Stable Gender Gap and Similar Gender Trend in Chronic Morbidities between 1997–2015 in Adult Canary Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Chronic Morbidity
2.3. Data Analysis
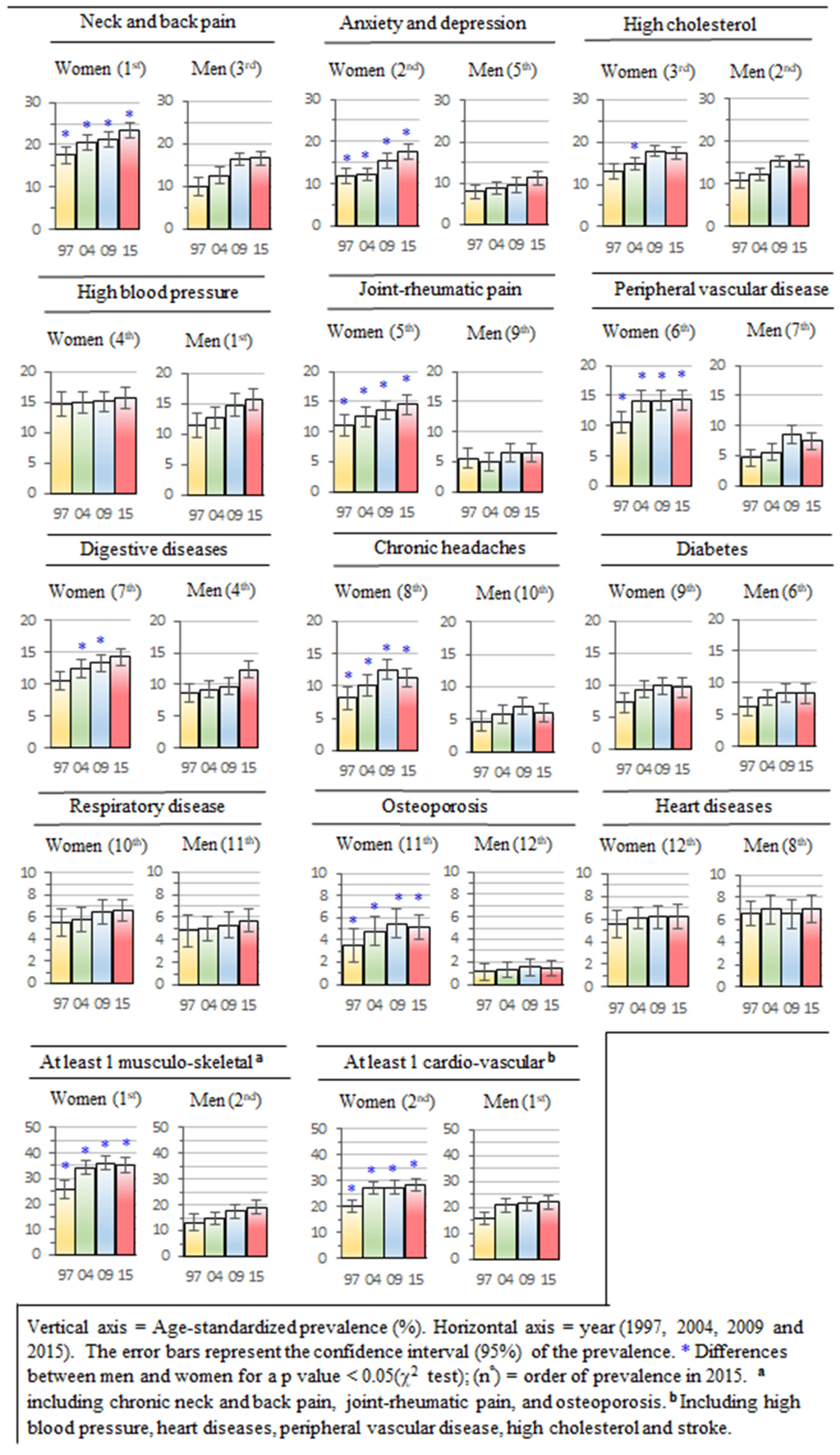
3. Results
3.1. Descriptive Analysis of Participants
3.2. Gender Differences in the Prevalence of Chronic Morbidities
3.3. Trend in the Prevalence of Chronic Morbidities for the Overall Sample
3.4. Trend of Prevalence in Chronic Morbidities for Age Groups
4. Discussion
4.1. Gender Gap in Chronic Morbidities
4.2. Trend in Chronic Morbidities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, L.; McBride, T.D.; Allen, P.; Jacob, R.R.; Jones, E.; Kerner, J.; Brownson, R.C. Health care system collaboration to address chronic diseases: A nationwide snapshot from state public health practitioners. Prev. Chronic Dis. 2014, 11, E152. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Touraine, C.; Georget, A.; Dartigues, J.F.; Commenges, D.; Jacqmin-Gadda, H. Prevalence projections of chronic diseases and impact of public health intervention. Biometrics 2013, 69, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Mayoral Cortes, J.M.; Aragones Sanz, N.; Godoy, P.; Sierra Moros, M.J.; Cano Portero, R.; Gonzalez Moran, F.; Pousa Ortega, A.; Grupo de Vigilancia Epidemiologica de la Sociedad Espanola de Epidemiologia. [Chronic diseases as a priority for the public health surveillance system in Spain]. Gac. Sanit. 2016, 30, 154–157. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preventing Chronic Diseases: A Vital Investment; World Health Organization: Geneve, Switzerland, 2005. [Google Scholar]
- Cheng, P.; Zhu, H.; Witteles, R.M.; Wu, J.C.; Quertermous, T.; Wu, S.M.; Rhee, J.W. Cardiovascular Risks in Patients with COVID-19: Potential Mechanisms and Areas of Uncertainty. Curr. Cardiol. Rep. 2020, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, E16–E25. [Google Scholar] [CrossRef] [PubMed]
- CDC. About Chronic Diseases. Available online: https://www.cdc.gov/chronicdisease/about/index.htm (accessed on 24 May 2022).
- Gorman, B.K.; Read, J.N.G. Gender Disparities in Adult Health: An Examination of Three Measures of Morbidity. J. Health Soc. Behav. 2006, 47, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Nusselder, W.J.; Cambois, E.M.; Wapperom, D.; Mesle, F.; Looman, C.W.N.; Yokota, R.T.C.; Van Oyen, H.; Jagger, C.; Robine, J.M. Women’s excess unhealthy life years: Disentangling the unhealthy life years gap. Eur. J. Public Health 2019, 29, 914–919. [Google Scholar] [CrossRef]
- Verbrugge, L.M. Gender and health: An update on hypotheses and evidence. J. Health Soc. Behav. 1985, 26, 156–182. [Google Scholar] [CrossRef] [PubMed]
- Mechakra-Tahiri, S.D.; Freeman, E.E.; Haddad, S.; Samson, E.; Zunzunegui, M.V. The gender gap in mobility: A global cross-sectional study. BMC Public Health 2012, 12, 598. [Google Scholar] [CrossRef]
- Wijnhoven, H.A.H.; de Vet, H.C.W.; Picavet, S.J.H. Explaining sex differences in chronic musculoskeletal pain in a general population. Pain 2006, 124, 158–166. [Google Scholar] [CrossRef]
- Case, A.; Paxson, C. Sex differences in morbidity and mortality. Demography 2005, 42, 189–214. [Google Scholar] [CrossRef] [PubMed]
- Kynyk, J.A.; Mastronarde, J.G.; McCallister, J.W. Asthma, the sex difference. Curr. Opin. Pulm. Med. 2011, 17, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E.K.; Weiss, S.T.; Drazen, J.M.; Chapman, H.A.; Carey, V.; Campbell, E.J.; Denish, P.; Silverman, R.A.; Celedon, J.C.; Reilly, J.J.; et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med 2000, 162, 2152–2158. [Google Scholar] [CrossRef]
- Arber, S.; Cooper, H. Gender differences in health in later life: The new paradox? Soc. Sci. Med. 1999, 48, 61–76. [Google Scholar] [CrossRef]
- Bird, C.E.; Rieker, P.P. Gender matters: An integrated model for understanding men’s and women’s health. Soc. Sci. Med. 1999, 48, 745–755. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Guéguen, A.; Ferrie, J.; Shipley, M.; Martikainen, P.; Bonenfant, S.; Goldberg, M.; Marmot, M. Gender Differences in the Association Between Morbidity and Mortality Among Middle-Aged Men and Women. Am. J. Public Health 2008, 98, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A.; Lamassa, M.; Baldereschi, M.; Pracucci, G.; Basile, A.M.; Wolfe, C.D.; Giroud, M.; Rudd, A.; Ghetti, A.; Inzitari, D. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: Data from a multicenter multinational hospital-based registry. Stroke 2003, 34, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Fraticelli, L.; Freyssenge, J.; Claustre, C.; Buisson, M.; Bischoff, M.; Nighoghossian, N.; Derex, L.; El Khoury, C. Sex-Related Differences in Management and Outcome of Acute Ischemic Stroke in Eligible Patients to Thrombolysis. Cereb. Dis. 2019, 47, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Denton, M.; Prus, S.; Walters, V. Gender differences in health: A Canadian study of the psychosocial, structural and behavioural determinants of health. Soc. Sci. Med. 2004, 58, 2585–2600. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K. A generation apart? Gender-related experiences and health in women in early and late mid-life. Soc. Sci. Med. 2002, 54, 663–676. [Google Scholar] [CrossRef]
- Ross, C.E.; Bird, C.E. Sex stratification and health lifestyle: Consequences for men’s and women’s perceived health. J. Health Soc. Behav. 1994, 35, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M.; Beltran-Sanchez, H. Mortality and morbidity trends: Is there compression of morbidity? J. Gerontol. B Psychol. Sci. Soc. Sci. 2011, 66, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Perenboom, R.; van Herten, L.; Boshuizen, H.; van den Bos, G. Life Expectancy without Chronic Morbidity: Trends in Gender and Socioeconomic Disparities. Public Health Rep. 2005, 120, 46–54. [Google Scholar] [CrossRef]
- United Nations. Changing Levels and Trends in Mortality: The Role of Patterns of Death by Cause; Report nº ST/ESA/SER.A/318; United Nations: New York, NY, USA, 2012. [Google Scholar]
- Vallin, J.; Meslé, F. The Segmented Trend Line of Highest Life Expectancies. Popul. Dev. Rev. 2009, 35, 159–187. [Google Scholar] [CrossRef]
- Atlantis, E.; Lange, K.; Wittert, G.A. Chronic disease trends due to excess body weight in Australia. Obes. Rev. 2009, 10, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N. Physical inactivity: The biggest public health problem of the 21st century. Br. J. Sports Med. 2009, 43, 1232–1234. [Google Scholar]
- Blair, S.N.; Church, T.S. The fitness, obesity, and health equation: Is physical activity the common denominator? J. Am. Med. Assoc. 2004, 292, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Carson, V.; Lee, I.M.; Katzmarzyk, P.T.; Blair, S.N. Years of life gained due to leisure-time physical activity in the U.S. Am. J. Prev. Med. 2013, 44, 23–29. [Google Scholar] [CrossRef]
- GBD DALYs; Hale Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef]
- Jivraj, S.; Goodman, A.; Pongiglione, B.; Ploubidis, G.B. Living longer but not necessarily healthier: The joint progress of health and mortality in the working-age population of England. Popul. Stud. 2020, 74, 399–414. [Google Scholar] [CrossRef]
- Salomon, J.A.; Wang, H.; Freeman, M.K.; Vos, T.; Flaxman, A.D.; Lopez, A.D.; Murray, C.J. Healthy life expectancy for 187 countries, 1990-2010: A systematic analysis for the Global Burden Disease Study 2010. Lancet 2012, 380, 2144–2162. [Google Scholar] [CrossRef]
- Steensma, C.; Loukine, L.; Choi, B.C. Evaluating compression or expansion of morbidity in Canada: Trends in life expectancy and health-adjusted life expectancy from 1994 to 2010. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 68–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sole-Auro, A.; Alcaniz, M. Are we living longer but less healthy? Trends in mortality and morbidity in Catalonia (Spain), 1994–2011. Eur. J. Ageing 2015, 12, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Beltrán-Sánchez, H.; Regidor, E.; Gomez-Martin, C.; Del-Barrio, J.L.; Gil-de-Miguel, A.; Subramanian, S.V.; Gil-Prieto, R. No evidence of morbidity compression in Spain: A time series study based on national hospitalization records. Int. J. Public Health 2016, 61, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Zueras, P.; Renteria, E. Trends in disease-free life expectancy at age 65 in Spain: Diverging patterns by sex, region and disease. PLoS ONE 2020, 15, e0240923. [Google Scholar] [CrossRef]
- Nusselder, W.J.; Wapperom, D.; Looman, C.W.N.; Yokota, R.T.C.; van Oyen, H.; Jagger, C.; Robine, J.M.; Cambois, E.M. Contribution of chronic conditions to disability in men and women in France. Eur. J. Public Health 2018, 29, 99–104. [Google Scholar] [CrossRef]
- Doblhammer, G.; Kytir, J. Compression or expansion of morbidity? Trends in healthy-life expectancy in the elderly Austrian population between 1978 and 1998. Soc. Sci. Med. 2001, 52, 385–391. [Google Scholar] [CrossRef]
- Olshansky, S.J.; Rudberg, M.A.; Carnes, B.A.; Cassel, C.K.; Brody, J.A. Trading Off Longer Life for Worsening Health: The Expansion of Morbidity Hypothesis. J. Aging Health 1991, 3, 194–216. [Google Scholar] [CrossRef]
- Instituto Canario de Estadística. Encuesta de Salud de Canarias. Metodología. Available online: http://www.gobiernodecanarias.org/istac/descargas/C00035A/ESC-2015-Metodologia.pdf (accessed on 2 July 2020).
- Requena, M.L.; Suárez, M.; Pérez, Ó.; Grupo Técnico de Encuestas de Salud de la Subcomisión de Sistemas de Información del Consejo Interterritorial del Sistema Nacional de Salud. Encuestas de salud en España: Situación actual [Current situation of health surveys in Spain]. Rev. Española Salud Pública 2013, 87, 549–573. [Google Scholar] [CrossRef][Green Version]
- Beidas, R.S.; Williams, N.J.; Becker-Haimes, E.M.; Aarons, G.A.; Barg, F.K.; Evans, A.C.; Jackson, K.; Jones, D.; Hadley, T.; Hoagwood, K.; et al. A repeated cross-sectional study of clinicians’ use of psychotherapy techniques during 5 years of a system-wide effort to implement evidence-based practices in Philadelphia. Implement. Sci. 2019, 14, 67. [Google Scholar] [CrossRef]
- Steel, D. Repeated cross-sectional design. In Encyclopedia of Survery Research Methods; Layrakas, P., Ed.; SAGE Publications: Thousand Oaks, CA, USA, 2008; Volume 2, pp. 714–716. [Google Scholar]
- Instituto Canario de Estadística. Encuesta de Salud de Canarias. Cuestionario de Adultos. Available online: http://www.gobiernodecanarias.org/istac/descargas/C00035A/ESC-2015-Cuestionario-adultos.pdf (accessed on 2 July 2020).
- Rothman, K.; Greenland, S.; Lash, T. Moderm Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; 733p. [Google Scholar]
- Gordis, L. Epidemiology E-Book, 5th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; 79p. [Google Scholar]
- Chan, I.S.F.; Zhang, Z. Test-Based Exact Confidence Intervals for the Difference of Two Binomial Proportions. Biometrics 1999, 55, 1202–1209. [Google Scholar] [CrossRef]
- Lin, C.C.; Li, C.I.; Hsiao, C.Y.; Liu, C.S.; Yang, S.Y.; Lee, C.C.; Li, T.C. Time trend analysis of the prevalence and incidence of diagnosed type 2 diabetes among adults in Taiwan from 2000 to 2007: A population-based study. BMC Public Health 2013, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.E.; Lin, C.L.; Tsai, C.H.; Tang, C.H.; Hwang, W.L.; Cheng, Y.W.; Sung, F.C.; Chung, C.J. Time trend of multiple myeloma and associated secondary primary malignancies in Asian patients: A Taiwan population-based study. PLoS ONE 2013, 8, e68041. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, J.C. Logistic regression: A brief primer. Acad. Emerg. Med. 2011, 18, 1099–1104. [Google Scholar] [CrossRef]
- Jaccard, J. Interaction Effects in Logistic Regression; Sage: Thousand Oaks, CA, USA, 2001. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Schumacker, R. Log-linear regression. In Learning Statistics Using R; SAGE Publications, Inc.: London, UK, 2015; pp. 481–521. [Google Scholar]
- Crimmins, E.M.; Kim, J.K.; Solé-Auró, A. Gender differences in health: Results from SHARE, ELSA and HRS. Eur. J. Public Health 2010, 21, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Van Oyen, H.; Nusselder, W.; Jagger, C.; Kolip, P.; Cambois, E.; Robine, J.M. Gender differences in healthy life years within the EU: An exploration of the “health-survival” paradox. Int. J. Public Health 2013, 58, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Canary Institute of Statistics. Mortality Analysis [Análisis de la Mortalidad]. Servicio Canario de Salud. 2021. Available online: http://www.gobiernodecanarias.org/istac/estadisticas/sociedad/salud/analisismortalidad/.html (accessed on 1 January 2020).
- Okunrintemi, V.; Tibuakuu, M.; Virani, S.S.; Sperling, L.S.; Volgman, A.S.; Gulati, M.; Cho, L.; Leucker, T.M.; Blumenthal, R.S.; Michos, E.D. Sex Differences in the Age of Diagnosis for Cardiovascular Disease and Its Risk Factors Among US Adults: Trends From 2008 to 2017, the Medical Expenditure Panel Survey. J. Am. Heart Assoc. 2020, 9, e018764. [Google Scholar] [CrossRef] [PubMed]
- Kuan, V.; Denaxas, S.; Gonzalez-Izquierdo, A.; Direk, K.; Bhatti, O.; Husain, S.; Sutaria, S.; Hingorani, M.; Nitsch, D.; Parisinos, C.A.; et al. A chronological map of 308 physical and mental health conditions from 4 million individuals in the English National Health Service. Lancet Digit. Health 2019, 1, e63–e77. [Google Scholar] [CrossRef]
- Emslie, C.; Hunt, K.; Macintyre, S. Gender differences in minor morbidity among full time employees of a British university. J Epidemiol. Commun. Health 1999, 53, 465–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denton, M.; Walters, V. Gender differences in structural and behavioral determinants of health: An analysis of the social production of health. Soc. Sci. Med. 1999, 48, 1221–1235. [Google Scholar] [CrossRef]
- McDonough, P.; Walters, V. Gender and health: Reassessing patterns and explanations. Soc. Sci. Med. 2001, 52, 547–559. [Google Scholar] [CrossRef]
- Prus, S.G.; Gee, E. Gender differences in the influence of economic, lifestyle, and psychosocial factors on later-life health. Can. J. Public Health 2003, 94, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Rojas-Rueda, D.; Alonso, J.; Anto, J.M.; Cardona, P.J.; Fernandez, E.; Garcia-Basteiro, A.L.; Benavides, F.G.; Glenn, S.D.; Krish, V.; et al. The burden of disease in Spain: Results from the Global Burden of Disease 2016. Med. Clin. 2018, 151, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Juel, K.; Sørensen, J.; Brønnum-Hansen, H. Risk factors and public health in Denmark. Scand. J. Public Health 2008, 36 (Suppl. S1), 11–227. [Google Scholar] [CrossRef] [PubMed]
- Abades Porcel, M.; Rayón Valpuesta, E. Aging in Spain: A challenge or social problem? [El envejecimiento en España: ¿un reto o problema social?]. Gerokomos 2012, 23, 151–155. [Google Scholar] [CrossRef]
- García González, J.M.; Grande, r. From “dying old” to “dying old”: Mortality trends in the elderly population in Spain [De morirse de viejo a morirse viejo, tendencias de la mortalidad de la población de edades avanzadas en España]. In Informe España 2020 (Cap. 2); Cátedra Jose María Martín Patino, Ed.; Universidad Pontificia Comillas: Madrid, Spain, 2019; pp. 89–175. [Google Scholar]
- Jacobsen, G.D.; Jacobsen, K.H. Health awareness campaigns and diagnosis rates: Evidence from National Breast Cancer Awareness Month. J. Health Econ. 2011, 30, 55–61. [Google Scholar] [CrossRef]
- Kronborg, C.N.; Hallas, J.; Jacobsen, I.A. Prevalence, awareness, and control of arterial hypertension in Denmark. J. Am. Soc. Hypertens 2009, 3, 19–24.e12. [Google Scholar] [CrossRef]
- Singh, J. Greater awareness: Better health. World Health 1996, 49, 22. Available online: https://apps.who.int/iris/handle/10665/330505 (accessed on 1 January 2021).
- Narayan, K.M.V.; Chan, J.; Mohan, V. Early Identification of Type 2 Diabetes. Diabetes Care 2011, 34, 244. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.I.; Ekholm, O.; Gray, L.; Glumer, C.; Juel, K. What is wrong with non-respondents? Alcohol-, drug- and smoking-related mortality and morbidity in a 12-year follow-up study of respondents and non-respondents in the Danish Health and Morbidity Survey. Addiction 2015, 110, 1505–1512. [Google Scholar] [CrossRef]
- Basterra-Gortari, F.J.; Beunza, J.J.; Bes-Rastrollo, M.; Toledo, E.; García-López, M.; Martínez-González, M.A. Increasing Trend in the Prevalence of Morbid Obesity in Spain: From 1.8 to 6.1 per Thousand in 14 Years. Rev. Española Cardiol. 2011, 64, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Elosua, R.; Cabrera de León, A.; Guembe, M.J.; Baena-Díez, J.M.; Vega Alonso, T.; Javier Félix, F.; Zorrilla, B.; Rigo, F.; Lapetra, J.; et al. Cardiovascular Risk Factors in Spain in the First Decade of the 21st Century, a Pooled Analysis With Individual Data From 11 Population-Based Studies: The DARIOS Study [Factores de riesgo cardiovascular en España en la primera década del siglo XXI: Análisis agrupado con datos individuales de 11 estudios de base poblacional, estudio DARIOS]. Rev. Esp. Cardiol. 2011, 64, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Sanchez, J.A.; Bello-Lujan, L.M.; Auyanet-Batista, J.M.; Fernandez-Rodriguez, M.J.; Gonzalez-Henriquez, J.J. Lack of exercise of “moderate to vigorous” intensity in people with low levels of physical activity is a major discriminant for sociodemographic factors and morbidity. PLoS ONE 2014, 9, e115321. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Church, T.S.; Craig, C.L.; Bouchard, C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc. 2009, 41, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Young, D.R.; Hivert, M.-F.; Alhassan, S.; Camhi, S.M.; Ferguson, J.F.; Katzmarzyk, P.T.; Lewis, C.E.; Owen, N.; Perry, C.K.; Siddique, J.; et al. Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation 2016, 134, e262–e279. [Google Scholar] [CrossRef] [PubMed]
- George, E.S.; Rosenkranz, R.R.; Kolt, G.S. Chronic disease and sitting time in middle-aged Australian males: Findings from the 45 and Up Study. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 20. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Healy, G.N.; Dunstan, D.W.; Zderic, T.W.; Owen, N. Too Little Exercise and Too Much Sitting: Inactivity Physiology and the Need for New Recommendations on Sedentary Behavior. Curr. Cardiovasc. Risk Rep. 2008, 2, 292–298. [Google Scholar] [CrossRef]
- Nichols, M.; Townsend, N.; Luengo-Fernandez, R.; Lea, L.J.; Gray; Scarborough, P.; Rayner, M. European Cardiovascular Disease Statistics 2012; European Heart Network: Brussels, Belgium, 2012. [Google Scholar]
- Deaton, C.; Froelicher, E.S.; Wu, L.H.; Ho, C.; Shishani, K.; Jaarsma, T. The global burden of cardiovascular disease. J. Cardiovasc. Nurs. 2011, 26, S5–S14. [Google Scholar] [CrossRef]
- Lee, J.M.; Gebremariam, A.; Vijan, S.; Gurney, J.G. Excess body mass index-years, a measure of degree and duration of excess weight, and risk for incident diabetes. Arch. Pediatr. Adolesc. Med. 2012, 166, 42–48. [Google Scholar] [CrossRef]
- Serna Arnaiz, M.C.; Madrid Valls, M.; Cruz Esteve, I.; Gasco Eguiluz, E.; Ribelles, M.; Serra Majem, L. Estimation of the prevalence of diabetes mellitus in six regions of the province of Lleida [Estimación de la prevalencia de diabetes mellitus en seis comarcas de la provincia de Lleida]. Endocrinol. Nutr. 1999, 46, 83. Available online: https://www.elsevier.es/es-revista-endocrinologia-nutricion-12-articulo-estimacion-prevalencia-diabetes-mellitus-seis-8577#:~:text=En%8520el%8520estudio%8520que%8520nos,%8572C8572%8525%8520a%8520diabetes%8520desconocida (accessed on 1 January 2021).
- Cabrera de León, A.; Pérez, R.; del Cristo, M.; Almeida González, D.; Domínguez Coello, S.; Aguirre Jaime, A.; Brito Díaz, B.; González Hernández, A.; Pérez Méndez, L.I. Presentation of the Canary CDC cohort: Objectives, design and preliminary results [Presentación de la cohorte “CDC de Canarias”: Objetivos, diseño y resultados preliminares]. Rev. Española Salud Pública 2008, 82, 519–534. [Google Scholar] [CrossRef] [PubMed]
- OECD/European Observatory on Health Systems and Policies. Spain: Country Health Profile 2017, State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2017. [Google Scholar] [CrossRef]
- Cylus, J.; Papanicolas, I. An analysis of perceived access to health care in Europe: How universal is universal coverage? Health Policy 2015, 119, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, E.A.; Van Ameijden, E.J.; Grobbee, D.E.; Numans, M.E. Comparison of routine care self-reported and biometrical data on hypertension and diabetes: Results of the Utrecht Health Project. Eur. J. Public Health 2007, 17, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.M.; Byers, T.; Freedman, D.S.; Mokdad, A. Validity of self-reported diagnoses leading to hospitalization: A comparison of self-reports with hospital records in a prospective study of American adults. Am. J. Epidemiol. 1998, 147, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Goldman, N.; Lin, I.F.; Weinstein, M.; Lin, Y.H. Evaluating the quality of self-reports of hypertension and diabetes. J. Clin. Epidemiol. 2003, 56, 148–154. [Google Scholar] [CrossRef]
- Fregel, R.; Ordóñez, A.C.; Serrano, J.G. The demography of the Canary Islands from a genetic perspective. Hum. Mol. Genet. 2020, 30, R64–R71. [Google Scholar] [CrossRef] [PubMed]
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| 1997 | 2004 | 2009 | 2015 | 1997 | 2004 | 2009 | 2015 | |
| (n = 1124) | (n = 2513) | (n = 2655) | (n = 2591) | (n = 1043) | (n = 1791) | (n = 1887) | (n = 1969) | |
| % | % | % | % | % | % | % | % | |
| Age | ||||||||
| 16–30 | 30.6 | 20.3 | 15.5 | 12.3 | 32.1 | 23.0 | 18.5 | 12.8 |
| 31–45 | 27.8 | 29.3 | 30.6 | 25.0 | 28.1 | 31.0 | 33.0 | 28.7 |
| 46–60 | 20.1 | 19.4 | 24.2 | 29.7 | 22.0 | 20.1 | 22.7 | 29.7 |
| >60 | 21.5 | 31.0 | 29.8 | 32.9 | 17.8 | 25.9 | 25.8 | 28.8 |
| Mean age (years) | 43.1 | 48.4 | 49.4 | 51.9 | 41.8 | 46.3 | 47.2 | 50.2 |
| Education a | ||||||||
| Primary or lower | 47.3 | 33.4 | 32.9 | 26.1 | 43.5 | 32.0 | 28.2 | 21.2 |
| High school | 39.6 | 43.8 | 43.0 | 44.2 | 42.5 | 47.3 | 47.1 | 50.1 |
| University | 13.1 | 17.5 | 23.9 | 29.3 | 14.0 | 19.2 | 24.6 | 28.3 |
| Occupation a | ||||||||
| Employed | 32.7 | 35.1 | 34.3 | 40.9 | 63.6 | 53.8 | 46.5 | 50.5 |
| Unemployed | 7.0 | 11.9 | 20 | 23.6 | 9.9 | 10.7 | 20.1 | 23.2 |
| Student | 9.7 | 6.0 | 4.0 | 6.8 | 9.7 | 6.8 | 4.0 | 4.6 |
| Housework | 31.2 | 17.8 | 17.2 | 6.1 | 0.2 | 0.7 | 0.2 | 0.2 |
| Retired | 16.3 | 28.3 | 22.4 | 30.4 | 19.4 | 26.7 | 26.5 | 29.1 |
| Prevalence Ratio (95% CI) a | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1997 | 2004 | 2009 | 2015 | |||||
| Musculoskeletal (2nd level) b | 1.95 (1.70; 2.23) | *,a–c | 2.30 (2.11; 2.51) | *,b | 2.06 (1.94; 2.19) | *,a,c | 1.90 (1.81; 2.08) | *,a,c |
| Cardiovascular (2nd level) c | 1.27 (1.02; 1.51) | *,a | 1.31 (1.08; 1.36) | *,a | 1.28 (1.09; 1.47) | *,a | 1.29 (1.19; 1.40) | *,a |
| Osteoporosis | 3.18 (1.66; 6.09) | *,a | 3.69 (2.38; 5.73) | *,a | 3.67 (2.46; 5.46) | *,a | 3.59 (2.44; 5.21) | *,a |
| Joint–rheumatic pain | 1.98 (1.67; 2.29) | *,a | 2.07 (1.89; 2.26) | *,a | 2.03 (1.88; 2.19) | *,a | 2.24 (2.08; 2.40) | *,a |
| Peripheral vascular disease | 2.30 (1.87; 2.74) | *,a–c | 2.56 (2.21; 2.94) | *,b | 1.67 (1.40; 1.99) | *,c | 1.94 (1.71; 2.15) | *,a,c |
| Chronic headaches | 1.72 (1.23; 2.41) | *,a | 1.74 (1.40; 2.17) | *,a | 1.79 (1.47; 2.16) | *,a | 1.88 (1.57; 2.29) | *,a |
| Neck and back pain | 1.79 (1.43; 2.23) | *,a | 1.75 (1.51; 2.02) | *,a | 1.40 (1.19; 1.61) | *,a | 1.86 (1.68; 2.05) | *,a |
| Anxiety, depression | 1.48 (1.14; 1.92) | *,a | 1.40 (1.17; 1.68) | *,a | 1.62 (1.43; 1.81) | *,a | 1.57 (1.38; 1.73) | *,a |
| Digestive diseases | 1.22 (0.94; 1.58) | a | 1.35 (1.13; 1.66) | *,a | 1.37 (1.18; 1.59) | *,a | 1.18 (0.98; 1.39) | a |
| High cholesterol | 1.21 (0.95; 1.49) | a | 1.23 (1.02; 1.45) | *,a | 1.16 (0.99; 1.31) | a | 1.12 (0.98; 1.26) | a |
| High blood pressure | 1.28 (0.99; 1.57) | a | 1.19 (0.98; 1.40) | a | 1.03 (0.87; 1.19) | a | 1.01 (0.89; 1.16) | a |
| Respiratory diseases | 1.15 (0.92; 1.43) | a | 1.14 (0.95; 1.32) | a | 1.21 (0.98; 1.44) | a | 1.17 (0.98; 1.37) | a |
| Diabetes | 1.14 (0.81; 1.59) | a | 1.21 (0.98; 1.44) | a | 1.16 (0.95 1.41) | a | 1.16 (0.97; 1.36) | a |
| Heart diseases | 0.85 (0.65; 1.03) | a | 0.88 (0.75; 1.14) | a | 0.96 (0.78; 1.19) | a | 0.90 (0.71; 1.19) | a |
| Overall (n = 8884) | Age Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | 16–30 years old (n = 1585) | 31–45 years old (n = 2510) | 46–60 years old (n = 2126) | >60 years old (n = 2664) | ||||||
| nº | Women | OR (95% IC) a | OR (95% IC) b | OR (95% IC) c | OR (95% IC) c | OR (95% IC) c | OR (95% IC) c | ||||
| Musculoskeletal (2nd level) | 1.043 (1.035; 1.051) | *** | 1.020 (1.007; 1.033) | * | 1.018 (0.981; 1.053) | 1.017 (0.991; 1.043) | 1.019 (0.991; 1.047) | 1.035 (1.010; 1.060) | ** | ||
| Cardiovascular (2nd level) | 1.046 (1.038; 1.054) | *** | 1.026 (1.012; 1.040) | ** | 1.016 (0.980; 1.050) | 1.019 (0.989; 1.049) | 1.024 (0.996; 1.052) | 1.045 (1.021; 1.069) | *** | ||
| 1 | Neck–back pain | 1.043 (1.035; 1.052) | *** | 1.019 (1.003; 1.035) | * | 1.012 (0.976; 1.048) | 1.014 (0.977; 1.041) | 1.015 (0.986; 1.044) | 1.033 (1.007; 1.059) | * | |
| 2 | Anxiety Depression | 1.037 (1.028; 1.045) | *** | 1.026 (1.012; 1.040) | ** | 1.021 (0.985; 1.057) | 1.014 (0.985; 1.044) | 1.034 (1.002; 1.066) | * | 1.041 (1.013; 1.069) | ** |
| 3 | High blood pressure | 1.028 (1.019; 1.037) | *** | 1.002 (0.987; 1.017) | 0.982 (0.943; 1.027) | 0.984 (0.956; 1.012) | 1.005 (0.973; 1.037) | 1.039 (1.014; 1.055) | ** | ||
| 4 | High cholesterol | 1.039 (1.027; 1.047) | *** | 1.016 (1.002; 1.030) | * | 1.007 (0.971; 1.045) | 1.009 (0.984; 1.035) | 1.025 (0.995; 1.055) | 1.024 (1.001; 1.047) | * | |
| 5 | Joint–rheumatic pain | 1.031 (1.022; 1.041) | *** | 1.015 (1.001; 1.029) | * | 0.987 (0.948; 1.023) | 0.995 (0.969; 1.021) | 1.014 (0.983; 1.045) | 1.037 (1.002; 1.073) | * | |
| 6 | Peripheral vascular disease | 1.044 (1.032; 1.056) | *** | 1.018 (1.002; 1.035) | * | 1.018 (0.979; 1.048) | 1.019 (0.989; 1.049) | 1.022 (0.989; 1.055) | 1.029 (1.001; 1.057) | * | |
| 7 | Digestive diseases | 1.036 (1.025; 1.047) | *** | 1.019 (1.004; 1.034) | * | 0.999 (0.961; 1.037) | 1.010 (0.980; 1.045) | 1.023 (0.991; 1.055) | 1.031 (1.003; 1.059) | * | |
| 8 | Headaches | 1.039 (1.030; 1.048) | *** | 1.021 (1.008; 1.033) | * | 1.030 (0.995; 1.065) | 1.027 (0.998; 1.056) | 1.020 (0.987; 1.053) | 1.015 (0.988; 1.042) | ||
| 9 | Diabetes | 1.024 (1.013; 1.035) | ** | 1.019 (1.003; 1.035) | * | 1.011 (0.973; 1.050) | 1.012 (0.984; 1.030) | 1.021 (0.988; 1.055) | 1.031 (1.004; 1.058) | * | |
| 10 | Respiratory diseases | 1.036 (1.024; 1.048) | ** | 1.012 (0.994; 1.030) | 1.018 (0.974; 1.058) | 1.010 (0.979; 1.041) | 1.012 (0.977; 1.047) | 1.015 (0.986; 1.044) | |||
| 11 | Osteoporosis | 1.047 (1.034; 1.060) | *** | 1.028 (1.009; 1.049) | ** | --- | 1.021 (0.988; 1.055) | 1.027 (0.989; 1.065) | 1.035 (1.003; 1.067) | * | |
| 12 | Heart diseases | 1.011 (0.999; 1.023) | 1.005 (0.988; 1.022) | 0.993 (0.952; 1.034) | 1.007 (0.973; 1.038) | 1.009 (0.973; 1.045) | 1.021 (0.982 1.061) | ||||
| nº | Men | Overall (n = 6690) | 16–30 years old (n = 1350) | 31–45 years old (n = 2038) | 46–60 years old (n = 1601) | >60 years old (n = 1701) | |||||
| Musculoskeletal (2nd level) | 1.035 (1.026; 1.044) | *** | 1.025 (1.010; 1.041) | ** | 1.018 (0.980; 1.056) | 1.019 (0.991; 1.046) | 1.031 (1.002; 1.059) | * | 1.036 (1.008; 1.066) | * | |
| Cardiovascular (2nd level) | 1.047 (1.038; 1.056) | *** | 1.022 (1.006; 1.038) | ** | 1.011 (0.974; 1.038) | 1.020 (0.993; 1.047) | 1.034 (1.004; 1.065) | * | 1.037 (1.008; 1.066) | * | |
| 3 | Neck–back pain | 1.043 (1.032; 1.054) | *** | 1.017 (1.001; 1.033) | * | 1.012 (0.973; 1.051) | 1.005 (0.971; 1.035) | 1.036 (1.003; 1.069) | * | 1.029 (0.998; 1.060) | |
| 5 | Anxiety Depression | 1.041 (1.030; 1.052) | *** | 1.023 (1.006; 1.041) | * | 1.021 (0.982; 1.060) | 1.019 (0.987; 1.051) | 1.040 (1.006; 1.074) | * | 1.028 (0.996; 1.061) | |
| 1 | High blood pressure | 1.038 (1.026; 1.050) | *** | 1.028 (1.010; 1.046) | * | 1.015 (0.974; 1.056) | 1.023 (0.990; 1.055) | 1.031 (0.995; 1.067) | 1.045 (1.010; 1.080) | ** | |
| 2 | High cholesterol | 1.055 (1.044; 1.067) | *** | 1.024 (1.007; 1.041) | * | 1.009 (0.969; 1.042) | 1.019 (0.979; 1.049) | 1.034 (1.001; 1.067) | * | 1.042 (1.009; 1.075) | * |
| 9 | Joint–rheumatic pain | 1.024 (1.012; 1.036) | ** | 1.010 (0.991; 1.029) | 1.005 (0.967; 1.047) | 1.011 (0.977; 1.045) | 1.008 (0.969; 1.047) | 1.012 (0.962; 1.052) | |||
| 7 | Peripheral vascular disease | 1.059 (1.045; 1.073) | *** | 1.031 (1.011; 1.052) | ** | 1.021 (0.978; 1.064) | 1.013 (0.979; 1.047) | 1.051 (1.013; 1.089) | ** | 1.045 (1.008; 1.082) | * |
| 4 | Digestive diseases | 1.044 (1.031; 1.057) | *** | 1.027 (1.009; 1.045) | ** | 1.024 (0.984; 1.063) | 1.020 (0.989; 1.021) | 1.038 (1.003; 1.073) | * | 1.033 (0.998; 1.066) | |
| 10 | Headaches | 1.039 (1.027; 1.051) | *** | 1.014 (0.997; 1.031) | 1.032 (0.995; 1.069) | 1.018 (0.985; 1.051) | 1.015 (0.979; 1.051) | 0.993 (0.957; 1.028) | |||
| 6 | Diabetes | 1.039 (1.026; 1.052) | *** | 1.020 (1.002; 1.039) | * | 0.973 (0.934; 1.021) | 1.014 (0.982; 1.046) | 1.036 (1.001; 1.071) | * | 1.045 (1.009; 1.081) | * |
| 11 | Respiratory diseases | 1.009 (0.995; 1.023) | 1.011 (0.988; 1.030) | 0.997 (0.946; 1.048) | 1.009 (0.971; 1.043) | 1.029 (0.992; 1.066) | 1.021 (0.985; 1.057) | ||||
| 12 | Osteoporosis | 1.022 (1.006; 1.038) | * | 1.015 (0.987; 1.037) | --- | 1.012 (0.974; 1.050) | 1.013 (0.972; 1.054) | 1.025 (0.985; 1.065) | |||
| 8 | Heart diseases | 1.024 (1.013; 1.037) | ** | 1.002 (0.982; 1.022) | 0.991 (0.938; 1.045) | 1.015 (0.981; 1.049) | 1.005 (0.966; 1.044) | 1.022 (0.983; 1.061) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello-Lujan, L.M.; Serrano-Sanchez, J.A.; Gonzalez-Henriquez, J.J. Stable Gender Gap and Similar Gender Trend in Chronic Morbidities between 1997–2015 in Adult Canary Population. Int. J. Environ. Res. Public Health 2022, 19, 9404. https://doi.org/10.3390/ijerph19159404
Bello-Lujan LM, Serrano-Sanchez JA, Gonzalez-Henriquez JJ. Stable Gender Gap and Similar Gender Trend in Chronic Morbidities between 1997–2015 in Adult Canary Population. International Journal of Environmental Research and Public Health. 2022; 19(15):9404. https://doi.org/10.3390/ijerph19159404
Chicago/Turabian StyleBello-Lujan, Luis Miguel, Jose Antonio Serrano-Sanchez, and Juan Jose Gonzalez-Henriquez. 2022. "Stable Gender Gap and Similar Gender Trend in Chronic Morbidities between 1997–2015 in Adult Canary Population" International Journal of Environmental Research and Public Health 19, no. 15: 9404. https://doi.org/10.3390/ijerph19159404
APA StyleBello-Lujan, L. M., Serrano-Sanchez, J. A., & Gonzalez-Henriquez, J. J. (2022). Stable Gender Gap and Similar Gender Trend in Chronic Morbidities between 1997–2015 in Adult Canary Population. International Journal of Environmental Research and Public Health, 19(15), 9404. https://doi.org/10.3390/ijerph19159404






