Ventilatory Muscle Training for Early Cardiac Rehabilitation Improved Functional Capacity and Modulated Vascular Function of Individuals Undergoing Coronary Artery Bypass Grafting: Pilot Randomized Clinical Trial
Abstract
:1. Introduction
2. Methods
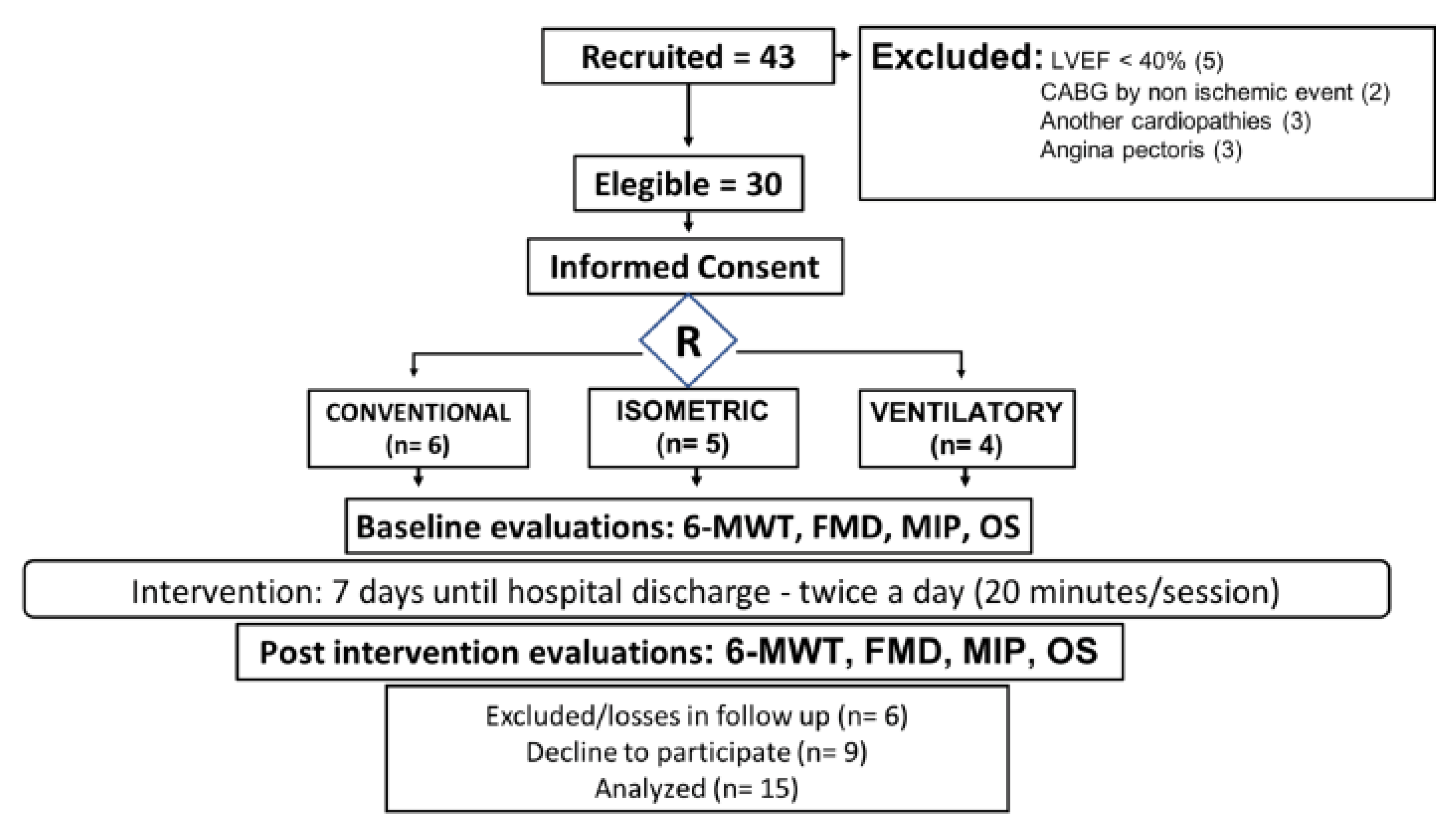
2.1. Study Design and Sample
2.2. Assessments
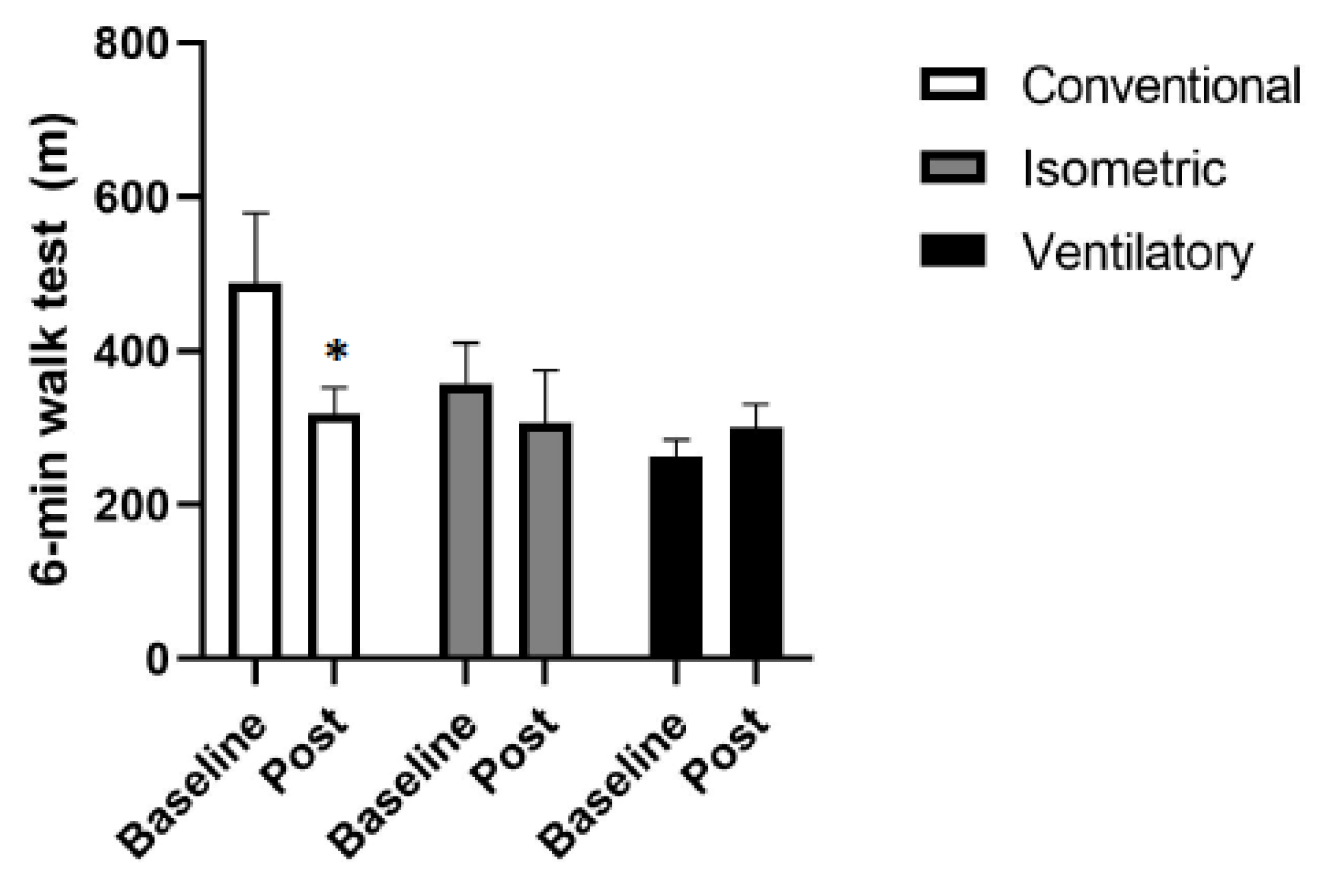
2.2.1. Six-Minute Walk Test
2.2.2. Brachial Artery Flow-Mediated Dilatation (FMD)
2.3. Data Analysis
2.4. Oxidative Stress Analysis
2.4.1. Biochemical Analysis
2.4.2. Carbonyl Measurements
2.4.3. Sulfhydryl Content
Nonenzymatic Antioxidant Defenses
2.5. Interventions
2.5.1. Isometric Handgrip Resistance Exercise
2.5.2. Ventilatory Muscle Training
2.5.3. Conventional Respiratory and Motor Physiotherapy
2.5.4. Sample Size
2.5.5. Statistical Analysis
3. Results
3.1. Functional Capacity
3.2. Endothelial Function
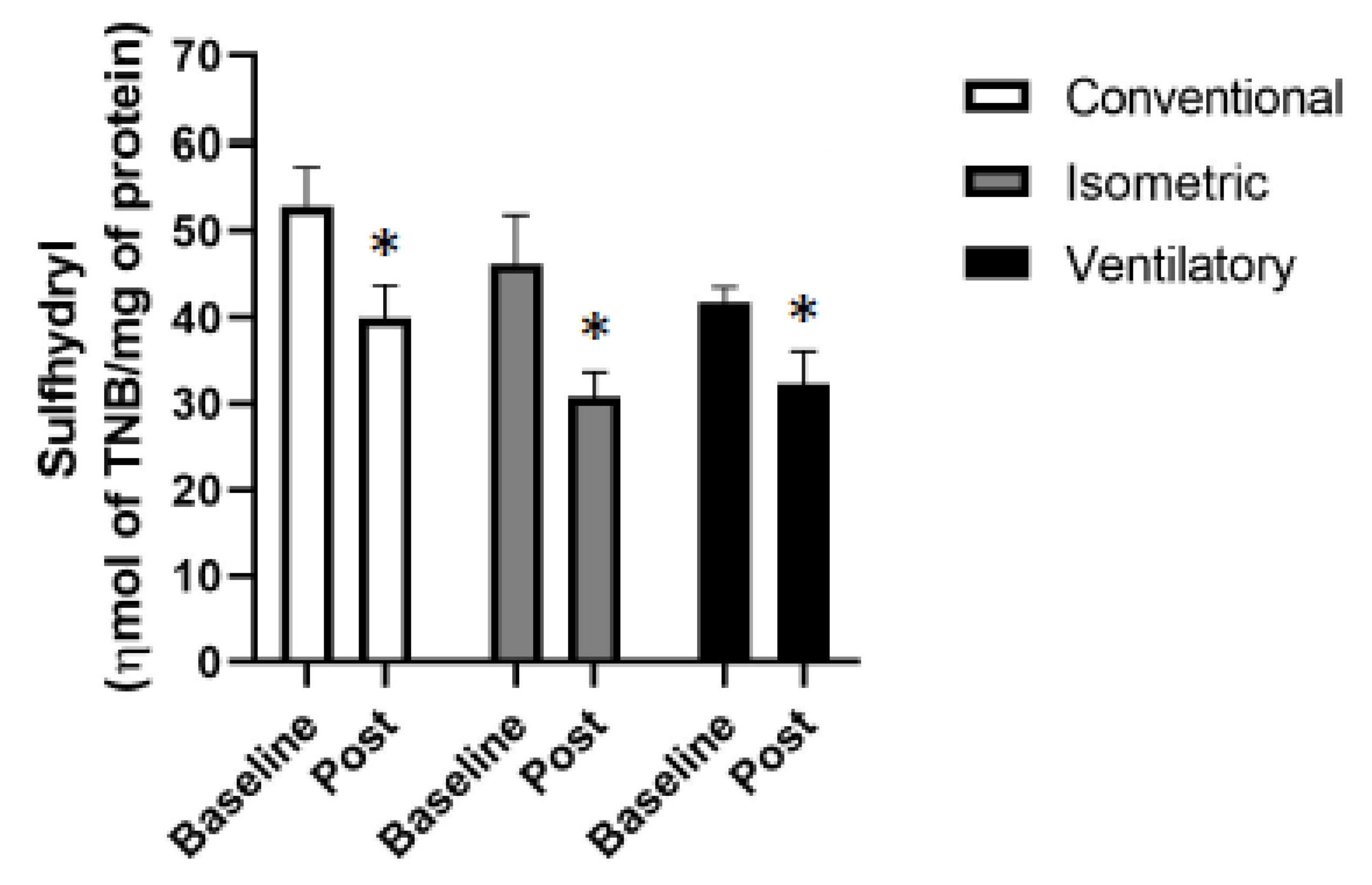
3.3. Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, R.; Maia, C.P.; Silveira, A.D.; Chiappa, G.R.; Myers, J.; Ribeiro, J.P. Inspiratory muscle strength as a determinant of functional capacity early after coronary artery bypass graft surgery. Arch. Phys. Med. Rehabil. 2009, 90, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Yang, L. Risk factors for postoperative pulmonary complications in coronary artery bypass graft surgery patients. Eur. J. Cardiovasc. Nurs. 2007, 6, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Hulzebos, E.H.J.; Van Meeteren, N.L.U.; De Bie, R.A.; Dagnelie, P.C.; Helders, P.J.M. Prediction of postoperative pulmonary complications on the basis of preoperative risk factors in patients who had undergone coronary artery bypass graft surgery. Phys. Ther. 2003, 83, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hulzebos, E.H.J.; Helders, P.J.M.; Favié, N.J.; De Bie, R.A.; de la Riviere, A.B.; Van Meeteren, N.L. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: A randomized clinical trial. JAMA 2006, 296, 1851–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, D.G.; Schulz, K.F.; Moher, D.; Egger, M.; Davidoff, F.; Elbourne, D.; Gøtzsche, P.C.; Lang, T. The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann. Intern. Med. 2001, 134, 663–694. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, M.Y.; Markesbery, W.R. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett. 2001, 302, 141–145. [Google Scholar] [CrossRef]
- Teare, J.; A Punchard, N.; Powell, J.J.; Lumb, P.J.; Mitchell, W.D.; Thompson, R.P. Automated spectrophotometric method for determining oxidized and reduced glutathione in liver. Clin. Chem. 1993, 39, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Ash, G.I.; Taylor, B.A.; Thompson, P.D.; MacDonald, H.; Lamberti, L.; Chen, M.-H.; Farinatti, P.; Kraemer, W.J.; Panza, G.A.; Zaleski, A.L.; et al. The antihypertensive effects of aerobic versus isometric handgrip resistance exercise. J. Hypertens. 2017, 35, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, J.B.; Plentz, R.D.; Stein, C.; Casali, K.R.; Arena, R.; Dal Lago, P. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: A randomized controlled trial. Int. J. Cardiol. 2013, 166, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, G.U.; Carvalho, V.O.; Cacau, L.P.D.A.; Filho, A.A.D.A.; Neto, M.L.D.C.; Da Silva, W.M.; Cerqueira, T.C.F.; Filho, V.J.D.S. Determinants of distance walked during the six-minute walk test in patients undergoing cardiac surgery at hospital discharge. J. Cardiothorac. Surg. 2014, 9, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minet, C.; Vivodtzev, I.; Tamisier, R.; Arbib, F.; Wuyam, B.; Timsit, J.-F.; Monneret, D.; Borel, J.-C.; Baguet, J.-P.; Lévy, P.; et al. Reduced six-minute walking distance, high fat-free-mass index and hypercapnia are associated with endothelial dysfunction in COPD. Respir. Physiol. Neurobiol. 2012, 183, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Nohria, A.; Gerhard-Herman, M.; Creager, M.A.; Hurley, S.; Mitra, D.; Ganz, P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J. Appl. Physiol. 2006, 101, 545–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musthafa, Q.A.; Shukor, M.F.A.; Ismail, N.A.S.; Ghazi, A.M.; Ali, R.M.; Nor, I.F.M.; Dimon, M.Z.; Ngah, W.Z.W. Oxidative status and reduced glutathione levels in premature coronary artery disease and coronary artery disease. Free. Radic. Res. 2017, 51, 787–798. [Google Scholar] [CrossRef]
- Early, K.S.; Stewart, A.; Johannsen, N.; Lavie, C.J.; Thomas, J.R.; Welsch, M. The Effects of Exercise Training on Brachial Artery Flow-Mediated Dilation: A Metaanalysis. J. Cardiopulm. Rehabil. Prev. 2017, 37, 77–89. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Conventional (n = 6) | Isometric (n = 5) | Ventilatory (n = 4) | p |
|---|---|---|---|---|
| Age (years) § | 58.8 ± 7.7 | 66.4 ± 4.3 | 63.7 ± 5.7 | 0.172 |
| Male † | 6 (100%) | 4 (80%) | 2 (50%) | 0.153 |
| SPB (mmHg) § | 132 ± 18 | 136 ± 23 | 129 ± 20 | 0.213 |
| DBP (mmHg) § | 78 ± 12 | 74 ± 14 | 76 ± 14 | 0.371 |
| MIP | 87 ± 55 | 73 ± 24 | 51 ± 8 | 0.047 |
| Comorbidities | ||||
| T2DM † | 0 (0%) | 1 (20%) | 2 (50%) | 0.153 |
| Obesity † | 1 (16.7%) | 1 (20%) | 1 (25%) | 0.949 |
| Smoking † | 0 (0%) | 1 (20%) | 0 (0%) | 0.343 |
| Sedentary lifestyle † | 6 (100%) | 5 (100%) | 4 (100%) | - a |
| Cardiovascular disease | ||||
| MI † | 6 (100%) | 5 (100%) | 4 (100%) | - a |
| HT † | 2 (33.3%) | 3 (60%) | 3 (75%) | 0.405 |
| Hemorrhagic stroke † | 0 (0%) | 0 (0%) | 1 (25%) | 0.229 |
| Drugs | ||||
| β-blockers † | 5 (83.3%) | 4 (80%) | 2 (50%) | 0.464 |
| Statins † | 6 (100%) | 5 (100%) | 4 (100%) | - a |
| ACE inhibitors † | 2 (33.3%) | 3 (60%) | 3 (75%) | 0.405 |
| Antiplatelet agents † | 6 (100%) | 5 (100%) | 4 (100%) | - a |
| Antianginal agentes † | 6 (100%) | 5 (100%) | 4 (100%) | - a |
| Diuretics † | 4 (66.7%) | 4 (80%) | 3 (75%) | 0.880 |
| Antidiabetics † | 0 (0%) | 1 (20%) | 2 (50%) | 0.153 |
| ARBs † | 2 (33.3%) | 3 (60%) | 3 (75%) | 0.405 |
| Anticoagulants † | 6 (100%) | 5 (100%) | 4 (100%) | - a |
| Conventional (n = 6) | Isometric (n = 5) | Ventilatory (n = 4) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | p | Baseline | Post | p | Baseline | Post | p | |
| FMD (%) | 9.2 ± 15.8 | 2.7 ± 2.6 | 0.710 | 10.4 ± 4.8 | 2.9 ± 2.5 | 0.152 | 9.8 ± 5.1 | 11.0 ± 6.1 | 0.825 |
| Baseline brachial diameter (mm) | 5.39 ± 1.19 | 4.86 ± 0.40 | 0.696 | 4.48 ± 0.08 | 4.51 ± 0.20 | 0.842 | 4.20 ± 0.35 | 4.16 ± 0.32 | 0.800 |
| Peak brachial diameter (mm) | 4.95 ± 0.29 | 4.94 ± 0.32 | 0.994 | 4.96 ± 0.30 | 4.66 ± 0.30 | 0.235 | 4.57 ± 0.31 | 4.58 ± 0.34 | 0.866 |
| Resting mean blood flow (mL/min) | 375.6 ± 183.7 | 192.8 ± 115.0 | 0.459 | 162.0 ± 55.0 | 129.9 ± 63.7 | 0.662 | 83.74 ± 12.4 | 58.7 ± 17.1 | 0.041 |
| Resting shear rate (s−1) | 87.3 ± 30.7 | 84.6 ± 36.1 | 0.932 | 166.6 ± 58.9 | 118.9 ± 62.0 | 0.564 | 98.5 ± 16.2 | 60.6 ± 17.1 | 0.072 |
| Mean blood flow AUC (mL/min) | 324.83 ± 115.7 | 371.78 ± 158.46 | 0.658 | 253.27 ± 15.59 | 348.08 ± 43.42 a | 0.103 | 413.76 ± 226.45 | 185.31 ± 24.42 | 0.341 |
| Shear rate AUC (s−1) | 292.2 ± 91.2 | 209.0 ± 43.0 | 0.232 | 241.5 ± 15.4 | 375.5 ± 58.3 | 0.003 | 217.9 ± 55.9 | 252.9 ± 79.6 | 0.495 |
| Conventional (n = 6) | Isometric (n = 5) | Ventilatory (n = 4) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | p | Baseline | Post | p | Baseline | Post | p | |
| Carbonyl (ηmol carbonyl/mg protein) | 10.4 ± 2.05 | 8.7 ± 0.907 | 0.449 | 0.816 ± 0.087 b | 1.05 ± 0.311 b | 0.361 | 1.23 ± 0.248 b | 0.864 ± 0.227 b | 0.372 |
| GSH (ηmol/mg protein) | 18.9 ± 0.087 | 19.3 ± 0.183 | 0.119 | 19.0 ± 0.036 | 19.1 ± 0.040 | 0.198 | 18.3 ± 0.533 | 19.0 ± 0.047 | 0.130 |
| GSSG (ηmol/mg protein) | 0.184 ± 0.003 | 0.196 ± 0.007 | 0.120 | 0.185 ± 0.001 | 0.188 ± 0.002 | 0.176 | 0.158 ± 0.021 | 0.187 ± 0.002 | 0.132 |
| GSH/GSSG | 103.4 ± 1.39 | 98.7 ± 2.32 | 0.095 | 102.5 ± 0.58 | 101.4 ± 0.61 | 0.197 | 125.9 ± 18.74 | 101.9 ± 0.74 | 0.189 |
| Sulfhydryl (ηmol of TNB/mg of protein) | 53.0 ± 4.07 | 39.9 ± 3.48 | 0.009 | 46.2 ± 5.11 | 30.8 ± 2.56 | 0.021 | 41.7 ± 1.48 a | 32.3 ± 3.28 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eibel, B.; Marques, J.R.; Dipp, T.; Waclawovsky, G.; Marschner, R.A.; Boll, L.C.; Kalil, R.A.K.; Lehnen, A.M.; Sales, A.R.K.; Irigoyen, M.C.C. Ventilatory Muscle Training for Early Cardiac Rehabilitation Improved Functional Capacity and Modulated Vascular Function of Individuals Undergoing Coronary Artery Bypass Grafting: Pilot Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 9340. https://doi.org/10.3390/ijerph19159340
Eibel B, Marques JR, Dipp T, Waclawovsky G, Marschner RA, Boll LC, Kalil RAK, Lehnen AM, Sales ARK, Irigoyen MCC. Ventilatory Muscle Training for Early Cardiac Rehabilitation Improved Functional Capacity and Modulated Vascular Function of Individuals Undergoing Coronary Artery Bypass Grafting: Pilot Randomized Clinical Trial. International Journal of Environmental Research and Public Health. 2022; 19(15):9340. https://doi.org/10.3390/ijerph19159340
Chicago/Turabian StyleEibel, Bruna, Juliana R. Marques, Thiago Dipp, Gustavo Waclawovsky, Rafael A. Marschner, Liliana C. Boll, Renato A. K. Kalil, Alexandre M. Lehnen, Allan R. K. Sales, and Maria Claudia Costa Irigoyen. 2022. "Ventilatory Muscle Training for Early Cardiac Rehabilitation Improved Functional Capacity and Modulated Vascular Function of Individuals Undergoing Coronary Artery Bypass Grafting: Pilot Randomized Clinical Trial" International Journal of Environmental Research and Public Health 19, no. 15: 9340. https://doi.org/10.3390/ijerph19159340
APA StyleEibel, B., Marques, J. R., Dipp, T., Waclawovsky, G., Marschner, R. A., Boll, L. C., Kalil, R. A. K., Lehnen, A. M., Sales, A. R. K., & Irigoyen, M. C. C. (2022). Ventilatory Muscle Training for Early Cardiac Rehabilitation Improved Functional Capacity and Modulated Vascular Function of Individuals Undergoing Coronary Artery Bypass Grafting: Pilot Randomized Clinical Trial. International Journal of Environmental Research and Public Health, 19(15), 9340. https://doi.org/10.3390/ijerph19159340






