Comparison of the Effects of Aerobic versus Resistance Exercise on the Autonomic Nervous System in Middle-Aged Women: A Randomized Controlled Study
Abstract
:1. Introduction
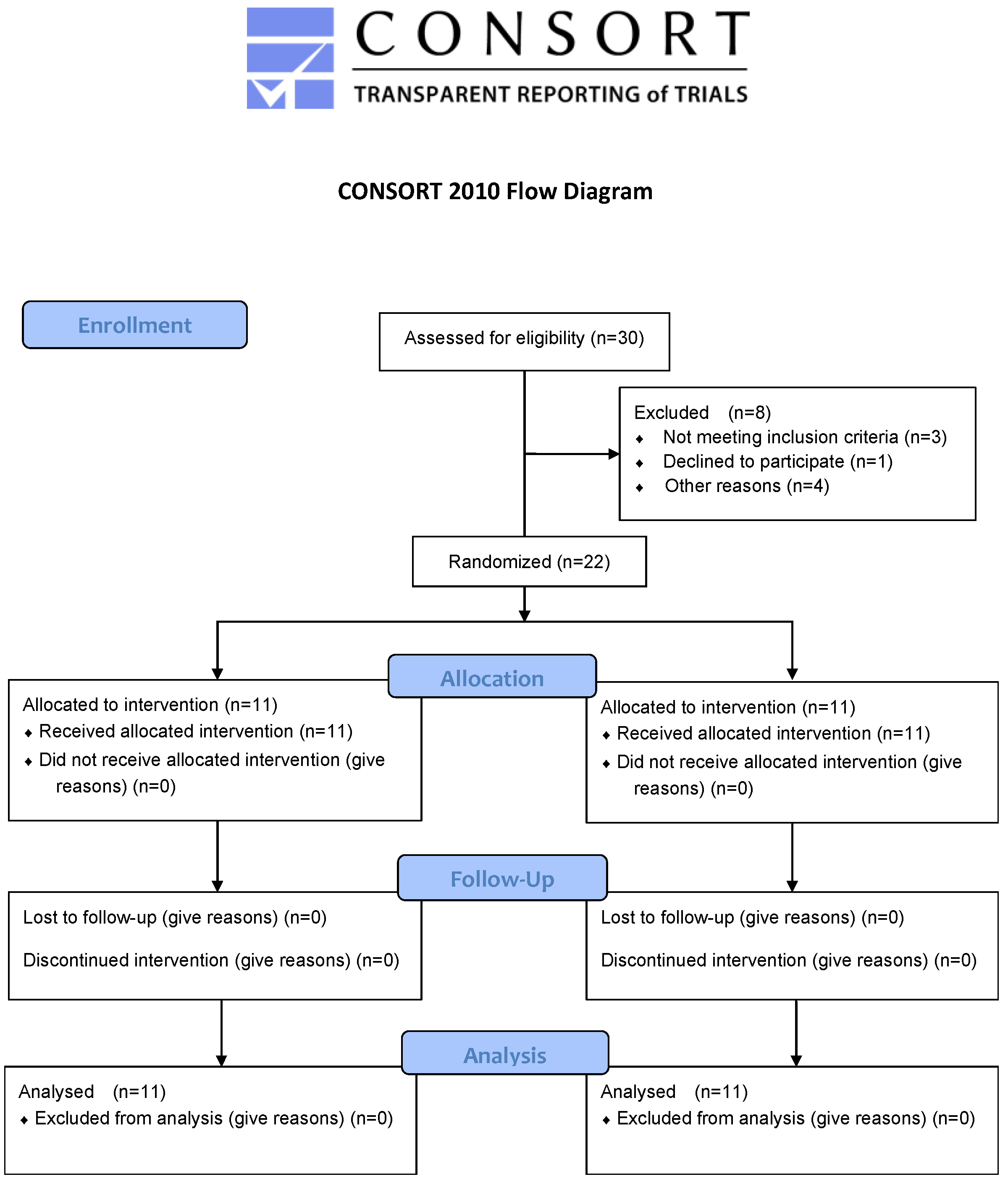
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Heart Rate Variability Measurement Device
2.4. Exercise Programs
2.5. Data Analysis
3. Results
3.1. LF Activity
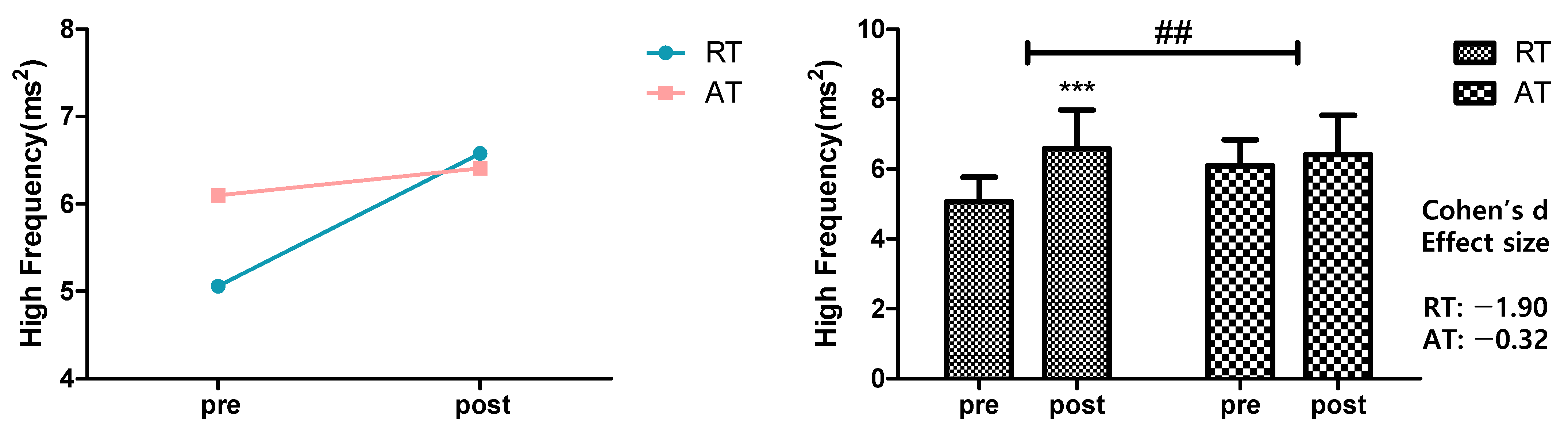
3.2. HF Activity
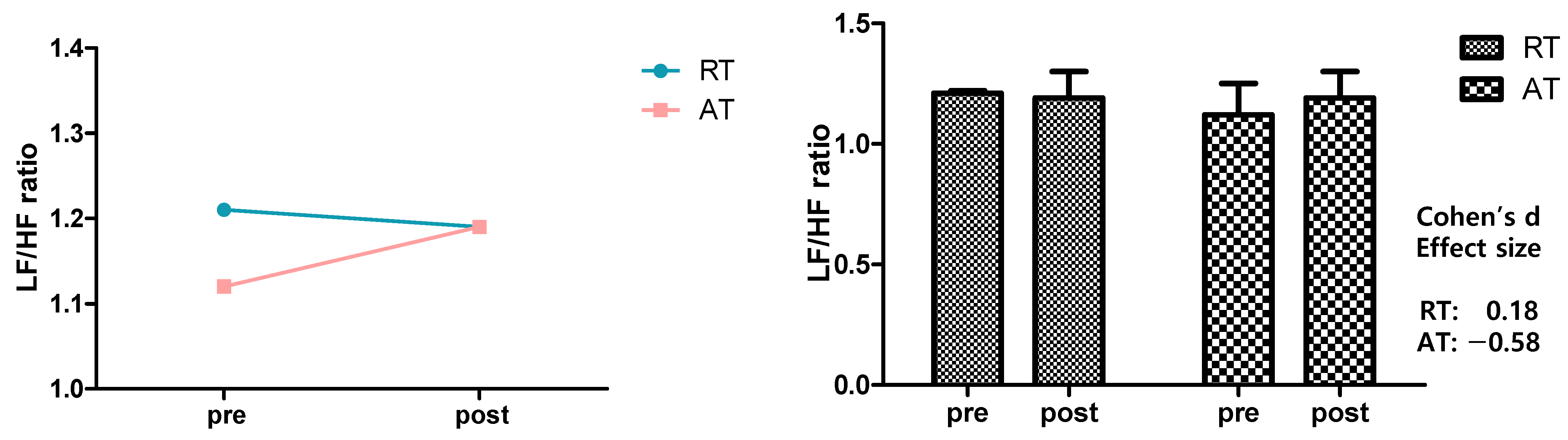
3.3. LF/HF Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascot, A.; Lemieux, S.; Lemieux, I.; Prud’Homme, D.; Tremblay, A.; Bouchard, C.; Nadeau, A.; Couillard, C.; Tchernof, A.; Bergeron, J.; et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care 1999, 22, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Lindle, R.S.; Metter, E.J.; Lynch, N.A.; Fleg, J.L.; Fozard, J.L.; Tobin, J.; Roy, T.A.; Hurley, B.F. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J. Appl. Physiol. 1997, 83, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Curtis, B.M.; O’Keefe, J. Autonomic Tone as a Cardiovascular Risk Factor: The Dangers of Chronic Fight or Flight. Mayo Clin. Proc. 2002, 77, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Moodithaya, S.; Avadhany, S.T. Gender Differences in Age-Related Changes in Cardiac Autonomic Nervous Function. J. Aging Res. 2011, 2012, 679345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.C.; Kuo, T.B.J.; Yang, C.C.H. Effects of estrogen on gender-related autonomic differences in humans. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2188–H2193. [Google Scholar] [CrossRef] [Green Version]
- Davy, K.P.; Desouza, C.A.; Jones, P.P.; Seals, D.R. Elevated Heart Rate Variability in Physically Active Young and Older Adult Women. Clin. Sci. 1998, 94, 579–584. [Google Scholar] [CrossRef]
- Parati, G.; Saul, J.P.; Di Rienzo, M.; Mancia, G. Power spectral Analysis of Blood Pressure and Heart Rate Variability in Evaluating Cardiovascular Regulation. Hypertension 1995, 25, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hughson, R.L.; Peterson, J.C. Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J. Appl. Physiol. 1991, 71, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Levine, B.D. Exercise and the autonomic nervous system. Handb Clin Neurol. 2013, 117, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Hudson, D.L.; Graitzer, H.M.; Raven, P.B. Exercise training bradycardia: The role of autonomic balance. Med. Sci. Sports Exerc. 1989, 21, 40–44. [Google Scholar] [CrossRef]
- Hautala, A.J.; Kiviniemi, A.M.; Mäkikallio, T.H.; Kinnunen, H.; Nissilä, S.; Huikuri, H.V.; Tulppo, M.P. Individual differences in the responses to endurance and resistance training. Eur. J. Appl. Physiol. 2005, 96, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.H.; Blair, S.N.; Murtagh, E.M. Accumulated versus continuous exercise for health benefit: A review of empirical studies. Sport. Med. 2009, 39, 29–43. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Program, K. Exercise for prevention and treatment of cardiovascular disease, type 2 diabetes, and metabolic syndrome. Curr. Diabetes Rep. 2007, 7, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bemben, D.A.; Fetters, N.L.; Bemben, M.G.; Nabavi, N.; Koh, E.T. Musculoskeletal responses to high- and low-intensity resistance training in early postmenopausal women. Med. Sci. Sports Exerc. 2000, 32, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Taylor, A.; Steptoe, A. The effect of acute aerobic exercise on stress related blood pressure responses: A systematic review and meta-analysis. Biol. Psychol. 2006, 71, 183–190. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, S.B.; Lenhard, A.; Mehanna, A.; de Souza, H.C.D.; de Aguiar Correa, F.M.; Hasser, E.M.; Martins-Pinge, M.C. Role of paraventricular nucleus in exercise training-induced autonomic modulation in conscious rats. Auton. Neurosci. 2009, 148, 28–35. [Google Scholar] [CrossRef]
- Mazzeo, R.S.; Marshall, P. Influence of plasma catecholamines on the lactate threshold during graded exercise. J. Appl. Physiol. 1989, 67, 1319–1322. [Google Scholar] [CrossRef]
- Iellamo, F.; Legramante, J.M.; Massaro, M.; Raimondi, G.; Galante, A. Effects of a Residential Exercise Training on Baroreflex Sensitivity and Heart Rate Variability in Patients with Coronary Artery Disease. Circulation 2000, 102, 2588–2592. [Google Scholar] [CrossRef] [Green Version]
- A Cornelissen, V.; Verheyden, B.; E Aubert, A.; Fagard, R.H. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J. Hum. Hypertens. 2010, 24, 175–182. [Google Scholar] [CrossRef]
- Hautala, A.J.; Kiviniemi, A.M.; Tulppo, M.P. Individual responses to aerobic exercise: The role of the autonomic nervous system. Neurosci. Biobehav. Rev. 2009, 33, 107–115. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S. Progressive Resistance Exercise and Resting Blood Pressure. Hypertension 2000, 35, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, J.D.; Figueroa, A. Acute and training effects of resistance exercise on heart rate variability. Clin. Physiol. Funct. Imaging 2016, 36, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H. Investigating collision risk factors perceived by navigation officers in a close-quarters situation using a ship bridge simulator. Cogn. Technol. Work 2021, 23, 419–428. [Google Scholar] [CrossRef]
- Kim, D.-H. Human factors influencing the ship operator’s perceived risk in the last moment of collision encounter. Reliab. Eng. Syst. Saf. 2020, 203, 107078. [Google Scholar] [CrossRef]
- Hedman, A.; Hartikainen, J.; Hakumaki, M. Physiological Background Underlying Short-Term Heart Rate Variability. Ann. Noninvasive Electrocardiol. 1998, 3, 267–280. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2017. [Google Scholar]
- Park, B.-S. The effects of strength, strength and walking, strength and yoga on obese middle-aged women’s physical fitness for 12 weeks. J. Korean Phys. Educ. Assoc. Girls Women 2008, 22, 53–65. [Google Scholar]
- Brzycki, M. Strength Testing—Predicting a One-Rep Max from Reps-to-Fatigue. J. Phys. Educ. Recreat. Dance 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MC, USA, 2013. [Google Scholar]
- Earnest, C.P.; Lavie, C.J.; Blair, S.N.; Church, T.S. Heart Rate Variability Characteristics in Sedentary Postmenopausal Women Following Six Months of Exercise Training: The DREW Study. PLoS ONE 2008, 3, e2288. [Google Scholar] [CrossRef]
- Jurca, R.; Church, T.S.; Morss, G.M.; Jordan, A.N.; Earnest, C.P. Eight weeks of moderate-intensity exercise training increases heart rate variability in sedentary postmenopausal women. Am. Heart J. 2004, 147, e8–e15. [Google Scholar] [CrossRef]
- Figueroa, A.; Kingsley, J.D.; McMillan, V.; Panton, L.B. Resistance exercise training improves heart rate variability in women with fibromyalgia. Clin. Physiol. Funct. Imaging 2007, 28, 49–54. [Google Scholar] [CrossRef]
- Hu, M.; Finni, T.; Zou, L.; Perhonen, M.; Sedliak, M.; Alen, M.; Cheng, S. Effects of strength training on work capacity and parasympathetic heart rate modulation during exercise in physically inactive men. Int. J. Sports Med. 2009, 30, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Malik, M. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, heart rate variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Borchard, U. The role of the sympathetic nervous system in cardiovascular disease. J. Clin. Basic Cardiol. 2001, 4, 175–177. [Google Scholar]
- Tsuji, H.; Larson, M.G.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of Reduced Heart Rate Variability on Risk for Cardiac Events. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, M.A.; Polessi, E.A.; Simioni, F.C. Evaluation of heart rate variability in trained and sedentary climacteric women. Arq. Bras. Cardiol. 2008, 90, 80–86. [Google Scholar] [CrossRef]
- Bhati, P.; Moiz, J.A.; Menon, G.R.; Hussain, M.E. Does resistance training modulate cardiac autonomic control? A systematic review and meta-analysis. Clin. Auton. Res. 2019, 29, 75–103. [Google Scholar] [CrossRef]

| Variables | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) | |
|---|---|---|---|---|---|
| Group | |||||
| RT (n = 11) | 50.4 ± 1.6 | 159.3 ± 2.8 | 63.6 ± 3.2 | 22.4 ± 2.3 | |
| AT (n = 11) | 49.1 ± 1.5 | 157.5 ± 3.9 | 61.1 ± 4.7 | 22.1 ± 3.0 | |
| Week | Order | Exercise | Intensity | Frequency | |
|---|---|---|---|---|---|
| Warn-Up (10 min) | Static Stretching | ||||
| 1–4 | Main exercise (40 min) | 1. Bench press 2. Leg press 3. Lat pull down 4. Leg curl 5. Shoulder Press 6. Leg extension 7. Biceps curl 8. Triceps extension | 3 set | 65% 1RM | 3 times/week |
| 5–8 | 3 set | 70% 1RM | |||
| 9–12 | 3 set | 75% 1RM | |||
| Cool-down (10 min) | Static stretching | ||||
| Week | Order | Exercise | Intensity | Frequency |
|---|---|---|---|---|
| Warn-Up (10 min) | Static Stretching | |||
| 1–4 | Main exercise (40 min) | Treadmill walking or jogging | 50–60% HRR | 3 times/week |
| 5–8 | 60–70% HRR | |||
| 9–12 | 70–75% HRR | |||
| Cool-down (10 min) | Static stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.K.; Lee, J.-H.; Ha, M.-S. Comparison of the Effects of Aerobic versus Resistance Exercise on the Autonomic Nervous System in Middle-Aged Women: A Randomized Controlled Study. Int. J. Environ. Res. Public Health 2022, 19, 9156. https://doi.org/10.3390/ijerph19159156
Lee CK, Lee J-H, Ha M-S. Comparison of the Effects of Aerobic versus Resistance Exercise on the Autonomic Nervous System in Middle-Aged Women: A Randomized Controlled Study. International Journal of Environmental Research and Public Health. 2022; 19(15):9156. https://doi.org/10.3390/ijerph19159156
Chicago/Turabian StyleLee, Chae Kwan, Jae-Hoon Lee, and Min-Seong Ha. 2022. "Comparison of the Effects of Aerobic versus Resistance Exercise on the Autonomic Nervous System in Middle-Aged Women: A Randomized Controlled Study" International Journal of Environmental Research and Public Health 19, no. 15: 9156. https://doi.org/10.3390/ijerph19159156
APA StyleLee, C. K., Lee, J.-H., & Ha, M.-S. (2022). Comparison of the Effects of Aerobic versus Resistance Exercise on the Autonomic Nervous System in Middle-Aged Women: A Randomized Controlled Study. International Journal of Environmental Research and Public Health, 19(15), 9156. https://doi.org/10.3390/ijerph19159156






