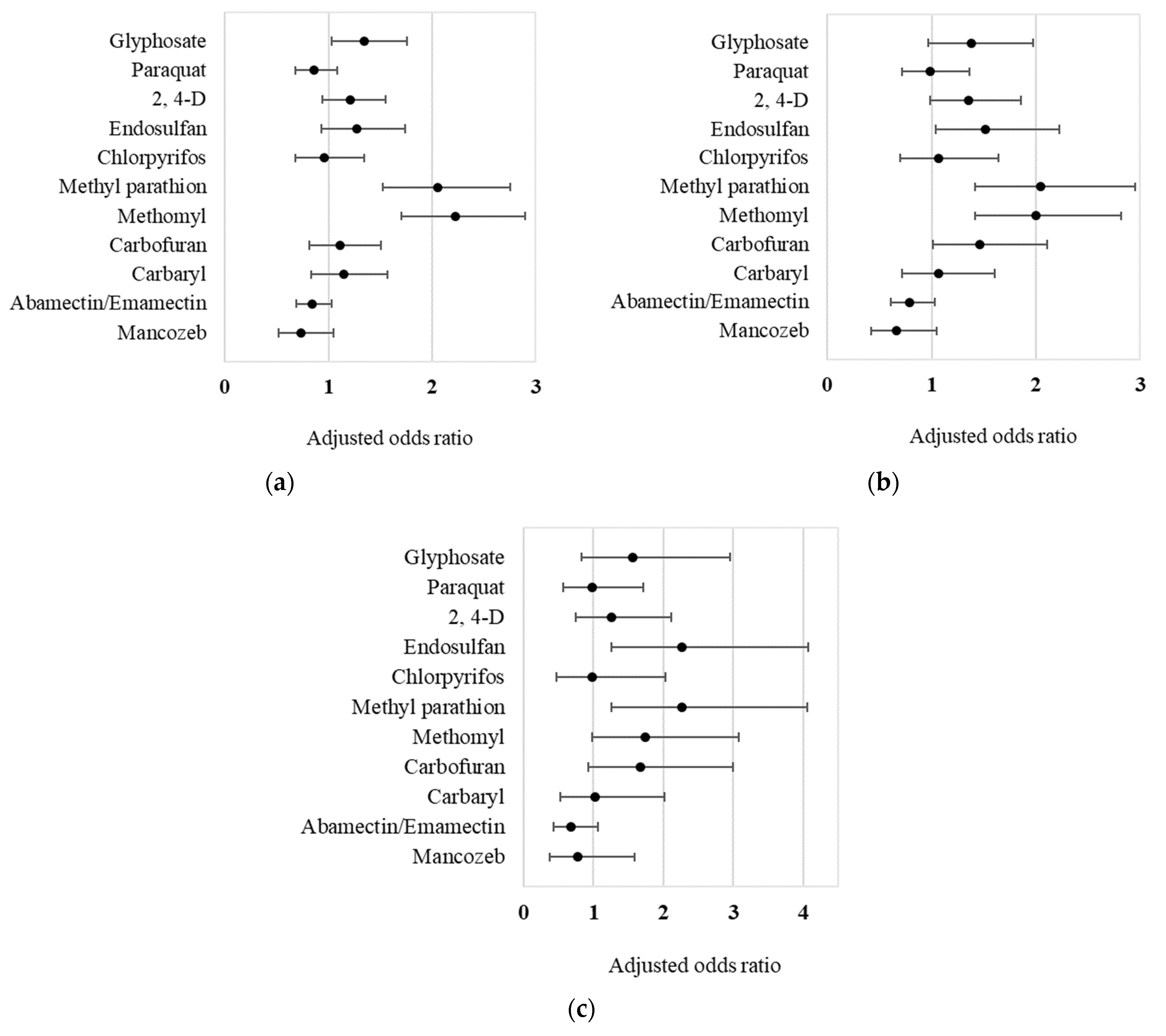
| Pesticide Active Ingredient | ≥2 Symptoms | ≥3 Symptoms | ≥6 Symptoms |
|---|---|---|---|
| (Ever Used vs. Never Used) | AOR (95%CI) | AOR (95%CI) | AOR (95%CI) |
| Single model | |||
| Glyphosate (n = 4489) | 1.57 (1.24, 2.00) * | 1.78 (1.29, 2.46) * | 2.11 (1.19, 3.76) * |
| Paraquat (n = 3953) | 1.24 (1.01, 1.52) * | 1.51 (1.14, 2.01) * | 1.67 (1.01, 2.74) * |
| 2,4-D (n = 1019) | 1.74 (1.38, 2.18) * | 2.12 (1.60, 2.81) * | 2.24 (1.41, 3.54) * |
| Endosulfan (n = 555) | 2.27 (1.74, 2.96) * | 2.97 (2.15, 4.10) * | 4.34 (2.66, 7.09) * |
| Chlorpyrifos (n = 524) | 1.36 (1.00, 1.85) * | 1.58 (1.08, 2.31) * | 1.51 (0.79, 2.88) |
| Methyl parathion (n = 521) | 3.13 (2.42, 4.04) * | 3.67 (2.68, 5.03) * | 4.44 (2.71, 7.29) * |
| Methomyl (n = 811) | 2.98 (2.39, 3.72) * | 3.22 (2.43, 4.27) * | 3.45 (2.18, 5.45) * |
| Carbofuran (n = 607) | 1.88 (1.43, 2.47) * | 2.62 (1.89, 3.63) * | 3.31 (1.97, 5.57) * |
| Carbaryl (n = 556) | 1.77 (1.34, 2.35) * | 1.89 (1.32, 2.71) * | 1.94 (1.06, 3.54) * |
| Abamectin/Emamectin (n = 2900) | 0.94 (0.77, 1.14) | 0.92 (0.72, 1.19) | 0.85 (0.56, 1.30) |
| Mancozeb (n = 547) | 1.11 (0.80, 1.54) | 1.11 (0.73, 1.71) | 1.31 (0.67, 2.56) |
| Multiple model | |||
| Glyphosate (n = 4489) | 1.35 (1.03, 1.75) * | 1.38 (0.96, 1.97) | 1.57 (0.83, 2.96) |
| Paraquat (n = 3953) | 0.86 (0.68, 1.09) | 0.99 (0.72, 1.36) | 0.99 (0.57, 1.71) |
| 2,4-D (n = 1019) | 1.21 (0.94, 1.55) | 1.35 (0.98, 1.85) | 1.26 (0.75, 2.11) |
| Endosulfan (n = 555) | 1.27 (0.93, 1.74) | 1.52 (1.04, 2.23) * | 2.27 (1.26, 4.08) * |
| Chlorpyrifos (n = 524) | 0.96 (0.68, 1.35) | 1.07 (0.70, 1.64) | 0.98 (0.47, 2.03) |
| Methyl parathion (n = 521) | 2.05 (1.53, 2.76) * | 2.04 (1.41, 2.95) * | 2.26 (1.26, 4.06) * |
| Methomyl (n = 811) | 2.22 (1.71, 2.89) * | 2.00 (1.42, 2.81) * | 1.74 (0.98, 3.09) |
| Carbofuran (n = 607) | 1.11 (0.82, 1.50) | 1.46 (1.01, 2.11) * | 1.66 (0.93, 2.99) |
| Carbaryl (n = 556) | 1.14 (0.83, 1.57) | 1.07 (0.71, 1.60) | 1.02 (0.52, 2.02) |
| Abamectin/Emamectin (n = 2900) | 0.84 (0.69, 1.03) | 0.79 (0.61, 1.03) | 0.67 (0.43, 1.06) |
| Mancozeb (n = 547) | 0.74 (0.52, 1.05) | 0.66 (0.42, 1.05) | 0.77 (0.38, 1.59) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong-Artborirak, P.; Boonchieng, W.; Juntarawijit, Y.; Juntarawijit, C. Potential Effects on Mental Health Status Associated with Occupational Exposure to Pesticides among Thai Farmers. Int. J. Environ. Res. Public Health 2022, 19, 9654. https://doi.org/10.3390/ijerph19159654
Ong-Artborirak P, Boonchieng W, Juntarawijit Y, Juntarawijit C. Potential Effects on Mental Health Status Associated with Occupational Exposure to Pesticides among Thai Farmers. International Journal of Environmental Research and Public Health. 2022; 19(15):9654. https://doi.org/10.3390/ijerph19159654
Chicago/Turabian StyleOng-Artborirak, Parichat, Waraporn Boonchieng, Yuwayong Juntarawijit, and Chudchawal Juntarawijit. 2022. "Potential Effects on Mental Health Status Associated with Occupational Exposure to Pesticides among Thai Farmers" International Journal of Environmental Research and Public Health 19, no. 15: 9654. https://doi.org/10.3390/ijerph19159654
APA StyleOng-Artborirak, P., Boonchieng, W., Juntarawijit, Y., & Juntarawijit, C. (2022). Potential Effects on Mental Health Status Associated with Occupational Exposure to Pesticides among Thai Farmers. International Journal of Environmental Research and Public Health, 19(15), 9654. https://doi.org/10.3390/ijerph19159654






