Digital Rehabilitation for Elbow Pain Musculoskeletal Conditions: A Prospective Longitudinal Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Outcomes
- Pain was assessed through an 11-point numerical pain rating scale (NPRS) through the question, “Please rate your average pain over the last 7 days: 0 (no pain at all) to 10 (worst pain imaginable)” [55];
- Analgesic use through the question: “Are you currently taking any pain medication?”;
- Self-reported surgery intent through the question, “How likely are you to seek surgery to address your condition in the next 12 months?” (range 0–100);
- Mental health including anxiety and depression levels through the Generalized Anxiety Disorder 7-item scale (GAD-7) (scores 0–21) [56,57] and Patient Health 9-item questionnaire (PHQ-9) (scores 0–27) [56,58], respectively. A cut-off threshold of ≥5 indicates at least mild anxiety/depression [59]. Fear-avoidance beliefs (FAB) were also assessed, through the 5-item questionnaire for physical activity (FABQ-PA), scored from 0 to 24 [60,61];
- Work productivity in employed participants through the Work Productivity and Activity Impairment questionnaire for general health (WPAI), assessed by the sub-scores: WPAI overall (combining presenteeism and absenteeism), WPAI work (presenteeism), WPAI time (absenteeism), and WPAI activities (activities of daily living impairment) (scores 0–100%) [62];
- Patient engagement of DCP was measured by completion of the program (completion rate), the number of completed exercise sessions, and the number of sessions performed per week;
- Overall satisfaction by the question: “On a scale from 0 to 10, how likely is it that you would recommend this intervention to a friend or neighbor?”.
2.5. Safety and Adverse Events
2.6. Data Availability
2.7. Statistical Analyses
3. Results
3.1. Participants
3.2. Clinical Outcomes
3.2.1. Primary Outcome
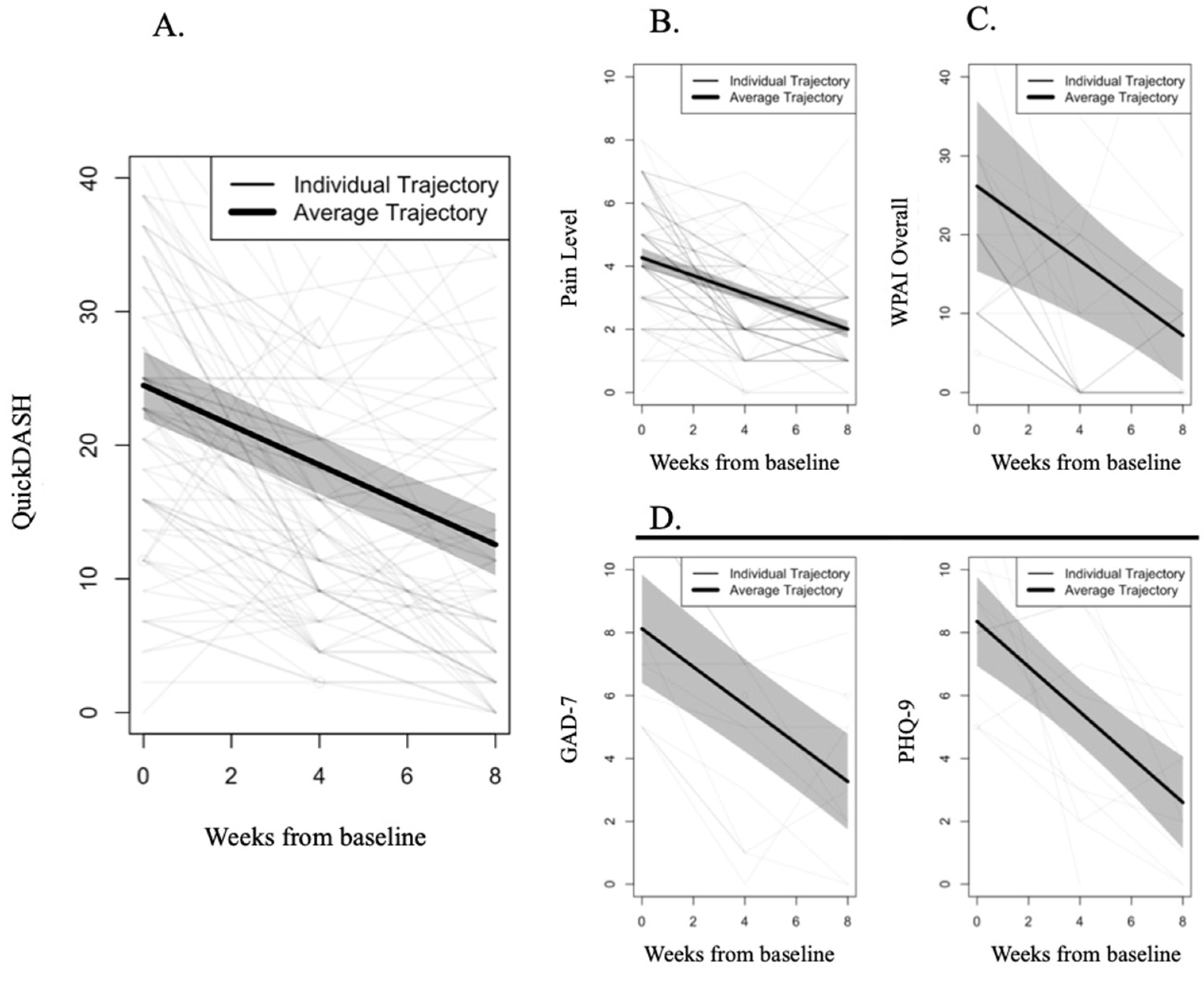
QuickDASH
3.2.2. Secondary Outcomes
Pain
Analgesics Consumption
Surgery Intent
Mental Health
Work Productivity
Engagement and Usability-Related Outcomes
4. Discussion
4.1. Main Findings
4.2. Comparison with Literature
4.3. Patient Engagement
4.4. Strengths, Limitations, and Future Studies Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordan, J.L.; Holden, M.A.; Mason, E.E.; Foster, N.E. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2010, 2010, Cd005956. [Google Scholar] [CrossRef]
- Govaerts, R.; Tassignon, B.; Ghillebert, J.; Serrien, B.; De Bock, S.; Ampe, T.; El Makrini, I.; Vanderborght, B.; Meeusen, R.; De Pauw, K. Prevalence and incidence of work-related musculoskeletal disorders in secondary industries of 21st century Europe: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2021, 22, 1–30. [Google Scholar] [CrossRef]
- Sanders, T.L., Jr.; Maradit Kremers, H.; Bryan, A.J.; Ransom, J.E.; Smith, J.; Morrey, B.F. The epidemiology and health care burden of tennis elbow: A population-based study. Am. J. Sports Med. 2015, 43, 1066–1071. [Google Scholar] [CrossRef]
- Degen, R.M.; Conti, M.S.; Camp, C.L.; Altchek, D.W.; Dines, J.S.; Werner, B.C. Epidemiology and Disease Burden of Lateral Epicondylitis in the USA: Analysis of 85,318 Patients. HSS J. Musculoskelet. J. Hosp. Spéc. Surg. 2018, 14, 9–14. [Google Scholar] [CrossRef]
- Hopkins, C.; Fu, S.C.; Chua, E.; Hu, X.; Rolf, C.; Mattila, V.M.; Qin, L.; Yung, P.S.; Chan, K.M. Critical review on the socio-economic impact of tendinopathy. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2016, 4, 9–20. [Google Scholar] [CrossRef]
- Pastora-Bernal, J.M.; Martín-Valero, R.; Barón-López, F.J. Cost analysis of telerehabilitation after arthroscopic subacromial decompression. J. Telemed. Telecare 2017, 24, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Walker-Bone, K.; Palmer, K.T.; Reading, I.; Coggon, D.; Cooper, C. Occupation and epicondylitis: A population-based study. Rheumatology 2012, 51, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Coombes, B.K.; Bisset, L.; Vicenzino, B. Management of Lateral Elbow Tendinopathy: One Size Does Not Fit All. J. Orthop. Sports Phys. Ther. 2015, 45, 938–949. [Google Scholar] [CrossRef]
- Aben, A.; De Wilde, L.; Hollevoet, N.; Henriquez, C.; Vandeweerdt, M.; Ponnet, K.; Van Tongel, A. Tennis elbow: Associated psychological factors. J. Shoulder Elb. Surg. 2018, 27, 387–392. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.M.; Huisstede, B.M.; Koes, B.W.; Burdorf, A. Associations between work-related factors and specific disorders at the elbow: A systematic literature review. Rheumatology 2009, 48, 528–536. [Google Scholar] [CrossRef]
- Miettinen, L.; Ryhänen, J.; Shiri, R.; Karppinen, J.; Miettunen, J.; Auvinen, J.; Hulkkonen, S. Work-related risk factors for ulnar nerve entrapment in the Northern Finland Birth Cohort of 1966. Sci. Rep. 2021, 11, 10010. [Google Scholar] [CrossRef] [PubMed]
- Hulkkonen, S.; Auvinen, J.; Miettunen, J.; Karppinen, J.; Ryhänen, J. Smoking is associated with ulnar nerve entrapment: A birth cohort study. Sci. Rep. 2019, 9, 9450. [Google Scholar] [CrossRef] [PubMed]
- Herquelot, E.; Bodin, J.; Roquelaure, Y.; Ha, C.; Leclerc, A.; Goldberg, M.; Zins, M.; Descatha, A. Work-related risk factors for lateral epicondylitis and other cause of elbow pain in the working population. Am. J. Ind. Med. 2013, 56, 400–409. [Google Scholar] [CrossRef]
- Curti, S.; Mattioli, S.; Bonfiglioli, R.; Farioli, A.; Violante, F.S. Elbow tendinopathy and occupational biomechanical overload: A systematic review with best-evidence synthesis. J. Occup. Health 2021, 63, e12186. [Google Scholar] [CrossRef]
- Seidel, D.H.; Ditchen, D.M.; Hoehne-Hückstädt, U.M.; Rieger, M.A.; Steinhilber, B. Quantitative Measures of Physical Risk Factors Associated with Work-Related Musculoskeletal Disorders of the Elbow: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- Descatha, A.; Albo, F.; Leclerc, A.; Carton, M.; Godeau, D.; Roquelaure, Y.; Petit, A.; Aublet-Cuvelier, A. Lateral Epicondylitis and Physical Exposure at Work? A Review of Prospective Studies and Meta-Analysis. Arthritis Care Res. 2016, 68, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Samagh, P.; Sudhakar, K.; Jindal, R. The impact of lateral epicondylitis on quality of life. Int. J. Physiother. 2015, 2, 627–632. [Google Scholar] [CrossRef]
- Bisset, L.M.; Vicenzino, B. Physiotherapy management of lateral epicondylalgia. J. Physiother. 2015, 61, 174–181. [Google Scholar] [CrossRef]
- Kim, Y.J.; Wood, S.M.; Yoon, A.P.; Howard, J.C.; Yang, L.Y.; Chung, K.C. Efficacy of Nonoperative Treatments for Lateral Epicondylitis: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2021, 147, 112–125. [Google Scholar] [CrossRef]
- Buchbinder, R.; Johnston, R.V.; Barnsley, L.; Assendelft, W.J.; Bell, S.N.; Smidt, N. Surgery for lateral elbow pain. Cochrane Database Syst. Rev. 2011, 2011, Cd003525. [Google Scholar] [CrossRef]
- Coombes, B.K.; Bisset, L.; Brooks, P.; Khan, A.; Vicenzino, B. Effect of Corticosteroid Injection, Physiotherapy, or Both on Clinical Outcomes in Patients with Unilateral Lateral Epicondylalgia. JAMA 2013, 309, 461. [Google Scholar] [CrossRef]
- Bisset, L.; Beller, E.; Jull, G.; Brooks, P.; Darnell, R.; Vicenzino, B. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: Randomised trial. BMJ 2006, 333, 939. [Google Scholar] [CrossRef]
- Cullinane, F.L.; Boocock, M.G.; Trevelyan, F.C. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin. Rehabil. 2014, 28, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef]
- Brennan, F.; Lohman, D.; Gwyther, L. Access to Pain Management as a Human Right. Am. J. Public Health 2019, 109, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Spink, A.; Wagner, I.; Orrock, P. Common reported barriers and facilitators for self-management in adults with chronic musculoskeletal pain: A systematic review of qualitative studies. Musculoskelet. Sci. Pract. 2021, 56, 102433. [Google Scholar] [CrossRef]
- Adamse, C.; Dekker-Van Weering, M.G.; van Etten-Jamaludin, F.S.; Stuiver, M.M. The effectiveness of exercise-based telemedicine on pain, physical activity and quality of life in the treatment of chronic pain: A systematic review. J. Telemed. Telecare 2018, 24, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, M.A.; Galea, O.A.; O’Leary, S.P.; Hill, A.J.; Russell, T.G. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: A systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Piga, M.; Cangemi, I.; Mathieu, A.; Cauli, A. Telemedicine for patients with rheumatic diseases: Systematic review and proposal for research agenda. Semin. Arthritis Rheum. 2017, 47, 121–128. [Google Scholar] [CrossRef]
- Shukla, H.; Nair, S.R.; Thakker, D. Role of telerehabilitation in patients following total knee arthroplasty: Evidence from a systematic literature review and meta-analysis. J. Telemed. Telecare 2017, 23, 339–346. [Google Scholar] [CrossRef]
- Wang, X.; Hunter, D.; Vesentini, G.; Pozzobon, D.; Ferreira, M. Technology-assisted rehabilitation following total knee or hip replacement for people with osteoarthritis: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 1–17. [Google Scholar] [CrossRef]
- Fiani, B.; Siddiqi, I.; Lee, S.C.; Dhillon, L. Telerehabilitation: Development, Application, and Need for Increased Usage in the COVID-19 Era for Patients with Spinal Pathology. Cureus 2020, 12, e10563. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Hefner, J.E.; Kodish-Wachs, J.E.; Iaccarino, M.A.; Paganoni, S. Telehealth in Physical Medicine and Rehabilitation: A Narrative Review. PM R 2017, 9, S51–S58. [Google Scholar] [CrossRef] [PubMed]
- Snoswell, C.L.; North, J.B.; Caffery, L.J. Economic Advantages of Telehealth and Virtual Health Practitioners: Return on Investment Analysis. JMIR Perioper. Med. 2020, 3, e15688. [Google Scholar] [CrossRef]
- Almojaibel, A.A.; Munk, N.; Goodfellow, L.T.; Fisher, T.F.; Miller, K.K.; Comer, A.R.; Bakas, T.; Justiss, M.D. Health Care Practitioners’ Determinants of Telerehabilitation Acceptance. Int. J. Telerehabilit. 2020, 12, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ramey, L.; Osborne, C.; Kasitinon, D.; Juengst, S. Apps and Mobile Health Technology in Rehabilitation: The Good, the Bad, and the Unknown. Phys. Med. Rehabil. Clin. N. Am. 2019, 30, 485–497. [Google Scholar] [CrossRef]
- Cranen, K.; Drossaert, C.H.; Brinkman, E.S.; Braakman-Jansen, A.L.; Ijzerman, M.J.; Vollenbroek-Hutten, M.M. An exploration of chronic pain patients’ perceptions of home telerehabilitation services. Health Expect. 2012, 15, 339–350. [Google Scholar] [CrossRef]
- Jiang, S.; Xiang, J.; Gao, X.; Guo, K.; Liu, B. The comparison of telerehabilitation and face-to-face rehabilitation after total knee arthroplasty: A systematic review and meta-analysis. J. Telemed. Telecare 2018, 24, 257–262. [Google Scholar] [CrossRef]
- Lade, H.; McKenzie, S.; Steele, L.; Russell, T.G. Validity and reliability of the assessment and diagnosis of musculoskeletal elbow disorders using telerehabilitation. J. Telemed. Telecare 2012, 18, 413–418. [Google Scholar] [CrossRef]
- Mayer, N.; Portnoy, S.; Palti, R.; Levanon, Y. The Efficacy of Tele-Rehabilitation Program for Improving Upper Limb Function among Adults Following Elbow Fractures: A Pilot Study. Appl. Sci. 2021, 11, 1708. [Google Scholar] [CrossRef]
- Slattery, B.W.; Haugh, S.; O’Connor, L.; Francis, K.; Dwyer, C.P.; O’Higgins, S.; Egan, J.; McGuire, B.E. An Evaluation of the Effectiveness of the Modalities Used to Deliver Electronic Health Interventions for Chronic Pain: Systematic Review with Network Meta-Analysis. J. Med. Internet Res. 2019, 21, e11086. [Google Scholar] [CrossRef]
- Correia, F.D.; Molinos, M.; Neves, C.; Janela, D.; Carvalho, D.; Luis, S.; Francisco, G.E.; Lains, J.; Bento, V. Digital Rehabilitation for Acute Ankle Sprains: Prospective Longitudinal Cohort Study. JMIR Rehabil. Assist. Technol. 2021, 8, e31247. [Google Scholar] [CrossRef] [PubMed]
- Correia, F.D.; Nogueira, A.; Magalhaes, I.; Guimaraes, J.; Moreira, M.; Barradas, I.; Molinos, M.; Teixeira, L.; Tulha, J.; Seabra, R.; et al. Medium-Term Outcomes of Digital Versus Conventional Home-Based Rehabilitation After Total Knee Arthroplasty: Prospective, Parallel-Group Feasibility Study. JMIR Rehabil. Assist. Technol. 2019, 6, e13111. [Google Scholar] [CrossRef] [PubMed]
- Correia, F.D.; Nogueira, A.; Magalhaes, I.; Guimaraes, J.; Moreira, M.; Barradas, I.; Teixeira, L.; Tulha, J.; Seabra, R.; Lains, J.; et al. Home-based Rehabilitation with A Novel Digital Biofeedback System versus Conventional In-person Rehabilitation after Total Knee Replacement: A feasibility study. Sci. Rep. 2018, 8, 11299. [Google Scholar] [CrossRef] [PubMed]
- Dias Correia, F.; Nogueira, A.; Magalhaes, I.; Guimaraes, J.; Moreira, M.; Barradas, I.; Molinos, M.; Teixeira, L.; Pires, J.; Seabra, R.; et al. Digital Versus Conventional Rehabilitation after Total Hip Arthroplasty: A Single-Center, Parallel-Group Pilot Study. JMIR Rehabil. Assist. Technol. 2019, 6, e14523. [Google Scholar] [CrossRef]
- Correia, F.D.; Molinos, M.; Luís, S.; Carvalho, D.; Carvalho, C.; Costa, P.; Seabra, R.; Francisco, G.; Bento, V.; Lains, J. Digitally assisted versus conventional home-based rehabilitation after Arthroscopic Rotator Cuff Repair: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2021, 101, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Janela, D.; Molinos, M.; Lains, J.; Francisco, G.E.; Bento, V.; Dias Correia, F. Telerehabilitation of acute musculoskeletal multi-disorders: Prospective, single-arm, interventional study. BMC Musculoskelet. Disord. 2022, 23, 29. [Google Scholar] [CrossRef]
- Janela, D.; Costa, F.; Molinos, M.; Moulder, R.G.; Lains, J.; Francisco, G.E.; Bento, V.; Cohen, S.P.; Correia, F.D. Asynchronous and Tailored Digital Rehabilitation of Chronic Shoulder Pain: A Prospective Longitudinal Cohort Study. J. Pain Res. 2022, 15, 53–66. [Google Scholar] [CrossRef]
- Evans, J.P.; Porter, I.; Gangannagaripalli, J.B.; Bramwell, C.; Davey, A.; Smith, C.D.; Fine, N.; Goodwin, V.A.; Valderas, J.M. Assessing Patient-Centred Outcomes in Lateral Elbow Tendinopathy: A Systematic Review and Standardised Comparison of English Language Clinical Rating Systems. Sports Med.-Open 2019, 5, 10. [Google Scholar] [CrossRef]
- Mintken, P.E.; Glynn, P.; Cleland, J.A. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J. Shoulder Elb. Surg. 2009, 18, 920–926. [Google Scholar] [CrossRef]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J. Orthop. Sports Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef]
- Randall, D.J.; Zhang, Y.; Harris, A.P.; Qiu, Y.; Li, H.; Stephens, A.R.; Kazmers, N.H. The minimal clinically important difference of the Patient-Reported Outcomes Measurement Information System (PROMIS) physical function and upper extremity computer adaptive tests and QuickDASH in the setting of elbow trauma. JSES Int. 2021, 5, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Budtz, C.R.; Andersen, J.H.; de Vos Andersen, N.-B.; Christiansen, D.H. Responsiveness and minimal important change for the quick-DASH in patients with shoulder disorders. Health Qual. Life Outcomes 2018, 16, 226. [Google Scholar] [CrossRef]
- Rysstad, T.; Grotle, M.; Klokk, L.P.; Tveter, A.T. Responsiveness and minimal important change of the QuickDASH and PSFS when used among patients with shoulder pain. BMC Musculoskelet. Disord. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Macdermid, J.C.; Silbernagel, K.G. Outcome Evaluation in Tendinopathy: Foundations of Assessment and a Summary of Selected Measures. J. Orthop. Sports Phys. Ther. 2015, 45, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Bijker, L.; Sleijser-Koehorst, M.L.S.; Coppieters, M.W.; Cuijpers, P.; Scholten-Peeters, G.G.M. Preferred Self-Administered Questionnaires to Assess Depression, Anxiety and Somatization in People with Musculoskeletal Pain—A Modified Delphi Study. J. Pain 2020, 21, 409–417. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef]
- George, S.Z.; Stryker, S.E. Fear-avoidance beliefs and clinical outcomes for patients seeking outpatient physical therapy for musculoskeletal pain conditions. J. Orthop. Sports Phys. Ther. 2011, 41, 249–259. [Google Scholar] [CrossRef]
- Mintken, P.E.; Cleland, J.A.; Whitman, J.M.; George, S.Z. Psychometric properties of the Fear-Avoidance Beliefs Questionnaire and Tampa Scale of Kinesiophobia in patients with shoulder pain. Arch. Phys. Med. Rehabil. 2010, 91, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Ospina, M.; Dennett, L.; Waye, A.; Jacobs, P. A systematic review of the measurement properties of self report instruments that assess presenteeism. Am. J. Manag. Care 2015, 21, e171–e185. [Google Scholar]
- McNeish, D.; Matta, T. Differentiating between mixed-effects and latent-curve approaches to growth modeling. Behav. Res. Methods 2018, 50, 1398–1414. [Google Scholar] [CrossRef]
- Pfaffel, A.; Schober, B.; Spiel, C. A Comparison of Three Approaches to Correct for Direct and Indirect Range Restrictions: A Simulation Study. Pract. Assess. 2016, 21, 1–15. [Google Scholar]
- Xiao, J.; Bulut, O. Evaluating the Performances of Missing Data Handling Methods in Ability Estimation from Sparse Data. Educ. Psychol. Meas. 2020, 80, 932–954. [Google Scholar] [CrossRef]
- Duncan, T.E.; Duncan, S.C. An introduction to latent growth curve modeling. Behav. Ther. 2004, 35, 333–363. [Google Scholar] [CrossRef]
- Ferrer, E.; Hamagami, F.; McArdle, J.J. Modeling Latent Growth Curves with Incomplete Data Using Different Types of Structural Equation Modeling and Multilevel Software. Struct. Equ. Model. A Multidiscip. J. 2004, 11, 452–483. [Google Scholar] [CrossRef]
- Kenneth, A.B.; Curran, P.J. Latent Curve Models: A Structural Equation Perspective; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Preacher, K.J.; Wichman, A.L.; MacCallum, R.C.; Briggs, N.E. Latent Growth Curve Modeling; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2008. [Google Scholar] [CrossRef]
- Iacobucci, D. Structural equations modeling: Fit Indices, sample size, and advanced topics. J. Consum. Psychol. 2010, 20, 90–98. [Google Scholar] [CrossRef]
- Brown, T.A. Confirmatory Factor Analysis for Applied Research; The Guilford Press: New York, NY, USA, 2006; pp. 81–88. [Google Scholar]
- Croisier, J.-L.; Foidart-Dessalle, M.; Tinant, F.; Crielaard, J.-M.; Forthomme, B. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br. J. Sports Med. 2007, 41, 269–275. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, T.-H.; Lim, K.-B. Effects of eccentric control exercise for wrist extensor and shoulder stabilization exercise on the pain and functions of tennis elbow. J. Phys. Ther. Sci. 2018, 30, 590–594. [Google Scholar] [CrossRef]
- Martinez-Silvestrini, J.A.; Newcomer, K.L.; Gay, R.E.; Schaefer, M.P.; Kortebein, P.; Arendt, K.W. Chronic lateral epicondylitis: Comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J. Hand Ther. 2005, 18, 411–419, quiz 420. [Google Scholar] [CrossRef]
- McQueen, K.S.; Powell, R.K.; Keener, T.; Whalley, R.; Calfee, R.P. Role of strengthening during nonoperative treatment of lateral epicondyle tendinopathy. J. Hand Ther. 2021, 34, 619–626. [Google Scholar] [CrossRef]
- Padasala, M.; Sharmila, B.; Bhatt, H.J.; D’Onofrio, R. Comparison of efficacy of the eccentric concentric training of wrist extensors with static stretching versus eccentric concentric training with supinator strengthening in patients with tennis elbow: A randomized clinical trial. Ital. J. Sports Rehabil. Posturology 2020, 7, 1597–1623. [Google Scholar]
- Park, J.Y.; Park, H.K.; Choi, J.H.; Moon, E.S.; Kim, B.S.; Kim, W.S.; Oh, K.S. Prospective evaluation of the effectiveness of a home-based program of isometric strengthening exercises: 12-month follow-up. Clin. Orthop. Surg. 2010, 2, 173–178. [Google Scholar] [CrossRef]
- Peterson, M.; Butler, S.; Eriksson, M.; Svärdsudd, K. A randomized controlled trial of exercise versus wait-list in chronic tennis elbow (lateral epicondylosis). Upsala J. Med. Sci. 2011, 116, 269–279. [Google Scholar] [CrossRef]
- Peterson, M.; Butler, S.; Eriksson, M.; Svärdsudd, K. A randomized controlled trial of eccentric vs. concentric graded exercise in chronic tennis elbow (lateral elbow tendinopathy). Clin. Rehabil. 2014, 28, 862–872. [Google Scholar] [CrossRef]
- Sethi, K.; Noohu, M.M. Scapular muscles strengthening on pain, functional outcome and muscle activity in chronic lateral epicondylalgia. J. Orthop. Sci. 2018, 23, 777–782. [Google Scholar] [CrossRef]
- Stasinopoulos, D.; Stasinopoulos, I. Comparison of effects of eccentric training, eccentric-concentric training, and eccentric-concentric training combined with isometric contraction in the treatment of lateral elbow tendinopathy. J. Hand Ther. 2017, 30, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Stasinopoulos, D.; Stasinopoulos, I.; Pantelis, M.; Stasinopoulou, K. Comparison of effects of a home exercise programme and a supervised exercise programme for the management of lateral elbow tendinopathy. Br. J. Sports Med. 2010, 44, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Tyler, T.F.; Nicholas, S.J.; Schmitt, B.M.; Mullaney, M.; Hogan, D.E. Clinical outcomes of the addition of eccentrics for rehabilitation of previously failed treatments of golfers elbow. Int. J. Sports Phys. 2014, 9, 365–370. [Google Scholar]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.J.; Coppieters, M.W.; Ross, L.; Hughes, I.; Vicenzino, B.; Schmid, A.B. Group education, night splinting and home exercises reduce conversion to surgery for carpal tunnel syndrome: A multicentre randomised trial. J. Physiother. 2020, 66, 97–104. [Google Scholar] [CrossRef]
- Rozmaryn, L.M.; Dovelle, S.; Rothman, E.R.; Gorman, K.; Olvey, K.M.; Bartko, J.J. Nerve and tendon gliding exercises and the conservative management of carpal tunnel syndrome. J. Hand Ther. 1998, 11, 171–179. [Google Scholar] [CrossRef]
- Modi, C.S.; Veillette, C.J.H.; Gandhi, R.; Perruccio, A.V.; Rampersaud, Y.R. Factors that influence the choice to undergo surgery for shoulder and elbow conditions. Clin. Orthop. Relat. Res. 2014, 472, 883–891. [Google Scholar] [CrossRef]
- Kuhn, J.E.; Dunn, W.R.; Sanders, R.; An, Q.; Baumgarten, K.M.; Bishop, J.Y.; Brophy, R.H.; Carey, J.L.; Holloway, B.G.; Jones, G.L.; et al. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: A multicenter prospective cohort study. J. Shoulder Elb. Surg. 2013, 22, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Haik, M.N.; Alburquerque-Sendín, F.; Fernandes, R.A.S.; Kamonseki, D.H.; Almeida, L.A.; Liebano, R.E.; Camargo, P.R. Biopsychosocial Aspects in Individuals with Acute and Chronic Rotator Cuff Related Shoulder Pain: Classification Based on a Decision Tree Analysis. Diagnostics 2020, 10, 928. [Google Scholar] [CrossRef]
- Kamper, S.J.; Logan, G.; Copsey, B.; Thompson, J.; Machado, G.C.; Abdel-Shaheed, C.; Williams, C.M.; Maher, C.G.; Hall, A.M. What is usual care for low back pain? A systematic review of health care provided to patients with low back pain in family practice and emergency departments. Pain 2020, 161, 694–702. [Google Scholar] [CrossRef]
- van Erp, R.M.A.; Huijnen, I.P.J.; Jakobs, M.L.G.; Kleijnen, J.; Smeets, R. Effectiveness of Primary Care Interventions Using a Biopsychosocial Approach in Chronic Low Back Pain: A Systematic Review. Pain Pract. 2019, 19, 224–241. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, S.; Young, L.; Li, F. The Effectiveness of Group-Based Physiotherapy-Led Behavioral Psychological Interventions on Adults with Chronic Low Back Pain: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2019, 98, 215–225. [Google Scholar] [CrossRef]
- Thiese, M.S.; Hegmann, K.T.; Kapellusch, J.; Merryweather, A.; Bao, S.; Silverstein, B.; Tang, R.; Garg, A. Psychosocial Factors Related to Lateral and Medial Epicondylitis: Results from Pooled Study Analyses. J. Occup. Environ. Med. 2016, 58, 588–593. [Google Scholar] [CrossRef][Green Version]
- Alhowimel, A.; AlOtaibi, M.; Radford, K.; Coulson, N. Psychosocial factors associated with change in pain and disability outcomes in chronic low back pain patients treated by physiotherapist: A systematic review. SAGE Open Med. 2018, 6, 2050312118757387. [Google Scholar] [CrossRef] [PubMed]
- Luque-Suarez, A.; Martinez-Calderon, J.; Falla, D. Role of kinesiophobia on pain, disability and quality of life in people suffering from chronic musculoskeletal pain: A systematic review. Br. J. Sports Med. 2019, 53, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Wertli, M.M.; Rasmussen-Barr, E.; Weiser, S.; Bachmann, L.M.; Brunner, F. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: A systematic review. Spine J. 2014, 14, 816–836.e814. [Google Scholar] [CrossRef]
- Bennell, K.L.; Marshall, C.J.; Dobson, F.; Kasza, J.; Lonsdale, C.; Hinman, R.S. Does a Web-Based Exercise Programming System Improve Home Exercise Adherence for People with Musculoskeletal Conditions? A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2019, 98, 850–858. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Entire Cohort |
|---|---|
| Age (years), mean (SD) | 51.3 (9.9) |
| Age categories (years), N (%): | |
| <25 | 0 (0.0) |
| 25–40 | 20 (15.2) |
| 40–60 | 89 (67.4) |
| >60 | 23 (17.4) |
| Sex, N (%) | |
| Female | 115 (60.8) |
| Male | 73 (38.6) |
| BMI, mean (SD) | 26.8 (5.3) |
| BMI categories, N (%): | |
| Underweight (<18.5) | 0 (0.0) |
| Normal (18.5–25) | 62 (47.0) |
| Overweight (25–30) | 38 (28.8) |
| Obese (30–40) | 29 (22.0) |
| Morbidly obese (>40) | 3 (2.3) |
| Laterality | |
| Left | 37 (28.0) |
| Right | 88 (66.7) |
| Bilateral | 7 (5.3) |
| Elbow pain-related condition, N (%): | |
| Tendinopathies | 100 (75.8) |
| Lateral elbow tendinopathy | 66 (50.0) |
| Medial elbow tendinopathy | 26 (19.7) |
| Other tendinopathies | 8 (6.1) |
| Elbow pain after non-traumatic injury | 9 (6.8) |
| Elbow pain after traumatic injury | 6 (4.5) |
| Distal nerve entrapment neuropathy | 11 (8.3) |
| Non-specific | 6 (4.6) |
| Pain duration, N (%): | |
| Acute (<12 weeks) | 72 (54.5) |
| 0–4 weeks | 12 (9) |
| 4–12 weeks | 60 (45) |
| Chronic (>12 weeks) | 60 (45.5) |
| <6 months | 29 (22) |
| 6–12 months | 19 (14) |
| ≥1 year | 12 (10) |
| Employment status, N (%): | |
| Employed (part-time or full-time) | 123 (93.2) |
| Unemployed (not working or retired) | 9 (6.8) |
| Occupation type, N (%): | |
| White collar | 68 (51.5) |
| Blue collar | 40 (30.3) |
| Other (e.g., retired) | 19 (14.4) |
| Not available | 5 (3.8) |
| Outcome, Mean (95% CI) | N | Baseline | End-Of-Program | Mean Change | % Change |
|---|---|---|---|---|---|
| QuickDASH | 130 | 24.48 (21.94; 27.01) | 12.56 (10.25; 14.87) | 11.92 (9.80; 14.04) | 48.7% |
| Pain Level | 132 | 4.27 (3.96; 4.57) | 2.00 (1.73; 2.27) | 2.27 (1.92; 2.61) | 53.1% |
| Surgery Intent > 0 | 50 | 7.58 (2.83; 12.33) | 3.22 (1.62; 4.83) | 4.36 (0.37; 8.35) | 57.5% |
| Surgery Intent | 132 | 3.70 (1.96; 5.44) | 1.34 (0.61; 2.06) | 2.36 (0.84; 3.89) | 63.9% |
| FABQ-PA | 132 | 12.21 (11.28; 13.15) | 8.03 (6.82; 9.24) | 4.18 (3.02; 5.34) | 34.2% |
| GAD-7 ≥ 5 | 16 | 8.12 (6.40; 9.85) | 3.26 (1.74; 4.79) | 4.86 (3.49; 6.23) | 59.8% |
| GAD-7 | 132 | 1.87 (1.37; 2.37) | 1.28 (0.83; 1.74) | 0.59 (0.12; 1.05) | 31.4% |
| PHQ-9 ≥ 5 | 19 | 8.36 (6.94; 9.77) | 2.60 (1.14; 4.06) | 5.76 (3.74; 7.77) | 68.9% |
| PHQ-9 | 132 | 1.86 (1.32; 2.41) | 0.99 (0.56; 1.43) | 0.87 (0.28; 1.46) | 46.7% |
| WPAI Overall > 0 | 46 | 26.14 (15.36; 36.92) | 7.24 (1.39; 13.08) | 18.90 (9.20; 28.60) | 72.3% |
| WPAI Overall | 117 | 7.74 (4.31; 11.16) | 3.71 (1.99; 5.43) | 4.03 (1.32; 6.74) | 52.1% |
| WPAI Work > 0 | 45 | 24.34 (16.15; 32.53) | 5.94 (0.83; 11.04) | 18.40 (11.52; 25.28) | 75.6% |
| WPAI Work | 117 | 7.50 (4.51; 10.49) | 3.31 (1.69; 4.94) | 4.19 (1.81; 6.57) | 55.8% |
| WPAI Activity > 0 | 104 | 30.08 (26.06; 34.11) | 9.78 (6.30; 13.25) | 20.30 (16.14; 24.27) | 67.5% |
| WPAI Activity | 132 | 23.03 (18.98; 27.08) | 8.77 (5.92; 11.62) | 14.26 (10.46; 18.07) | 61.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janela, D.; Costa, F.; Molinos, M.; Moulder, R.G.; Lains, J.; Bento, V.; Scheer, J.K.; Yanamadala, V.; Cohen, S.P.; Correia, F.D. Digital Rehabilitation for Elbow Pain Musculoskeletal Conditions: A Prospective Longitudinal Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 9198. https://doi.org/10.3390/ijerph19159198
Janela D, Costa F, Molinos M, Moulder RG, Lains J, Bento V, Scheer JK, Yanamadala V, Cohen SP, Correia FD. Digital Rehabilitation for Elbow Pain Musculoskeletal Conditions: A Prospective Longitudinal Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(15):9198. https://doi.org/10.3390/ijerph19159198
Chicago/Turabian StyleJanela, Dora, Fabíola Costa, Maria Molinos, Robert G. Moulder, Jorge Lains, Virgílio Bento, Justin K. Scheer, Vijay Yanamadala, Steven P. Cohen, and Fernando Dias Correia. 2022. "Digital Rehabilitation for Elbow Pain Musculoskeletal Conditions: A Prospective Longitudinal Cohort Study" International Journal of Environmental Research and Public Health 19, no. 15: 9198. https://doi.org/10.3390/ijerph19159198
APA StyleJanela, D., Costa, F., Molinos, M., Moulder, R. G., Lains, J., Bento, V., Scheer, J. K., Yanamadala, V., Cohen, S. P., & Correia, F. D. (2022). Digital Rehabilitation for Elbow Pain Musculoskeletal Conditions: A Prospective Longitudinal Cohort Study. International Journal of Environmental Research and Public Health, 19(15), 9198. https://doi.org/10.3390/ijerph19159198






