Comparison of Cholangiocarcinoma and Hepatocellular Carcinoma Incidence Trends from 1993 to 2012 in Lampang, Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
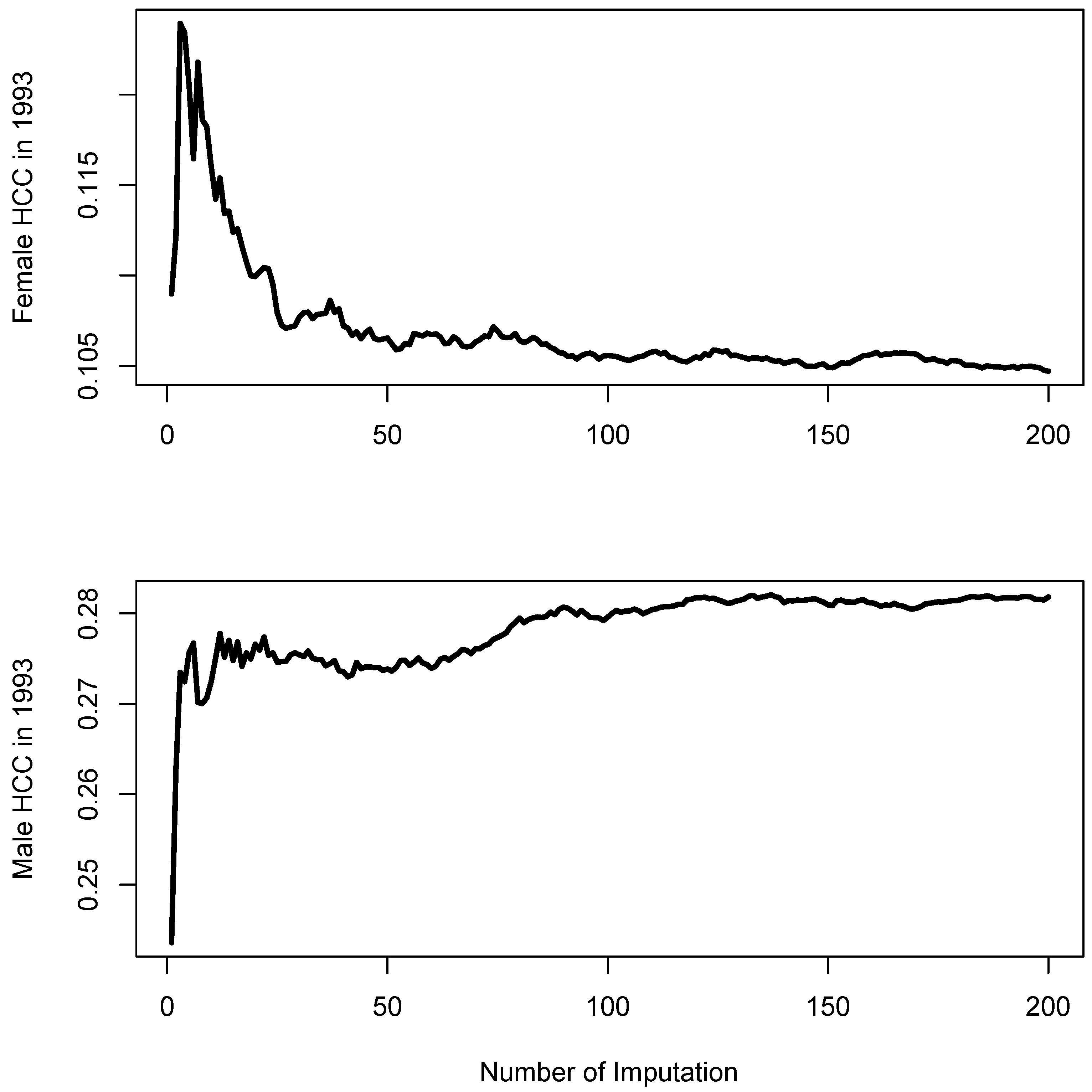
2.2. Multiple Imputation
2.3. Joinpoint Analysis
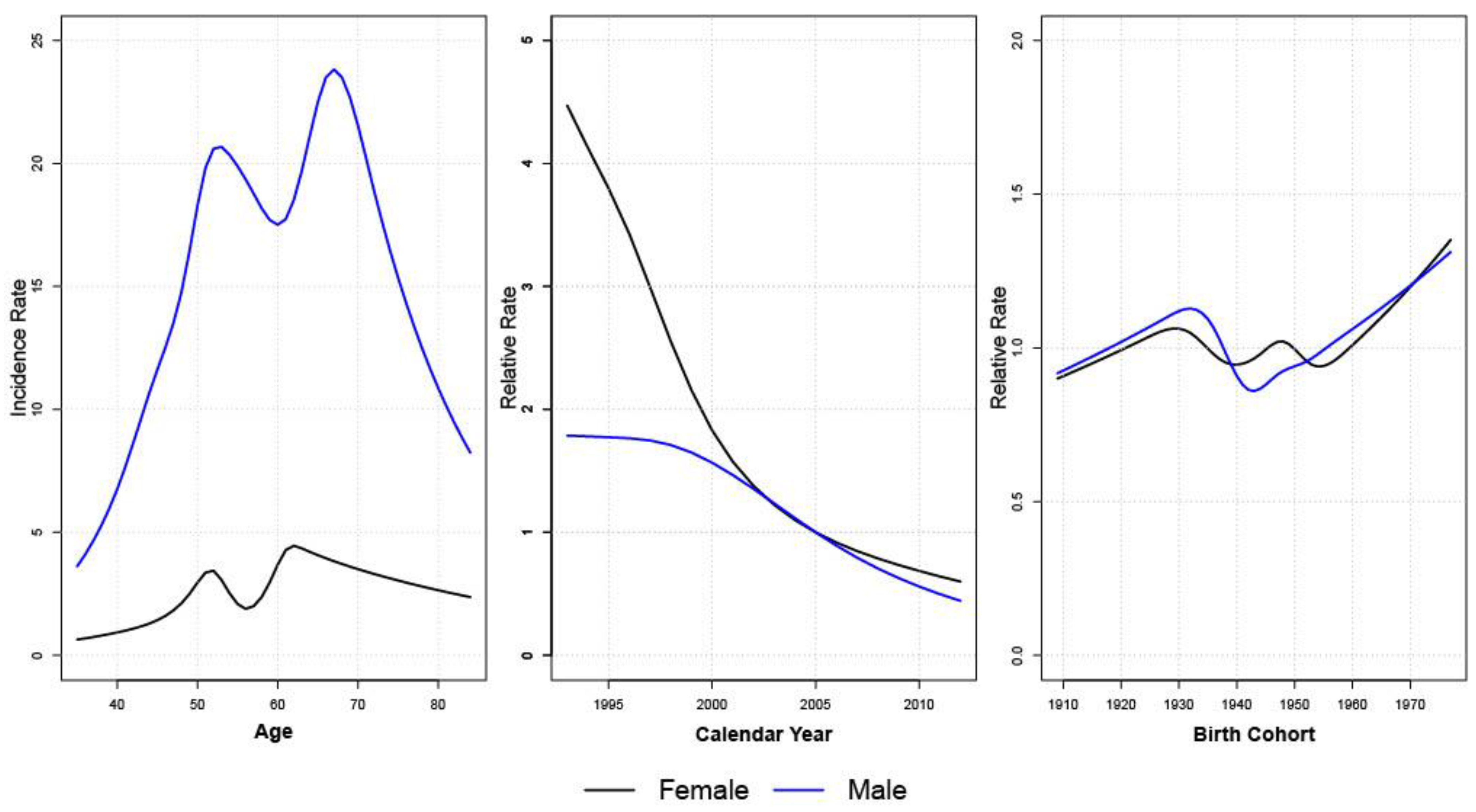
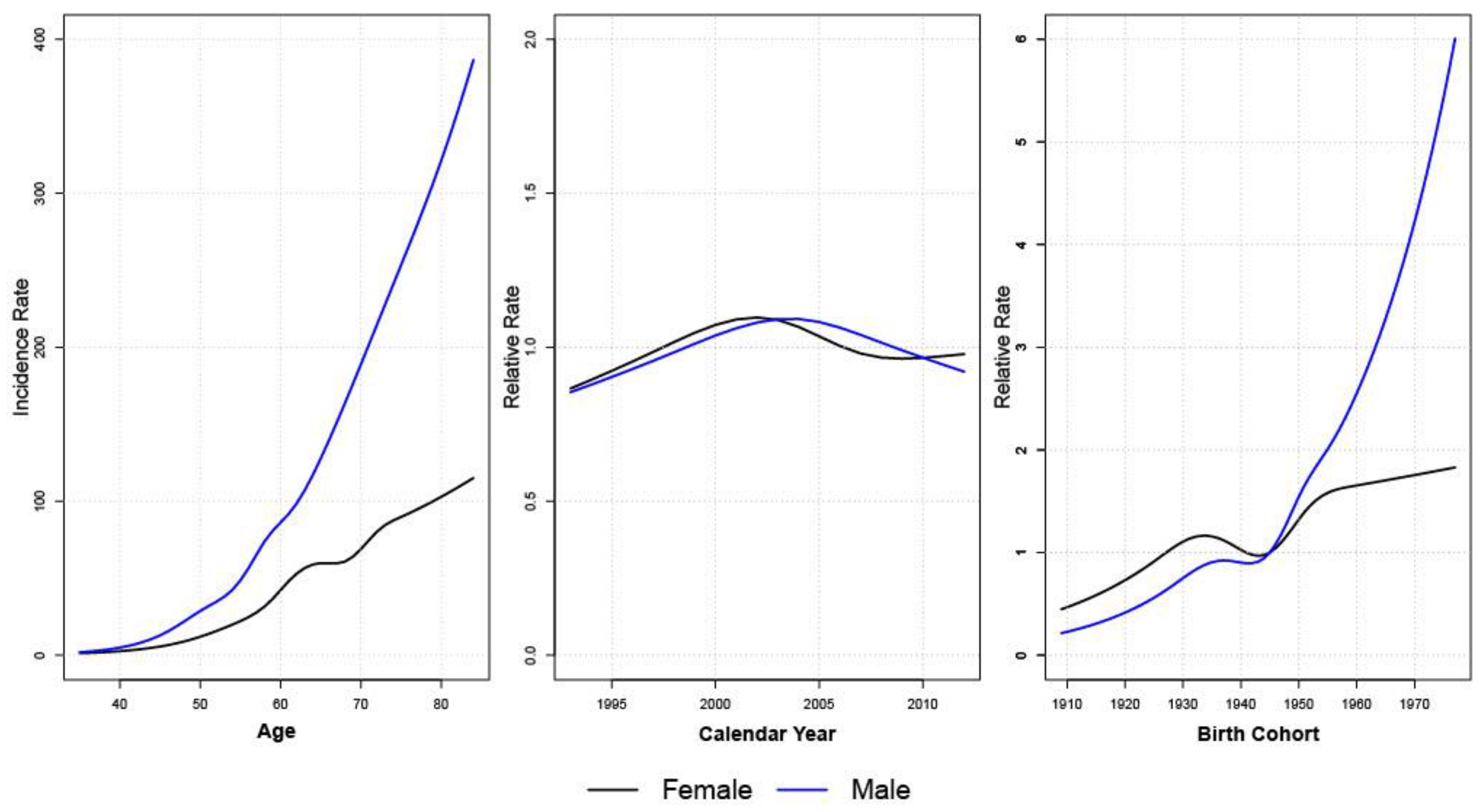
2.4. Age-Period-Cohort Analysis
3. Results
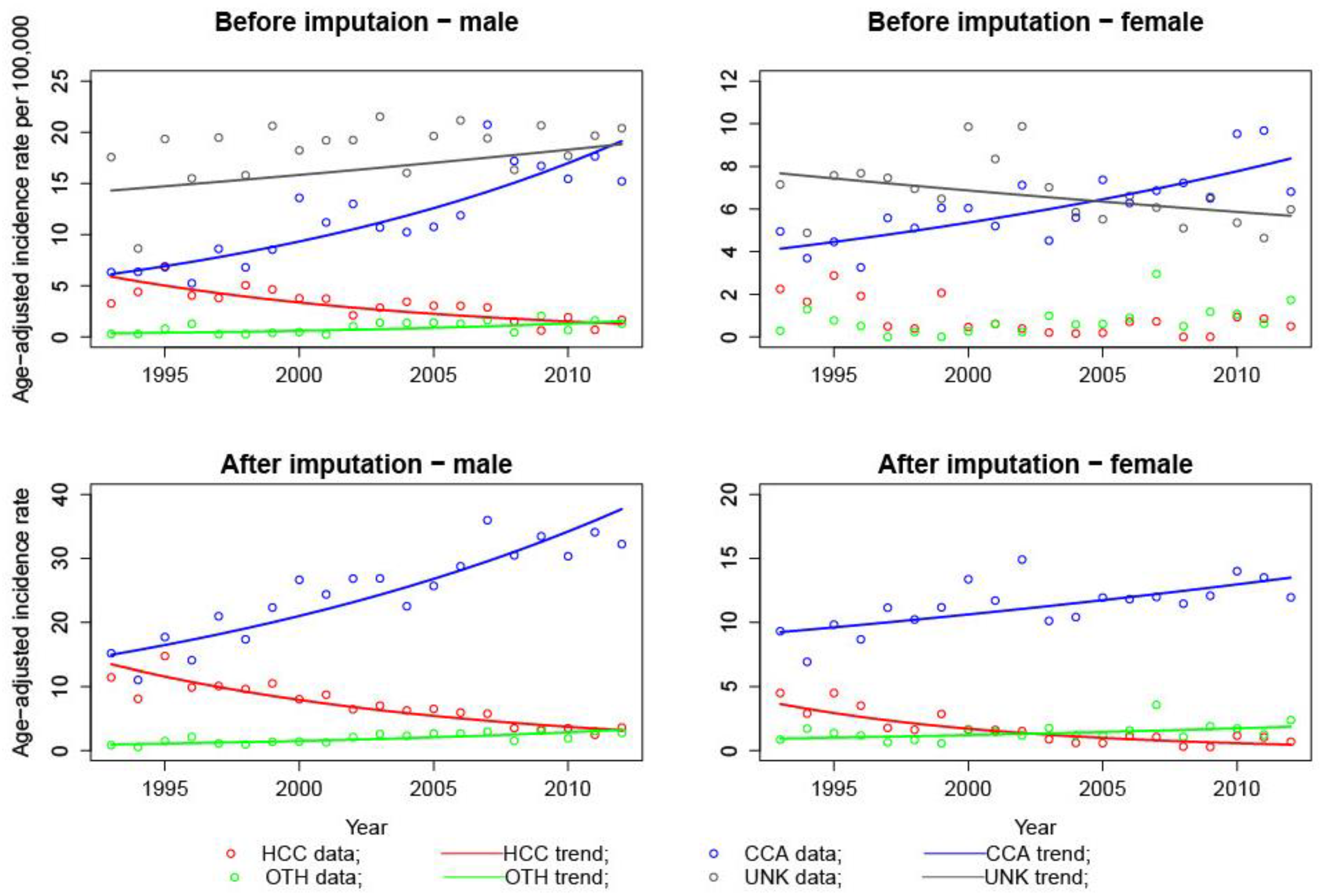
3.1. Multiple Imputation
3.2. Jointpoint Analyses
3.3. Age-Period-Cohort Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | All (N = 4921) | HCC (N = 370) | CCA (N = 1892) | Other (N = 172) | Unknown (N = 2487) | p-Value * |
|---|---|---|---|---|---|---|
| Age at diagnosis, Mean (SD) | 60.9 (13.3) | 54.6 (12.4) | 63.0 (12.3) | 61.0 (15.1) | 60.1 (13.6) | <0.001 |
| Sex, Number (%) | <0.001 | |||||
| Male | 3389 (68.9) | 294 (79.5) | 1222 (64.6) | 89 (51.7) | 1784 (71.7) | |
| Female | 1532 (31.1) | 76 (20.5) | 670 (35.4) | 83 (48.3) | 703 (28.3) | |
| Year of Diagnosis, Number (%) | <0.001 | |||||
| 1993–1997 | 817 (16.6) | 125 (33.8) | 214 (11.3) | 22 (12.8) | 456 (18.3) | |
| 1998–2002 | 1115 (22.7) | 106 (28.6) | 377 (19.9) | 17 (9.8) | 615 (24.7) | |
| 2003–2007 | 1353 (27.5) | 89 (24.1) | 519 (27.4) | 67 (39.0) | 678 (27.3) | |
| 2008–2012 | 1636 (33.2) | 50 (13.5) | 782 (41.3) | 66 (38.4) | 738 (29.7) | |
| Stage, Number (%) | <0.001 | |||||
| 1 | 59 (1.2) | 9 (2.4) | 28 (1.5) | 6 (3.5) | 16 (0.6) | |
| 2 | 987 (20.0) | 182 (49.2) | 418 (22.1) | 34 (19.8) | 353 (14.2) | |
| 3 | 123 (2.5) | 14 (3.8) | 63 (3.3) | 13 (7.6) | 33 (1.3) | |
| 4 | 1139 (23.1) | 70 (18.9) | 510 (27.0) | 68 (39.5) | 491 (19.7) | |
| Unknown | 2613 (53.1) | 95 (25.7) | 873 (46.1) | 51 (29.7) | 1594 (64.1) | |
| Living area ** | <0.001 |
| Sex | Histology | p-Value | Average Annual Percent Change Assuming Parallelism |
|---|---|---|---|
| Male | HCC | 0.92 | 7.5 |
| CCA | 0.38 | 5.6 | |
| OTH | 0.19 | 7.4 | |
| Female | HCC | N/A 1 | N/A 1 |
| CCA | <0.001 | N/A 2 | |
| OTH | N/A 1 | N/A 1 |
References
- Khuhaprema, T.; Attasara, P.; Sriplung, H.; Wiangnon, S.; Sangrajrang, S. Cancer in Thailand Volume VII, 2007–2009; National Cancer Institute: Bangkok, Thailand, 2013.
- Imsamran, W.; Chaiwerawattana, A.; Wiangnon, S.; Pongnikorn, D.; Suwanrungrung, K.; Sangrajrang, S.; Buasom, R. Cancer in Thailand Volume VIII, 2010–2012; National Cancer Institute: Bangkok, Thailand, 2015.
- Khuhaprema, T.; Srivatanakul, P.; Attasara, P.; Sriplung, H.; Wiangnon, S.; Sumitsawan, Y. Cancer in Thailand Volume V, 2001–2003; National Cancer Institute: Bangkok, Thailand, 2010.
- Khuhaprema, T.; Attasara, P.; Sriplung, H.; Wiangnon, S.; Sumitsawan, Y.; Sangrajrang, S. Cancer in Thailand Volumn VI, 2004–2006; National Cancer Institute: Bangkok, Thailand, 2012.
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini—multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Kaewpitoon, N.; Kaewpitoon, S.J.; Pengsaa, P. Opisthorchiasis in Thailand: Review and current status. World J. Gastroenterol. 2008, 14, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Suwannahitatorn, P.; Webster, J.; Riley, S.; Mungthin, M.; Donnelly, C.A. Uncooked fish consumption among those at risk of Opisthorchis viverrini infection in central Thailand. PLoS ONE 2019, 14, e0211540. [Google Scholar] [CrossRef] [PubMed]
- Yeesoonsang, S.; McNeil, E.; Virani, S.; Bilheem, S.; Pittayawonganon, C.; Jiraphongsa, C.; Sriplung, H. Trends in Incidence of Two Major Subtypes of Liver and Bile Duct Cancer: Hepatocellular Carcinoma and Cholangiocarcinoma in Songkhla, Southern Thailand, 1989–2030. J. Cancer Epidemiol. 2018, 2018, 8267059. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Tangkawattana, S.; Laha, T.; Kaewkes, S.; Mallory, F.F.; Smith, J.F.; Wilcox, B.A. Toward integrated opisthorchiasis control in northeast Thailand: The Lawa project. Acta Trop. 2015, 141, 361–367. [Google Scholar] [CrossRef]
- Bismuth, H.; Castaing, D.; Traynor, O. Resection or Palliation: Priority of Surgery in the Treatment of Hilar Cancer. World J. Surg. 1988, 12, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.R.A.; Clin, G.G.J. Cholangiocarcinoma. Clin. Liver Dis. 2008, 12, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Yeesoonsang, S.; Bilheem, S.; McNeil, E.; Iamsirithaworn, S.; Jiraphongsa, C.; Sriplung, H. Estimation of the Incidence of Hepatocellular Carcinoma and Cholangiocarcinoma in Songkhla, Thailand, 1989–2013, Using Multiple Imputation Method. Cancer Res. Treat. 2017, 49, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sriplung, H.; Yeesoonsang, S.; McNeil, E.; Bilheem, S. The use of a multiple imputation method to investigate the trends in Histologic types of lung cancer in Songkhla province, Thailand, 1989–2013. BMC Cancer 2016, 16, 389. [Google Scholar] [CrossRef]
- Graham, J.W.; Olchowski, A.E.; Gilreath, T.D. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev. Sci. 2007, 8, 206–213. [Google Scholar] [CrossRef]
- van Buuren, K.S. Groothuis-Oudshoorn, mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Joinpoint Regression Program. Statistical Methodology and Applications Branch, Surveillance Research Program; National Cancer Institute: Bethesda, MD, USA, 2020.
- Holford, T.R. The estimation of Age, Period and Cohort Effects for Vital Rates. Biometrics 1983, 39, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, B.; Plummer, M.; Laara, E.; Hills, M. Epi: A Package for Statistical Analysis in Epidemiology. R Package Version 2.44. 2021. Available online: https://CRAN.R-project.org/package=Epi (accessed on 8 July 2022).
- Wiangnon, S.; Kamsa-Ard, S.; Suwanrungruang, K.; Promthet, S.; Kamsa-Ard, S.; Mahaweerawat, S.; Khuntikeo, N. Trends in Incidence of Hepatocellular Carcinoma, 1990–2009, Khon Kaen, Thailand. Asian Pac. J. Cancer Prev. 2012, 13, 1065–1068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adami, H.O.; Hunter, D.; Trichopoulos, D. Textbook of Cancer Epidemiology; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Posuwan, N.; Wanlapakorn, N.; Sa-Nguanmoo, P.; Wasitthankasem, R.; Vichaiwattana, P.; Klinfueng, S.; Poovorawan, Y. The Success of a Universal Hepatitis B Immunization Program as Part of Thailand’s EPI after 22 Years’ Implementation. PLoS ONE 2016, 11, e0150499. [Google Scholar] [CrossRef] [PubMed]
- Wichajarn, K.; Kosalaraksa, P.; Wiangnon, S. Incidence of hepatocellular carcinoma in children in Khon Kaen before and after national hepatitis B vaccine program. Asian Pac. J. Cancer Prev. 2008, 9, 507–509. [Google Scholar] [PubMed]
- McAteer, J.P.; Goldin, A.B.; Healey, P.J.; Gow, K.W. Hepatocellular carcinoma in children: Epidemiology and the impact of regional lymphadenectomy on surgical outcomes. J. Pediatr. Surg. 2013, 48, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Wasitthankasem, R.; Posuwan, N.; Vichaiwattana, P.; Theamboonlers, A.; Klinfueng, S.; Vuthitanachot, V.; Poovorawan, Y. Decreasing Hepatitis C Virus Infection in Thailand in the Past Decade: Evidence from the 2014 National Survey. PLoS ONE 2016, 11, e0149362. [Google Scholar] [CrossRef]
- Rojanapithayakorn, W.; Hanenberg, R. The 100% condom program in Thailand. Aids 1996, 10, 1–7. [Google Scholar] [CrossRef]
- Sexual Transmission and Viral Hepatitis|CDC. 22 April 2021. Available online: https://www.cdc.gov/hepatitis/populations/stds.htm (accessed on 8 July 2022).
- Levy, D.T.; Benjakul, S.; Ross, H.; Ritthiphakdee, B. The role of tobacco control policies in reducing smoking and deaths in a middle income nation: Results from the Thailand SimSmoke simulation model. Tob. Control 2008, 17, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Asia Warms Up to American Fast Food: Trends: U.S. Chains are Most Prevalent in Japan, Where There are 350 McDonald’s Franchises and 649 Kentucky Fried Chicken Outlets. Available online: http://articles.latimes.com/1989-12-17/business/fi-1825_1_american-fast-food (accessed on 8 July 2022).
- Gallstones—Symptoms and Causes. Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/gallstones/symptoms-causes/syc-20354214 (accessed on 8 July 2022).
- Nordenstedt, H.; Mattsson, F.; El-Serag, H.; Lagergren, J. Gallstones and cholecystectomy in relation to risk of intra- and extrahepatic cholangiocarcinoma. Br. J. Cancer 2012, 106, 1011–1115. [Google Scholar] [CrossRef] [PubMed]
- Kamsa-ard, S.; Wiangnon, S.; Suwanrungruang, K.; Promthet, S.; Khuntikeo, N.; Kamsa-ard, S.; Mahaweerawat, S. Trends in liver cancer incidence between 1985 and 2009, Khon Kaen, Thailand: Cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2011, 12, 2209–2213. [Google Scholar] [PubMed]
- Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann. Surg. 1990, 211, 277–287. [Google Scholar]
- Multiple Imputation for Missing Data in Epidemiological and Clinical Research: Potential and Pitfalls|The BMJ. Available online: https://www.bmj.com/content/338/bmj.b2393 (accessed on 10 July 2022).
- Souverein, O.W.; Zwinderman, A.H.; Tanck, M.W.T. Multiple imputation of missing genotype data for unrelated individuals. Ann. Hum. Genet. 2006, 70, 372–381. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Male Number (%) | Female Number (%) | Total Number (%) |
|---|---|---|---|
| Before Imputation | |||
| HCC | 294 (8.7) | 76 (5.0) | 370 (7.5) |
| CCA | 1222 (36.1) | 670 (43.7) | 1892 (38.4) |
| OTH | 89 (2.6) | 83 (5.4) | 172 (3.5) |
| UNK | 1784 (52.6) | 703 (45.9) | 2487 (50.5) |
| After imputation | |||
| HCC | 609 (18.9) | 144 (10.0) | 753 (16.2) |
| CCA | 2428 (75.5) | 1159 (80.1) | 3587 (77.0) |
| OTH | 177 (5.5) | 144 (10.0) | 322 (6.9) |
| Gender | Histology | Year | AAPC (95% CI) Before Imputation | AAPC (95% CI) After Imputation |
|---|---|---|---|---|
| Male | HCC | 1993–2012 | −7.69 * (−10.6, −4.7) | −7.30 * (−8.8, −5.8) |
| CCA | 1993–2012 | 6.18 * (4.6, 7.8) | 5.00 * (3.7, 6.3) | |
| OTH | 1993–2012 | 8.19 * (2.9, 13.8) | 6.64 * (3.9, 9.5) | |
| UNK | 1993–2012 | 1.46 (−0.1, 3.0) | --- | |
| Female | HCC | 1993–2012 | N/A 1 | −10.29 * |
| CCA | 1993–2012 | 3.78 * (2.3, 5.3) | 2.02 * (0.9, 3.2) | |
| OTH | 1993–2012 | N/A 1 | 3.71 * (0.5, 7.0) | |
| UNK | 1993–2012 | −1.57 * (−3.1, −0.0) | --- |
| Model | Female HCC | Female CCA | Male HCC | Male CCA |
|---|---|---|---|---|
| Akaike information criteria (AIC) for AC, AP, and APC models relative (difference) to the Age only model | ||||
| Age Cohort | −49.0 | −10.4 | −95.7 | −151.7 |
| Age Period | −55.3 2 | −6.8 | −105.5 | −143.8 |
| Age Period Cohort | −41.8 | −6.4 | −100.3 | −154.7 |
| Residual deviance values for AC, AP, and APC models | ||||
| Age | 372.0 | 981.9 | 656.9 | 1045.1 |
| Age Cohort | 309.0 | 957.5 | 547.2 | 879.4 |
| Age Period | 308.7 | 967.1 | 543.4 | 893.2 |
| Age Period Cohort | 308.2 | 953.5 | 534.6 | 868.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, P.; Rozek, L.S.; Pongnikorn, D.; Sriplung, H.; Meza, R. Comparison of Cholangiocarcinoma and Hepatocellular Carcinoma Incidence Trends from 1993 to 2012 in Lampang, Thailand. Int. J. Environ. Res. Public Health 2022, 19, 9551. https://doi.org/10.3390/ijerph19159551
Cao P, Rozek LS, Pongnikorn D, Sriplung H, Meza R. Comparison of Cholangiocarcinoma and Hepatocellular Carcinoma Incidence Trends from 1993 to 2012 in Lampang, Thailand. International Journal of Environmental Research and Public Health. 2022; 19(15):9551. https://doi.org/10.3390/ijerph19159551
Chicago/Turabian StyleCao, Pianpian, Laura S. Rozek, Donsuk Pongnikorn, Hutcha Sriplung, and Rafael Meza. 2022. "Comparison of Cholangiocarcinoma and Hepatocellular Carcinoma Incidence Trends from 1993 to 2012 in Lampang, Thailand" International Journal of Environmental Research and Public Health 19, no. 15: 9551. https://doi.org/10.3390/ijerph19159551
APA StyleCao, P., Rozek, L. S., Pongnikorn, D., Sriplung, H., & Meza, R. (2022). Comparison of Cholangiocarcinoma and Hepatocellular Carcinoma Incidence Trends from 1993 to 2012 in Lampang, Thailand. International Journal of Environmental Research and Public Health, 19(15), 9551. https://doi.org/10.3390/ijerph19159551







