Nicotine Exerts Cytotoxic Effects in a Panel of Healthy Cell Lines and Strong Irritating Potential on Blood Vessels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cellular Viability Assessment
2.4. Cellular Morphology
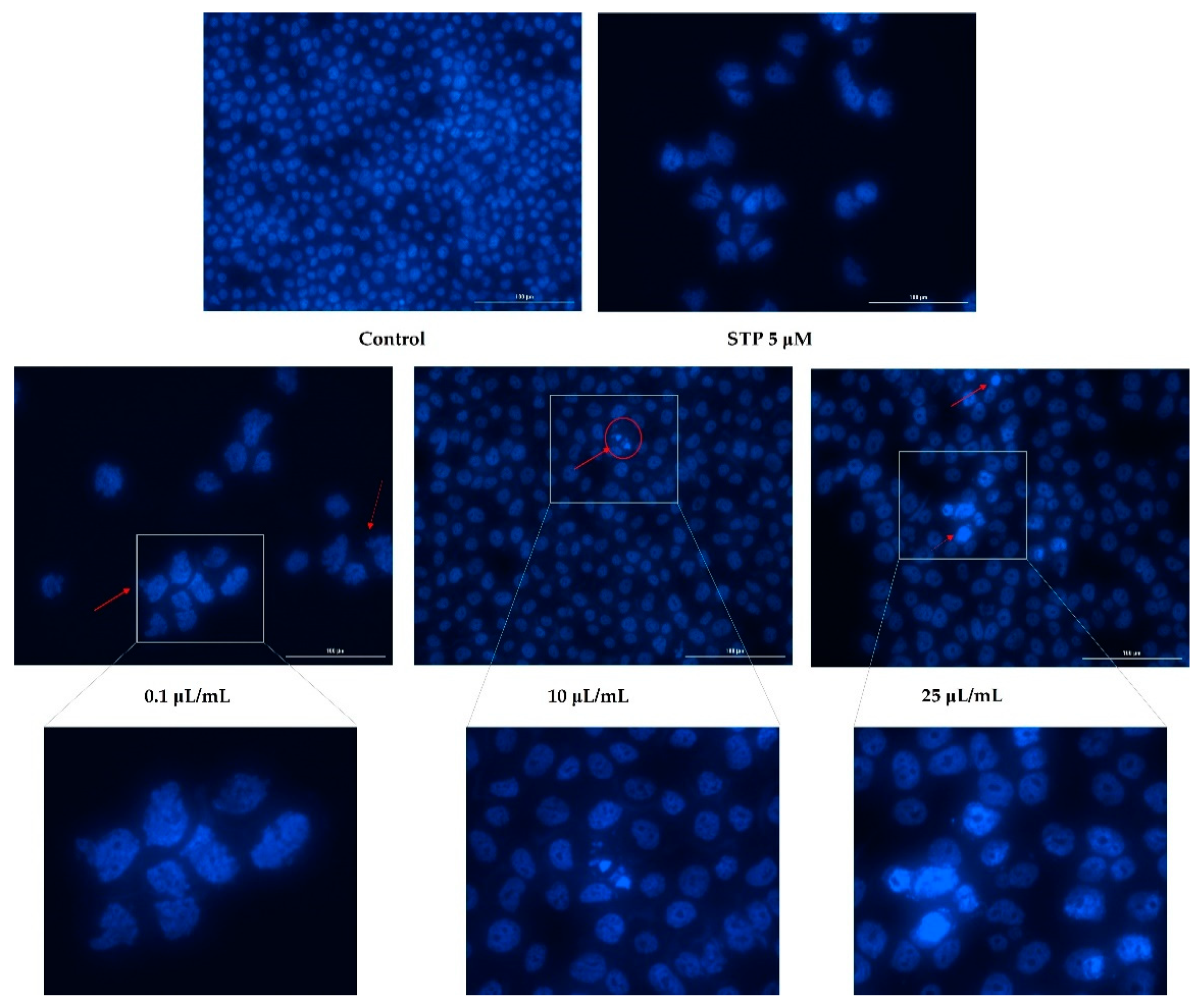
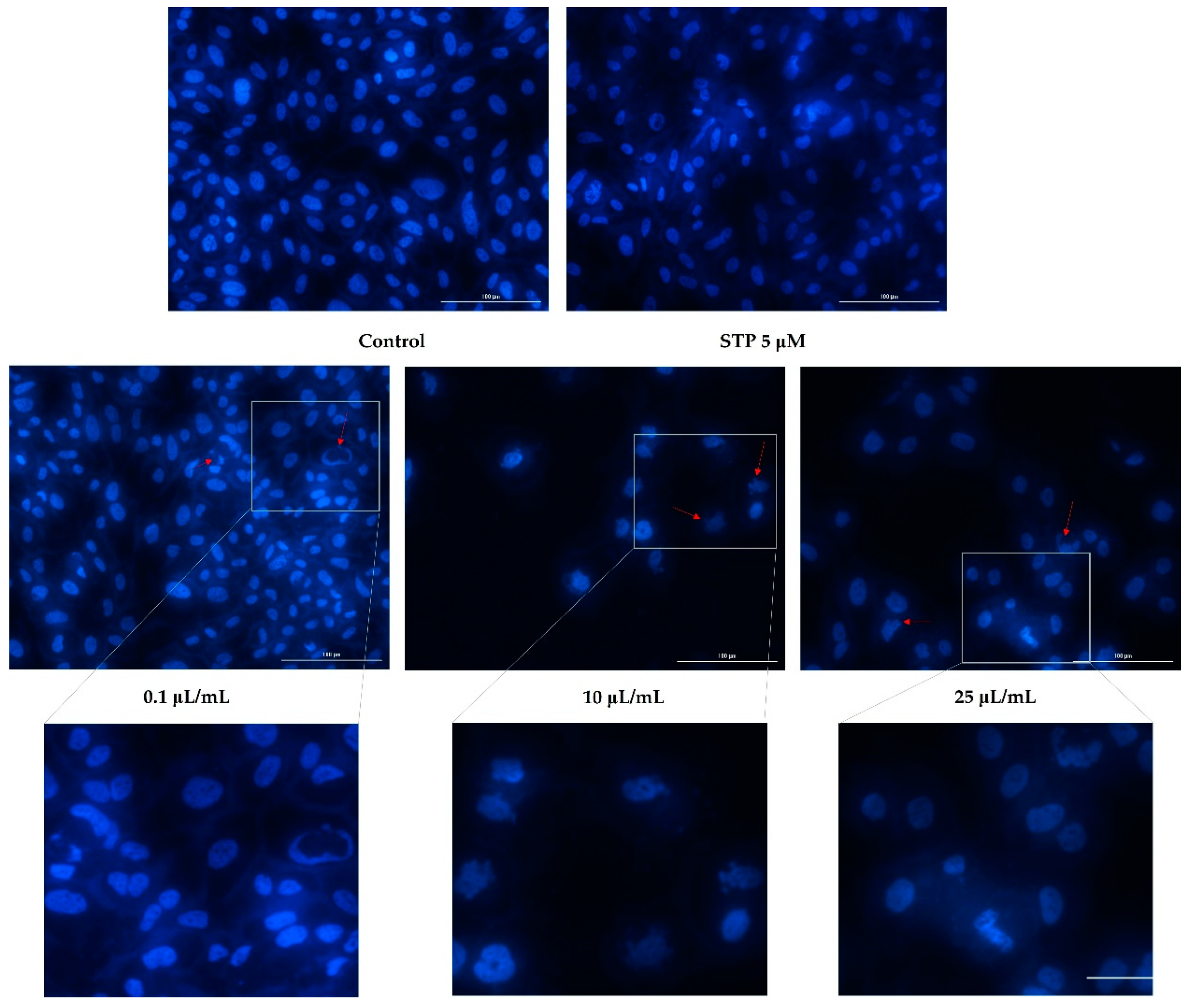
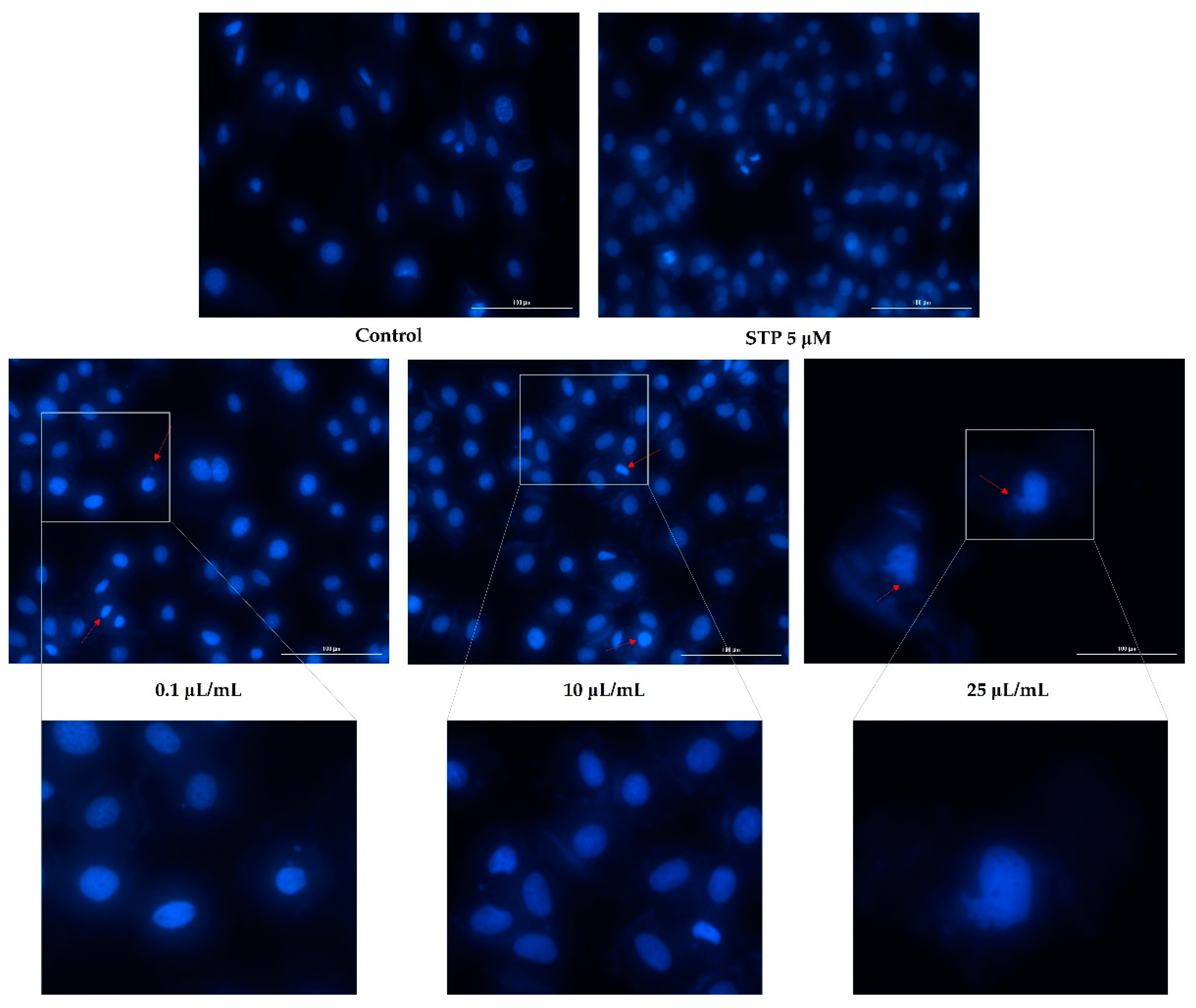
2.5. Nuclear Staining
2.6. Chorioallantoic Membrane (CAM) Assay
2.7. Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM) Assay
2.8. Statistical Analysis
3. Results
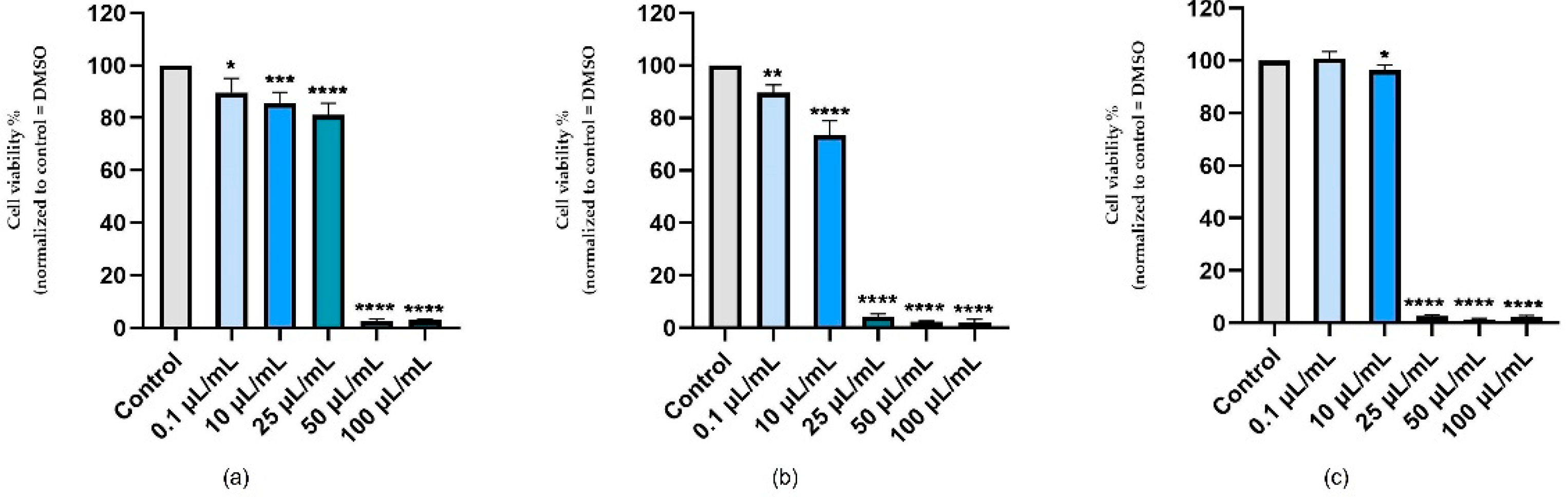
3.1. Cellular Viability Assessment
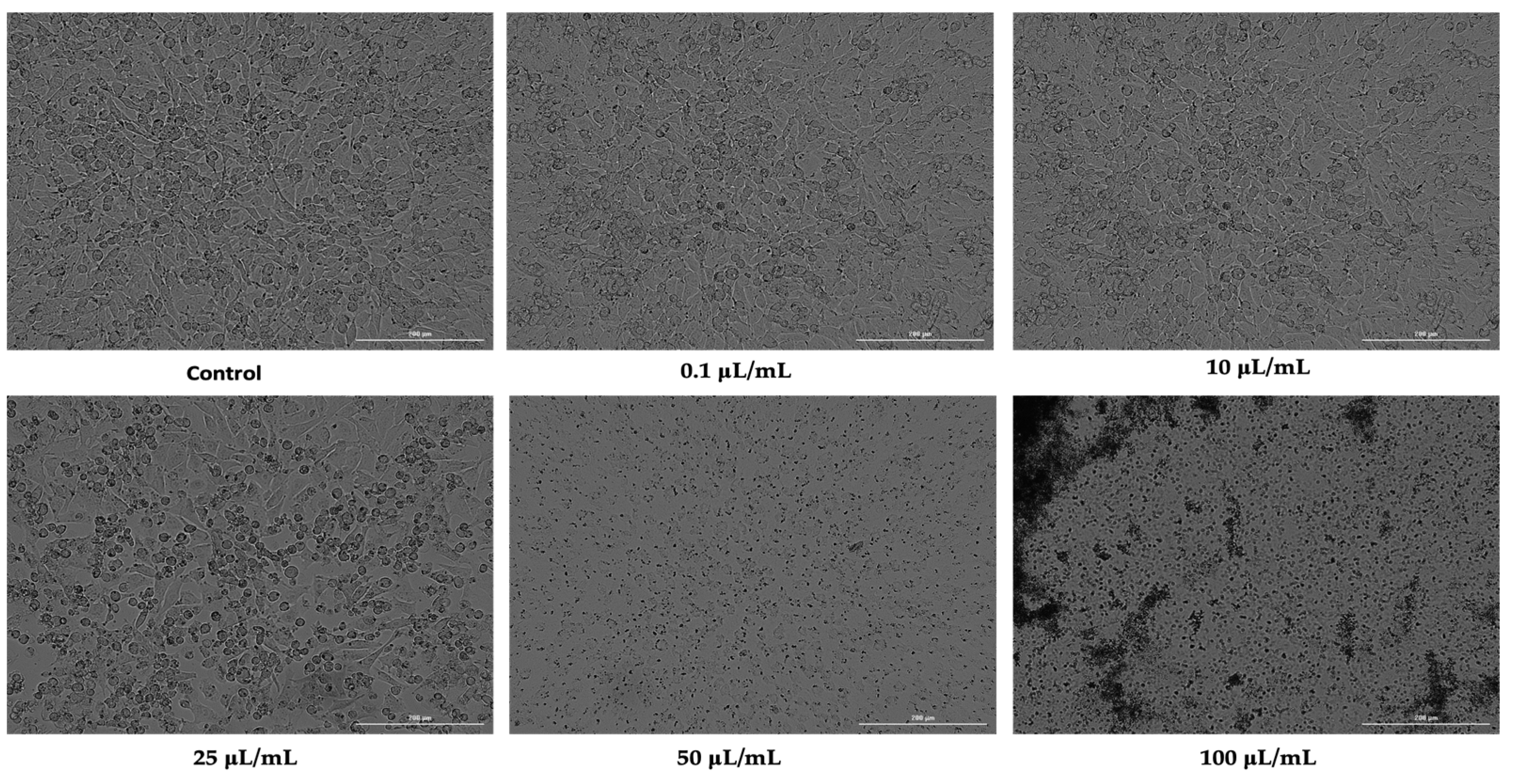
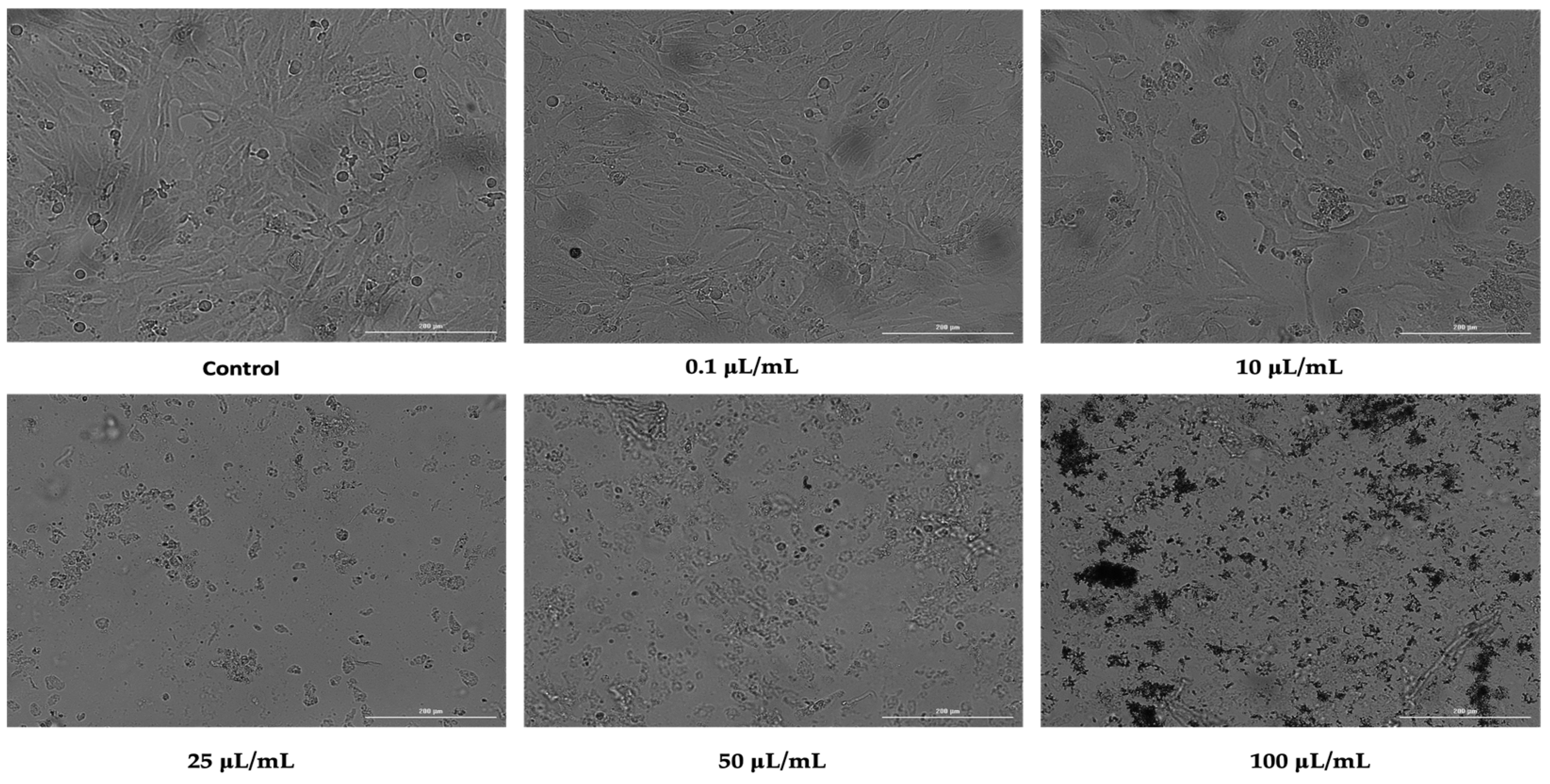
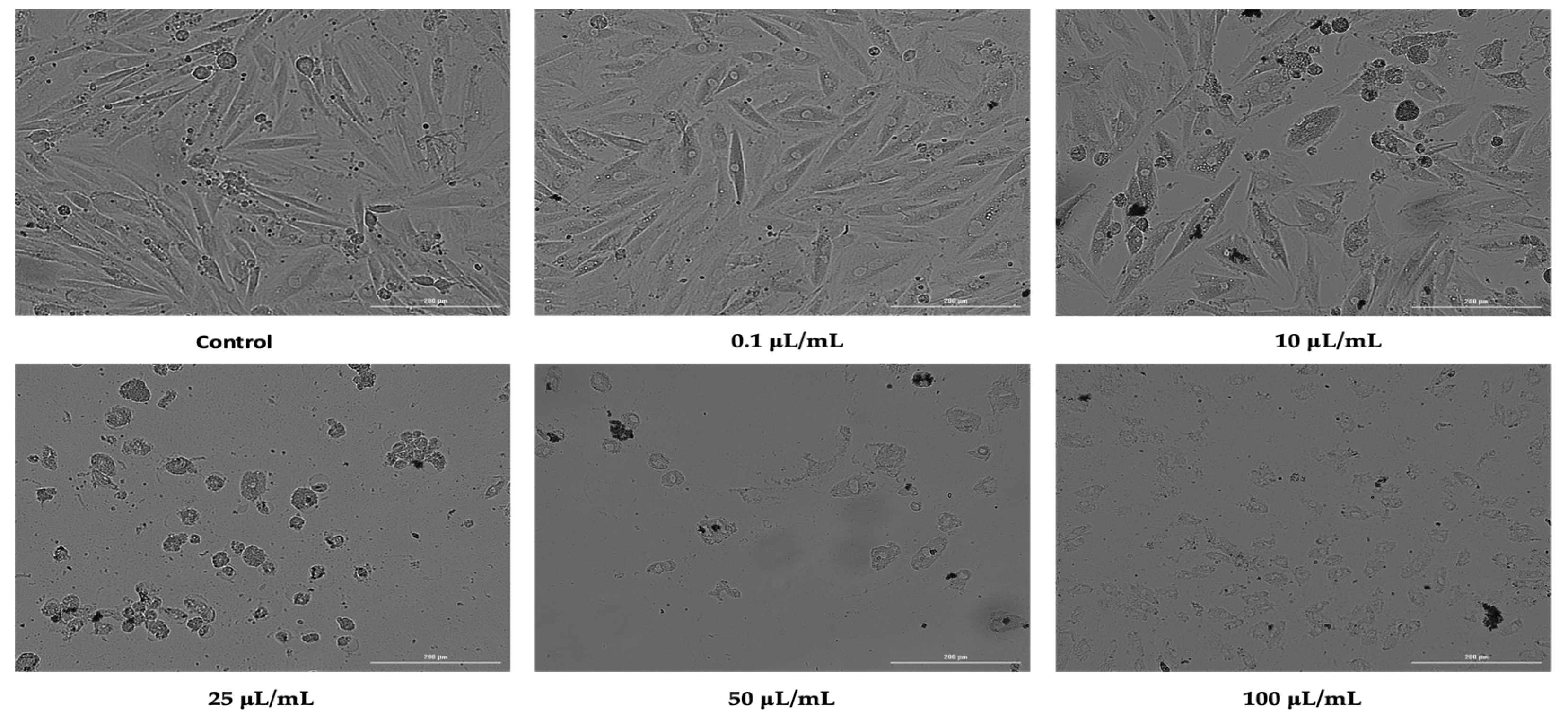
3.2. Cellular Morphology
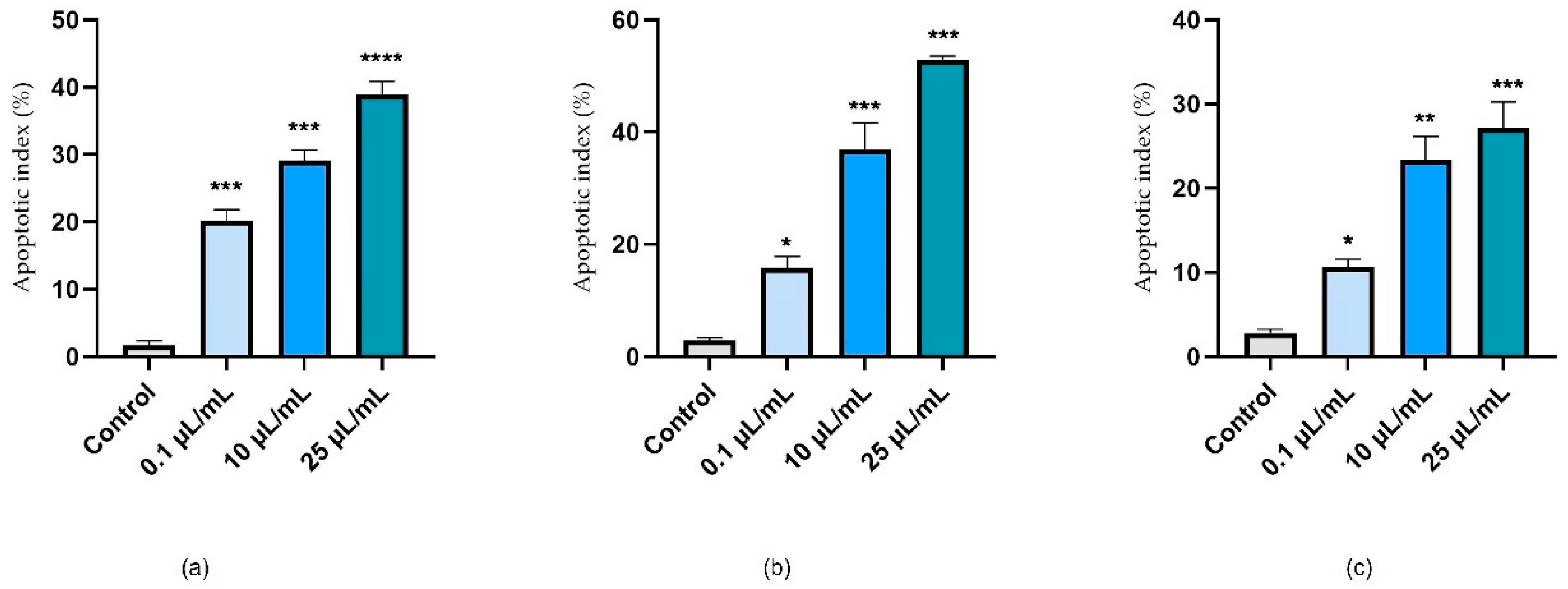
3.3. Nuclear Staining
3.4. HET-CAM Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Foll, B.; Piper, M.E.; Fowler, C.D.; Tonstad, S.; Bierut, L.; Lu, L.; Jha, P.; Hall, W.D. Tobacco and Nicotine Use. Nat. Rev. Dis. Prim. 2022, 8, 19. [Google Scholar] [CrossRef]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014.
- Marques, P.; Piqueras, L.; Sanz, M.-J. An Updated Overview of E-Cigarette Impact on Human Health. Respir. Res. 2021, 22, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-H.; Sun, J.Y.; Bonnevie, E.; Cummins, S.E.; Gamst, A.; Yin, L.; Lee, M. Four Hundred and Sixty Brands of E-Cigarettes and Counting: Implications for Product Regulation. Tob. Control 2014, 23, iii3–iii9. [Google Scholar] [CrossRef] [Green Version]
- Budzyńska, E.; Sielemann, S.; Puton, J.; Surminski, A.L.R.M. Analysis of E-Liquids for Electronic Cigarettes Using GC-IMS/MS with Headspace Sampling. Talanta 2020, 209, 120594. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Dunn, K.; Turfus, S. A Review of Nicotine-Containing Electronic Cigarettes—Trends in Use, Effects, Contents, Labelling Accuracy and Detection Methods. Drug Test. Anal. 2021, 13, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Chaturvedi, P.; Datta, S.; Sinukumar, S.; Joshi, P.; Garg, A. Harmful Effects of Nicotine. Indian J. Med. Paediatr. Oncol. 2015, 36, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Stepanov, I.; Carmella, S.G.; Briggs, A.; Hertsgaard, L.; Lindgren, B.; Hatsukami, D.; Hecht, S.S. Presence of the Carcinogen N’-Nitrosonornicotine in the Urine of Some Users of Oral Nicotine Replacement Therapy Products. Cancer Res. 2009, 69, 8236–8240. [Google Scholar] [CrossRef] [Green Version]
- Hall, B.J.; Wells, C.; Allenby, C.; Lin, M.Y.; Hao, I.; Marshall, L.; Rose, J.E.; Levin, E.D. Differential Effects of Non-Nicotine Tobacco Constituent Compounds on Nicotine Self-Administration in Rats. Pharmacol. Biochem. Behav. 2014, 120, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Misery, L. Nicotine Effects on Skin: Are They Positive or Negative? Exp. Dermatol. 2004, 13, 665–670. [Google Scholar] [CrossRef]
- Eltony, S.A.; Ali, S.S. Histological Study on the Effect of Nicotine on Adult Male Guinea Pig Thin Skin. Anat. Cell Biol. 2017, 50, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Rutledge, S.M.; Bao, M.; Asgharpour, A.; Rutledge, S.M. Smoking and Liver Disease. Gastroenterol. Hepatol. 2020, 16, 617–625. [Google Scholar]
- Xie, C.; Zhu, J.; Wang, X.; Chen, J.; Geng, S.; Wu, J.; Zhong, C.; Li, X. Tobacco Smoke Induced Hepatic Cancer Stem Cell-like Properties through IL-33/P38 Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 39. [Google Scholar] [CrossRef] [Green Version]
- Nouri-Shirazi, M.; Guinet, E. Evidence for the Immunosuppressive Role of Nicotine on Human Dendritic Cell Functions. Immunology 2003, 109, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular Toxicity of Nicotine: Implications for Electronic Cigarette Use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; US Government Printing Office: Atlanta, GA, USA, 2010; ISBN 0160840783.
- Zhou, X.; Sheng, Y.; Yang, R.; Kong, X. Nicotine Promotes Cardiomyocyte Apoptosis via Oxidative Stress and Altered Apoptosis-Related Gene Expression. Cardiology 2010, 115, 243–250. [Google Scholar] [CrossRef]
- Pinzaru, I.; Chioibas, R.; Marcovici, I.; Coricovac, D.; Susan, R.; Predut, D.; Georgescu, D.; Dehelean, C. Rutin Exerts Cytotoxic and Senescence-Inducing Properties in Human Melanoma Cells. Toxics 2021, 9, 226. [Google Scholar] [CrossRef]
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab–Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef]
- Afolalu, E.F.; Spies, E.; Bacso, A.; Clerc, E.; Abetz-Webb, L.; Gallot, S.; Chrea, C. Impact of Tobacco and/or Nicotine Products on Health and Functioning: A Scoping Review and Findings from the Preparatory Phase of the Development of a New Self-Report Measure. Harm Reduct. J. 2021, 18, 79. [Google Scholar] [CrossRef]
- Kesimer, M. Another Warning Sign: High Nicotine Content in Electronic Cigarettes Disrupts Mucociliary Clearance, the Essential Defense Mechanism of the Lung. Am. J. Respir. Crit. Care Med. 2019, 200, 1082–1084. [Google Scholar] [CrossRef]
- Taghavi, S.; Khashyarmanesh, Z.; Moalemzadeh-Haghighi, H.; Nassirli, H.; Eshraghi, P.; Jalali, N.; Hassanzadeh-Khayyat, M. Nicotine Content of Domestic Cigarettes, Imported Cigarettes and Pipe Tobacco in Iran. Addict. Health. 2012, 4, 28–35. [Google Scholar]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P., III. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. In Nicotine Psychopharmacology. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 29–60. [Google Scholar] [CrossRef] [Green Version]
- Theilig, C.; Bernd, A.; Ramirez-Bosca, A.; Görmar, F.F.; Bereiter-Hahn, J.; Keller-Stanislawski, B.; Sewell, A.C.; Rietbrock, N.; Holzmann, H. Reactions of Human Keratinocytes in Vitro after Application of Nicotine. Skin Pharmacol. 1994, 7, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Pozuelos, G.L.; Rubin, M.; Vargas, S.; Ramirez, E.; Bandaru, D.; Sha, J.; Wohlschlegel, J.; Talbot, P. Nicotine Affects Multiple Biological Processes in EpiDermTM Organotypic Tissues and Keratinocyte Monolayers. Atmosphere 2022, 13, 810. [Google Scholar] [CrossRef]
- Lee, H.-J.; Guo, H.-Y.; Lee, S.-K.; Jeon, B.-H.; Jun, C.-D.; Lee, S.-K.; Park, M.-H.; Kim, E.-C. Effects of Nicotine on Proliferation, Cell Cycle, and Differentiation in Immortalized and Malignant Oral Keratinocytes. J. Oral Pathol. Med. 2005, 34, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.; Rahimy, M.; Korrapati, A.; Xuan, Y.; Zou, A.E.; Krishnan, A.R.; Tsui, T.; Aguilera, J.A.; Advani, S.; Crotty Alexander, L.E.; et al. Electronic Cigarettes Induce DNA Strand Breaks and Cell Death Independently of Nicotine in Cell Lines. Oral Oncol. 2016, 52, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Cervellati, F.; Muresan, X.M.; Sticozzi, C.; Gambari, R.; Montagner, G.; Forman, H.J.; Torricelli, C.; Maioli, E.; Valacchi, G. Comparative Effects between Electronic and Cigarette Smoke in Human Keratinocytes and Epithelial Lung Cells. Toxicol. Vitro. 2014, 28, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, Z.N.; Alqahtani, A.; Almela, T.; Franklin, K.; Tayebi, L.; Moharamzadeh, K. Effects of Electronic Cigarette Liquid on Monolayer and 3D Tissue-Engineered Models of Human Gingival Mucosa. J. Adv. Periodontol. Implant Dent. 2019, 11, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Nagata, H.; Takagi, N.; Inoue, S.; Mizutani, Y. Nicotine Affects Tight Junction Barriers via Alpha7 Nicotine-like Acetylcholine Receptor in Keratinocytes. J. Dermatol. Sci. 2021, 103, 183–185. [Google Scholar] [CrossRef]
- Arredondo, J.; Chernyavsky, A.I.; Jolkovsky, D.L.; Pinkerton, K.E.; Grando, S.A. Receptor-Mediated Tobacco Toxicity: Acceleration of Sequential Expression of Alpha5 and Alpha7 Nicotinic Receptor Subunits in Oral Keratinocytes Exposed to Cigarette Smoke. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 1356–1368. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-Y.; Jiang, S.-K.; Wang, L.-L.; Zhang, M.-Z.; Wang, S.; Jiang, Z.-F.; Liu, Y.-L.; Cheng, H.; Zhang, M.; Zhao, R.; et al. A7-NAChR Activation Has an Opposite Effect on Healing of Covered and Uncovered Wounds. Inflammation 2018, 41, 474–484. [Google Scholar] [CrossRef]
- Rickard, B.P.; Ho, H.; Tiley, J.B.; Jaspers, I.; Brouwer, K.L.R. E-Cigarette Flavoring Chemicals Induce Cytotoxicity in HepG2 Cells. ACS Omega 2021, 6, 6708–6713. [Google Scholar] [CrossRef]
- Wieczorek, R.; Phillips, G.; Czekala, L.; Trelles Sticken, E.; O’Connell, G.; Simms, L.; Rudd, K.; Stevenson, M.; Walele, T. A Comparative in Vitro Toxicity Assessment of Electronic Vaping Product E-Liquids and Aerosols with Tobacco Cigarette Smoke. Toxicol. Vitro. 2020, 66, 104866. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, M.R.; Mahmoudian, Z.G.; Roshankhah, S.; Farokhi, M.; Jalili, C. Harmine Shows Therapeutic Activity on Nicotine-Induced Liver Failure in Mice. Histol. Histopathol. 2019, 34, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Azza, M.G.; Aliaa, M.I.; Nahed, S.B.; Sherin, M.M. Role of Green Tea on Nicotine Toxicity on Liver and Lung of Mice: Histological and Morphometrical Studies. Afr. J. Biotechnol. 2012, 11, 2013–2025. [Google Scholar] [CrossRef] [Green Version]
- Glickman, M.S.; Schluger, N. Adding Insult to Injury: Exacerbating TB Risk with Smoking. Cell Host Microbe 2016, 19, 432–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Guo, X.; Zhao, H.; An, R.; Lian, K.; Zhang, X.; Zhang, J.; Yan, F.; Xie, H.; Wang, S.; et al. Nicotine Induces H9C2 Cell Apoptosis via Akt Protein Degradation. Mol. Med. Rep. 2017, 16, 6269–6275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.X.; Liu, C.H.; Guo, R.; Meng, Z.J.; Gao, J.W.Y. Nicotine Promotes Apoptosis of Rat Cardiomyocyte Line H9C2 Cultured with High Glucose and High Fat. Basic Clin. Med. 2020, 40, 24–29. [Google Scholar]
- Farsalinos, K.E.; Romagna, G.; Allifranchini, E.; Ripamonti, E.; Bocchietto, E.; Todeschi, S.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Comparison of the Cytotoxic Potential of Cigarette Smoke and Electronic Cigarette Vapour Extract on Cultured Myocardial Cells. Int. J. Environ. Res. Public Health 2013, 10, 5146–5162. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Zhang, X.Q.; Kadono, T.; Matsuoka, N.; Rollins, D.; Badger, T.; Rodesch, C.K.; Barry, W.H. Direct Toxic Effects of Aqueous Extract of Cigarette Smoke on Cardiac Myocytes at Clinically Relevant Concentrations. Toxicol. Appl. Pharmacol. 2009, 236, 71–77. [Google Scholar] [CrossRef]
- Tippetts, T.S.; Winden, D.R.; Swensen, A.C.; Nelson, M.B.; Thatcher, M.O.; Saito, R.R.; Condie, T.B.; Simmons, K.J.; Judd, A.M.; Reynolds, P.R.; et al. Cigarette Smoke Increases Cardiomyocyte Ceramide Accumulation and Inhibits Mitochondrial Respiration. BMC Cardiovasc. Disord. 2014, 14, 165. [Google Scholar] [CrossRef] [Green Version]
- Kamceva, G.; Arsova-Sarafinovska, Z.; Ruskovska, T.; Zdravkovska, M.; Kamceva-Panova, L.; Stikova, E. Cigarette Smoking and Oxidative Stress in Patients with Coronary Artery Disease. Open Access Maced. J. Med. Sci. 2016, 4, 636–640. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, J.W.; Nasir, K.; DeFilippis, A.P.; Lima, J.A.C.; Bluemke, D.A.; Hundley, W.G.; Barr, R.G.; Budoff, M.J.; Szklo, M.; Navas-Acien, A.; et al. Relationship of Cigarette Smoking With Inflammation and Subclinical Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1002–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.; Itani, H.; Richmond, B.; Arslanbaeva, L.; Vergeade, A.; Rahman, S.M.J.; Boutaud, O.; Blackwell, T.; Massion, P.P.; Harrison, D.G.; et al. Tobacco Smoking Induces Cardiovascular Mitochondrial Oxidative Stress, Promotes Endothelial Dysfunction, and Enhances Hypertension. Am. J. Physiol. Circ. Physiol. 2019, 316, H639–H646. [Google Scholar] [CrossRef] [PubMed]
- Intachai, K.; C Chattipakorn, S.; Chattipakorn, N.; Shinlapawittayatorn, K. Revisiting the Cardioprotective Effects of Acetylcholine Receptor Activation against Myocardial Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2018, 19, 2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippini, P.; Cesario, A.; Fini, M.; Locatelli, F.; Rutella, S. The Yin and Yang of Non-Neuronal A7-Nicotinic Receptors in Inflammation and Autoimmunity. Curr. Drug Targets 2012, 13, 644–655. [Google Scholar] [CrossRef]
- Mousa, S.; Mousa, S.A. Cellular and Molecular Mechanisms of Nicotine’s pro-Angiogenesis Activity and Its Potential Impact on Cancer. J. Cell. Biochem. 2006, 97, 1370–1378. [Google Scholar] [CrossRef]
- Brown, K.C.; Perry, H.E.; Lau, J.K.; Jones, D.V.; Pulliam, J.F.; Thornhill, B.A.; Crabtree, C.M.; Luo, H.; Chen, Y.C.; Dasgupta, P. Nicotine Induces the Up-Regulation of the A7-Nicotinic Receptor (A7-NAChR) in Human Squamous Cell Lung Cancer Cells via the Sp1/GATA Protein Pathway. J. Biol. Chem. 2013, 288, 33049–33059. [Google Scholar] [CrossRef] [Green Version]
- Ashour, A.; Alhussain, H.; Rashid, U.B.; Abughazzah, L.; Gupta, I.; Malki, A.; Vranic, S.; Al Moustafa, A.-E. E-Cigarette Liquid Provokes Significant Embryotoxicity and Inhibits Angiogenesis. Toxics 2020, 8, 38. [Google Scholar] [CrossRef]
- England, L.J.; Aagaard, K.; Bloch, M.; Conway, K.; Cosgrove, K.; Grana, R.; Gould, T.J.; Hatsukami, D.; Jensen, F.; Kandel, D.; et al. Developmental toxicity of nicotine: A transdisciplinary synthesis and implications for emerging tobacco products. Neurosci. Biobehav. Rev. 2017, 72, 176–189. [Google Scholar] [CrossRef] [Green Version]
- Proctor, R.N. The history of the discovery of the cigarette–lung cancer link: Evidentiary traditions, corporate denial, global toll. Tob. Control 2012, 21, 87–91. [Google Scholar] [CrossRef]

| H2O | SDS 1% | 0.1 μL/mL | 50 μL/mL | 100 μL/mL | |
|---|---|---|---|---|---|
| IS | 0.13 | 19.68 | 4.13 | 14.01 | 17.75 |
| tH (s) | 300 | 21 | 224 | 135 | 56 |
| tL (s) | 300 | 18 | 287 | 98 | 47 |
| tC (s) | 299 | 15 | 217 | 84 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chioran, D.; Sitaru, A.; Macasoi, I.; Pinzaru, I.; Sarau, C.A.; Dehelean, C.; Dinu, S.; Szuhanek, C.; Zetu, I.N.; Serafin, A.C.; et al. Nicotine Exerts Cytotoxic Effects in a Panel of Healthy Cell Lines and Strong Irritating Potential on Blood Vessels. Int. J. Environ. Res. Public Health 2022, 19, 8881. https://doi.org/10.3390/ijerph19148881
Chioran D, Sitaru A, Macasoi I, Pinzaru I, Sarau CA, Dehelean C, Dinu S, Szuhanek C, Zetu IN, Serafin AC, et al. Nicotine Exerts Cytotoxic Effects in a Panel of Healthy Cell Lines and Strong Irritating Potential on Blood Vessels. International Journal of Environmental Research and Public Health. 2022; 19(14):8881. https://doi.org/10.3390/ijerph19148881
Chicago/Turabian StyleChioran, Doina, Adrian Sitaru, Ioana Macasoi, Iulia Pinzaru, Cristian Andrei Sarau, Cristina Dehelean, Stefania Dinu, Camelia Szuhanek, Irina Nicoleta Zetu, Andra Cristine Serafin, and et al. 2022. "Nicotine Exerts Cytotoxic Effects in a Panel of Healthy Cell Lines and Strong Irritating Potential on Blood Vessels" International Journal of Environmental Research and Public Health 19, no. 14: 8881. https://doi.org/10.3390/ijerph19148881
APA StyleChioran, D., Sitaru, A., Macasoi, I., Pinzaru, I., Sarau, C. A., Dehelean, C., Dinu, S., Szuhanek, C., Zetu, I. N., Serafin, A. C., Rivis, M., Poenaru, M., & Dragoi, R. (2022). Nicotine Exerts Cytotoxic Effects in a Panel of Healthy Cell Lines and Strong Irritating Potential on Blood Vessels. International Journal of Environmental Research and Public Health, 19(14), 8881. https://doi.org/10.3390/ijerph19148881











