Passive Smoking Is Associated with Multiple Heavy Metal Concentrations among Housewives in Shanxi Province, China
Abstract
:1. Introduction
2. Methods
2.1. Participants and Recruitment
2.2. Hair Sample Collection and Laboratory Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Ji, Y.; Dong, H.; Chang, C. The prevalence of smoking, second-hand smoke exposure, and knowledge of the health hazards of smoking among internal migrants in 12 provinces in China: A cross-sectional analysis. BMC Public Health 2018, 18, 655. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yang, C.; Gan, Y.; Lu, Z. The Health Effects of Passive Smoking: An Overview of Systematic Reviews Based on Observational Epidemiological Evidence. PLoS ONE 2015, 10, e0139907. [Google Scholar] [CrossRef]
- Li, N.; Li, Z.; Chen, S.; Yang, N.; Ren, A.; Ye, R. Effects of passive smoking on hypertension in rural Chinese nonsmoking women. J. Hypertens. 2015, 33, 2210–2214. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Newsroom: Tobacco-Key Facts. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 5 June 2022).
- Seidman, D.S. Health effects of involuntary smoking. N. Engl. J. Med. 1989, 320, 1287. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, F.; Dobaradaran, S.; De-la-Torre, G.E.; Schmidt, T.C.; Saeedi, R. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: A comprehensive systematic review. Sci. Total Environ. 2022, 813, 152667. [Google Scholar] [CrossRef]
- Zhang, G.; Zhan, J.; Fu, H. Trends in Smoking Prevalence and Intensity between 2010 and 2018: Implications for Tobacco Control in China. Int. J. Environ. Res. Public Health 2022, 19, 670. [Google Scholar] [CrossRef]
- Chinese Centers for Diases Control and Prevention (CDC). Summary of 2018 China Adult Tobacco Survey. 2019. Available online: https://www.chinacdc.cn/jkzt/sthd_3844/slhd_12885/201905/t20190530_202932.html (accessed on 5 June 2022).
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke; Routledge: London, UK, 2008. [Google Scholar]
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, S.E.; Aaron, J.J. Toxic heavy metals: Impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ. Sci. Pollut. Res. 2020, 27, 29927–29942. [Google Scholar] [CrossRef]
- Goutam Mukherjee, A.; Ramesh Wanjari, U.; Renu, K.; Vellingiri, B.; Valsala Gopalakrishnan, A. Heavy metal and metalloid-induced reproductive toxicity. Environ. Toxicol. Pharmacol. 2022, 92, 103859. [Google Scholar] [CrossRef]
- Pinto, E.; Cruz, M.; Ramos, P.; Santos, A.; Almeida, A. Metals transfer from tobacco to cigarette smoke: Evidences in smokers’ lung tissue. J. Hazard. Mater. 2017, 325, 31–35. [Google Scholar] [CrossRef]
- Shakeri, M.T.; Nezami, H.; Nakhaee, S.; Aaseth, J.; Mehrpour, O. Assessing Heavy Metal Burden Among Cigarette Smokers and Non-smoking Individuals in Iran: Cluster Analysis and Principal Component Analysis. Biol. Trace Elem. Res. 2021, 199, 4036–4044. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, Y.; Wang, C.; Jiang, Y.; Yang, L. Survey on status of smoking, passive smoking and quitting smoking in rural areas of the midwestern provinces in China. Chin. J. Epidemiol. 2013, 34, 137–139. [Google Scholar] [PubMed]
- Esteban, M.; Castaño, A. Non-invasive matrices in human biomonitoring: A review. Environ. Int. 2009, 35, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, Y.; Pang, Y.; Xie, J.; Hao, Y.; Yan, H.; Li, Z.; Ye, R. Indoor air pollution affects hypertension risk in rural women in Northern China by interfering with the uptake of metal elements: A preliminary cross-sectional study. Environ. Pollut. 2018, 240, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Wennig, R. Potential problems with the interpretation of hair analysis results. Forensic Sci. Int. 2000, 107, 5–12. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Ge, S.; Yan, L.; Liu, Y.; Li, Z.; Ren, A. A simultaneous analysis method of polycyclic aromatic hydrocarbons, nicotine, cotinine and metals in human hair. Environ. Pollut. 2016, 219, 66–71. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Patelarou, E.; Grandér, M.; Chatzi, L.; Palm, B.; Fthenou, E.; Roumeliotaki, T.; Koutis, A.; Kafatos, A.; Vrijheid, M.; et al. The association between active/passive smoking and toxic metals among pregnant women in Greece. Xenobiotica Fate Foreign Compd. Biol. Syst. 2011, 41, 456–463. [Google Scholar] [CrossRef]
- Bergman, A.; Heindel, J.; Jobling, S.; Kidd, K.; Zoeller, R.T. State-of-the-science of endocrine disrupting chemicals, 2012. Toxicol. Lett. 2012, 211, S3. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Cloonan, S.M.; Mumby, S.; Adcock, I.M.; Choi, A.M.K.; Chung, K.F.; Quinlan, G.J. The “Iron”-y of Iron Overload and Iron Deficiency in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.K.; Kim, R.Y.; Karim, R.; Mayall, J.R.; Martin, K.L.; Shahandeh, A.; Abbasian, F.; Starkey, M.R.; Loustaud-Ratti, V.; Johnstone, D.; et al. Role of iron in the pathogenesis of respiratory disease. Int. J. Biochem. Cell Biol. 2017, 88, 181–195. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Butler, J.J.; Cloonan, S.M. Smoking-induced iron dysregulation in the lung. Free Radic. Biol. Med. 2019, 133, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Fresquez, M.R.; Watson, C.H.; Valentin-Blasini, L.; Steven Pappas, R. Characterizing the Transport of Aluminum-, Silicon- and Titanium-Containing Particles and Nanoparticles in Mainstream Tobacco Smoke. J. Anal. Toxicol. 2021, 45, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Jacobsen, N.R.; Folkmann, J.K.; Danielsen, P.H.; Mikkelsen, L.; Hemmingsen, J.G.; Vesterdal, L.K.; Forchhammer, L.; Wallin, H.; Loft, S. Role of oxidative damage in toxicity of particulates. Free Radic. Res. 2010, 44, 1–46. [Google Scholar] [CrossRef]
- Arts, J.H.; Til, H.P.; Kuper, C.F.; de Neve, R.; Swennen, B. Acute and subacute inhalation toxicity of germanium dioxide in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1994, 32, 1037–1046. [Google Scholar] [CrossRef]
- Dixon, C.; Hagemeister, F.; Legha, S.; Bodey, G. Pulmonary toxicity associated with spirogermanium. Cancer Treat. Rep. 1984, 68, 907–908. [Google Scholar]
- Schauss, A.G. Nephrotoxicity and neurotoxicity in humans from organogermanium compounds and germanium dioxide. Biol. Trace Elem. Res. 1991, 29, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Goullé, J.P.; Mahieu, L.; Castermant, J.; Neveu, N.; Bonneau, L.; Lainé, G.; Bouige, D.; Lacroix, C. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci. Int. 2005, 153, 39–44. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, B.; Li, L.; Chen, X.; An, J.; Liu, X. Heavy metal contamination and ecological risk assessment of the agricultural soil in Shanxi Province, China. R. Soc. Open Sci. 2020, 7, 200538. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.; Roh, J.; Won, J.U.; Yoon, J.H. The Association between Involuntary Smoking Exposure with Urine Cotinine Level and Blood Cadmium Level in General Non-Smoking Populations. J. Korean Med. Sci. 2017, 32, 568–575. [Google Scholar] [CrossRef]
- Li, L.; Guo, L.; Chen, X.; Xiang, M.; Yang, F.; Ren, J.C.; Zhang, G.H. Secondhand smoke is associated with heavy metal concentrations in children. Eur. J. Pediatr. 2018, 177, 257–264. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Pang, Y.; Huo, W.; Li, N.; Li, Z.; Zhang, J.; Ye, R.; Wang, B. Association Between Chronic Exposure to Tobacco Smoke and Accumulation of Toxic Metals in Hair Among Pregnant Women. Biol. Trace Elem. Res. 2018, 185, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Gunay, F.; Cullas Ilarslan, N.E.; Bakar-Ates, F.; Deniz, K.; Kadioglu, Y.K.; Kiran, S.; Bakirarar, B.; Cobanoglu, N. Evaluation of hair cotinine and toxic metal levels in children who were exposed to tobacco smoke. Pediatric Pulmonol. 2020, 55, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Karatela, S.; Coomarasamy, C.; Paterson, J.; Ward, N.I. Household Smoking Status and Heavy Metal Concentrations in Toenails of Children. Int. J. Environ. Res. Public Health 2019, 16, 3871. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.Y.; Tu, M.; Wang, J.; Wang, L.; Jiang, Y. Summary of 2015 China Adult Tobacco Survey. Chin. J. Health Manag. 2016, 10, 85–87. [Google Scholar] [CrossRef]
- Bravo-Gutiérrez, O.A.; Falfán-Valencia, R.; Ramírez-Venegas, A.; Sansores, R.H.; Ponciano-Rodríguez, G.; Pérez-Rubio, G. Lung Damage Caused by Heated Tobacco Products and Electronic Nicotine Delivery Systems: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 4079. [Google Scholar] [CrossRef]
- Gunier, R.B.; Horn-Ross, P.L.; Canchola, A.J.; Duffy, C.N.; Reynolds, P.; Hertz, A.; Garcia, E.; Rull, R.P. Determinants and within-person variability of urinary cadmium concentrations among women in northern California. Environ. Health Perspect. 2013, 121, 643–649. [Google Scholar] [CrossRef]
| Variable | Number (%) |
|---|---|
| Age (year, mean ± S.D.) | 52.6 ± 10.4 |
| <45 | 112 (29.2) |
| 45–55 | 109 (28.4) |
| 55–65 | 105 (27.3) |
| ≥65 | 58 (15.1) |
| Body mass index (kg/m2, mean ± S.D.) | 24.9 ± 3.0 |
| <25 | 214 (55.7) |
| ≥25 | 170 (44.3) |
| Occupation | |
| Non-farmer | 85 (22.1) |
| Farmer | 299 (77.9) |
| Education | |
| Primary or lower | 200 (52.1) |
| Junior high | 113 (29.4) |
| High school or junior college | 38 (9.9) |
| Above junior college | 33 (8.6) |
| Using a stove for heating | |
| No | 98 (25.5) |
| Yes | 286 (74.5) |
| Second-hand smoking status | |
| No | 267 (69.5) |
| Yes | 117 (30.5) |
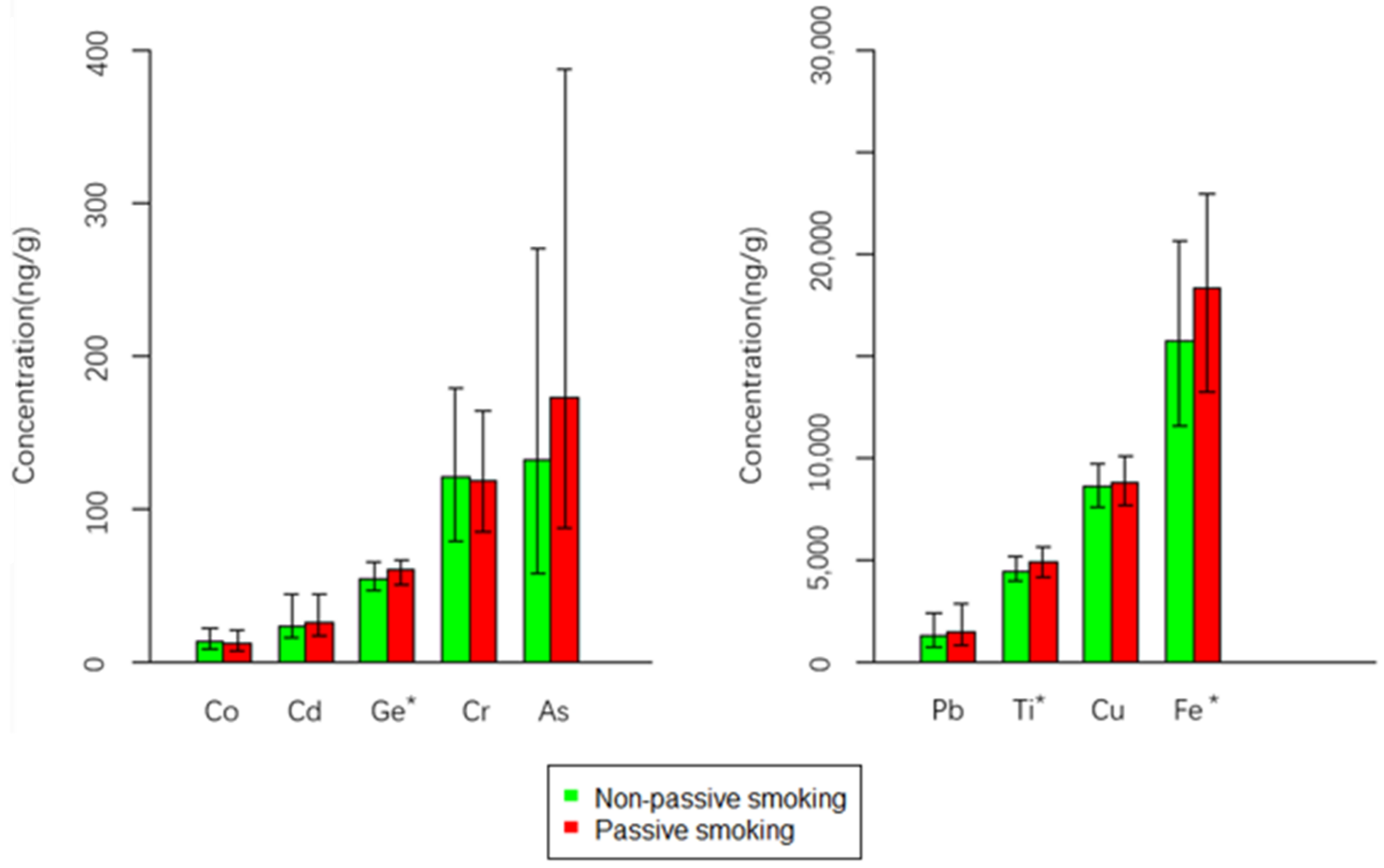
| Metals | P25 | Median | P75 | Range | LOD | LOQ | DF (%) | p Value a |
|---|---|---|---|---|---|---|---|---|
| Co | 8.14 | 12.48 | 20.74 | 1.72–27595.60 | 0.001 | 0.003 | 100 | |
| NPS | 8.46 | 12.73 | 21.45 | 1.72–469.72 | 0.271 | |||
| PS | 7.50 | 12.12 | 20.15 | 1.80–27595.60 | ||||
| Cd | 15.97 | 23.86 | 43.78 | 4.85–6546.20 | 0.003 | 0.009 | 100 | |
| NPS | 15.41 | 23.34 | 43.45 | 5.04–6546.2 | 0.432 | |||
| PS | 16.58 | 25.74 | 44.03 | 4.85–1430.95 | ||||
| Ge | 47.52 | 55.38 | 66.02 | 30.80–157.80 | 0.009 | 0.027 | 100 | |
| NPS | 46.69 | 53.60 | 64.74 | 30.8–129.63 | 0.007 | |||
| PS | 49.67 | 59.96 | 66.59 | 40.26–157.8 | ||||
| Cr | 79.34 | 120.16 | 172.60 | 35.38–908.44 | 0.002 | 0.006 | 100 | |
| NPS | 79.19 | 120.47 | 179.00 | 36.32–908.44 | 0.627 | |||
| PS | 85.09 | 118.09 | 163.93 | 35.38–888.6 | ||||
| As | 63.23 | 146.85 | 309.54 | 4.40–3214.49 | 0.05 | 0.15 | 100 | |
| NPS | 58.14 | 131.55 | 270.79 | 12.27–3214.49 | 0.028 | |||
| PS | 87.23 | 172.28 | 388.49 | 4.40–3073.75 | ||||
| Pb | 748.87 | 1342.55 | 2433.78 | 206.92–17,948.57 | 0.012 | 0.036 | 100 | |
| NPS | 716.64 | 1260.75 | 2351.50 | 206.92–17,948.57 | 0.186 | |||
| PS | 789.73 | 1468.74 | 2860.55 | 275.55–15,527.87 | ||||
| Ti | 4012.96 | 4527.54 | 5347.90 | 3038.81–17,023.87 | 0.01 | 0.03 | 100 | |
| NPS | 3970.24 | 4418.62 | 5171.90 | 3038.81–15,998.49 | 0.019 | |||
| PS | 4140.87 | 4861.90 | 5660.70 | 3315.92–17,023.87 | ||||
| Cu | 7628.89 | 8616.68 | 9762.22 | 3812.92–211,613.19 | 0.03 | 0.09 | 100 | |
| NPS | 7558.78 | 8584.14 | 9741.35 | 3812.92–211,613.19 | 0.460 | |||
| PS | 7703.29 | 8776.28 | 10,051.64 | 5156.21–14,8879.4 | ||||
| Fe | 12,095.61 | 16,405.81 | 21,933.06 | 5946.29–98,278.89 | 0.9 | 2.7 | 100 | |
| NPS | 11,568.85 | 15,714.06 | 20,615.38 | 5946.29–98,278.89 | 0.035 | |||
| PS | 13,251.20 | 18,331.57 | 22,947.21 | 6079.57–55,322.95 |
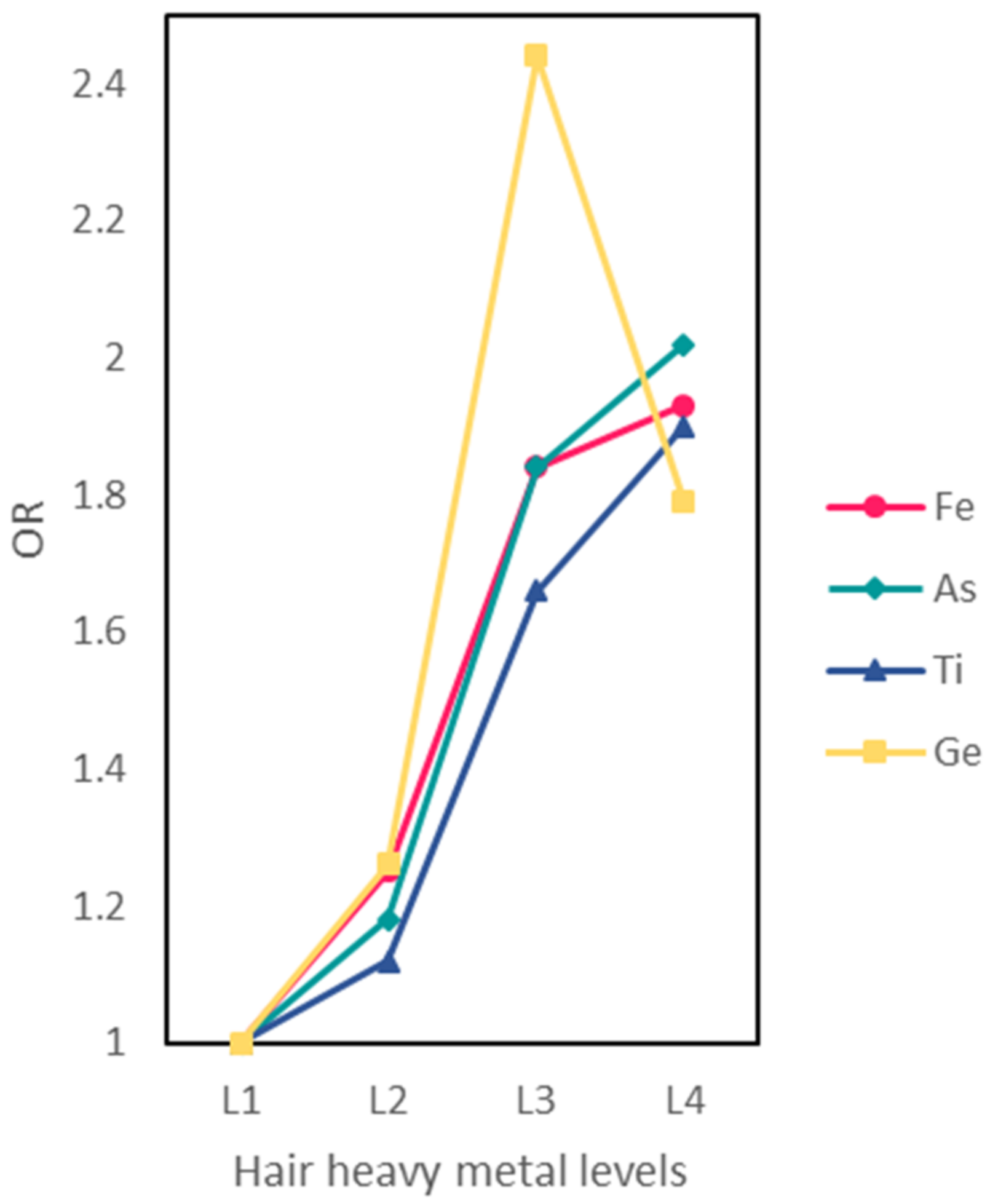
| Metals | Crude OR a | Crude OR 95%CI a | Adjusted OR b | Adjusted OR 95%CI b |
|---|---|---|---|---|
| Co | 0.88 | 0.57–1.37 | 0.88 | 0.56–1.36 |
| Cd | 1.25 | 0.81–1.93 | 1.21 | 0.78–1.89 |
| Ge * | 1.86 | 1.20–2.91 | 1.78 | 1.14–2.80 |
| Cr | 0.93 | 0.60–1.43 | 0.93 | 0.59–1.44 |
| As * | 1.77 | 1.14–2.76 | 1.80 | 1.13–2.90 |
| Pb | 1.38 | 0.89–2.14 | 1.31 | 0.84–2.05 |
| Ti * | 1.68 | 1.09–2.62 | 1.70 | 1.09–2.67 |
| Cu | 1.08 | 0.70–1.66 | 1.05 | 0.67–1.63 |
| Fe * | 1.68 | 1.09–2.62 | 1.67 | 1.07–2.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Na, J.; An, H.; Jin, M.; Jia, X.; Yan, L.; Li, N.; Li, Z. Passive Smoking Is Associated with Multiple Heavy Metal Concentrations among Housewives in Shanxi Province, China. Int. J. Environ. Res. Public Health 2022, 19, 8606. https://doi.org/10.3390/ijerph19148606
Chen H, Na J, An H, Jin M, Jia X, Yan L, Li N, Li Z. Passive Smoking Is Associated with Multiple Heavy Metal Concentrations among Housewives in Shanxi Province, China. International Journal of Environmental Research and Public Health. 2022; 19(14):8606. https://doi.org/10.3390/ijerph19148606
Chicago/Turabian StyleChen, Huiting, Jigen Na, Hang An, Ming Jin, Xiaoqian Jia, Lailai Yan, Nan Li, and Zhiwen Li. 2022. "Passive Smoking Is Associated with Multiple Heavy Metal Concentrations among Housewives in Shanxi Province, China" International Journal of Environmental Research and Public Health 19, no. 14: 8606. https://doi.org/10.3390/ijerph19148606
APA StyleChen, H., Na, J., An, H., Jin, M., Jia, X., Yan, L., Li, N., & Li, Z. (2022). Passive Smoking Is Associated with Multiple Heavy Metal Concentrations among Housewives in Shanxi Province, China. International Journal of Environmental Research and Public Health, 19(14), 8606. https://doi.org/10.3390/ijerph19148606






