The Association between Blood Concentrations of PCDD/DFs, DL-PCBs and the Risk of Type 2 Diabetes Mellitus and Thyroid Cancer in South Korea
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Measurements
2.2.1. Data Source
2.2.2. Analysis of Dioxins
2.3. Statistical Analysis
3. Results
3.1. Study Population and PCDD/DFs, DL-PCBs, and Total Dioxins in Blood
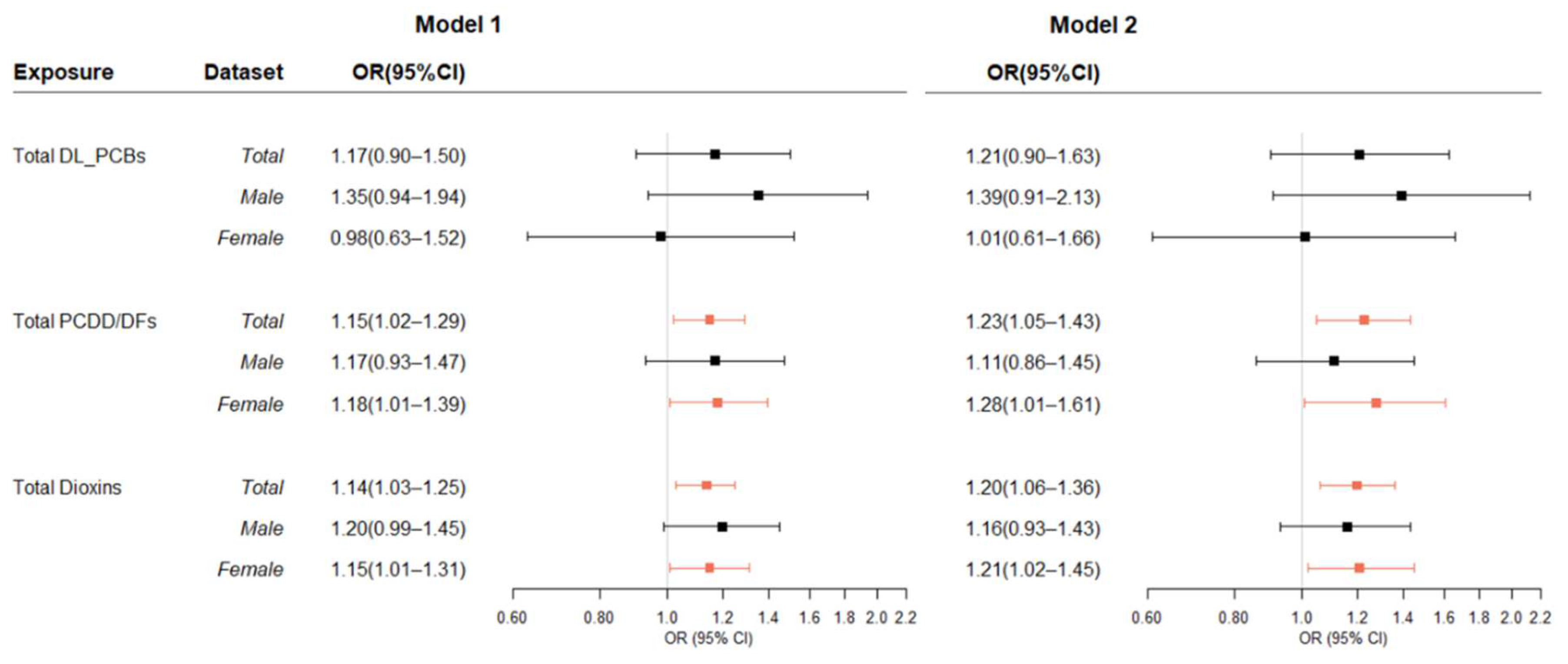
3.2. Association between Dioxins in Blood and T2DM
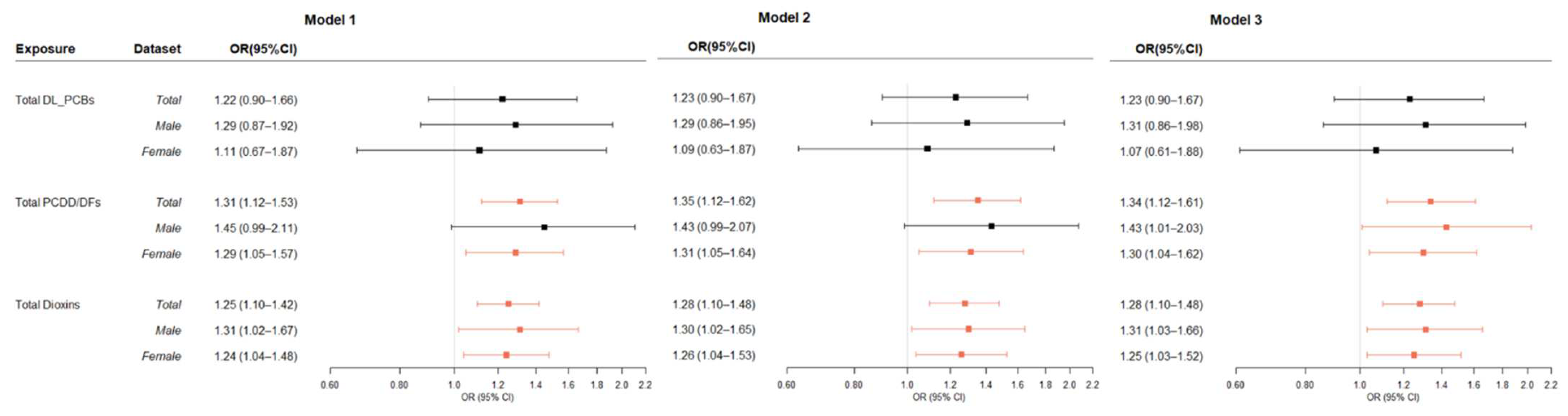
3.3. Association between Dioxins in Blood and Thyroid Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersson, P.; McGuire, J.; Rubio, C.; Gradin, K.; Whitelaw, M.L.; Pettersson, S.; Hanberg, A.; Poellinger, L. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 9990–9995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birnbaum, L.S.; Couture, L.A. Disposition of octachlorodibenzo-p-dioxin (OCDD) in male rats. Toxicol. Appl. Pharm. 1988, 93, 22–30. [Google Scholar] [CrossRef]
- Geyer, H.; Scheunert, I.; Korte, F. Bioconcentration potential of organic environmental chemicals in humans. Regul. Toxicol. Pharm. 1986, 6, 313–347. [Google Scholar] [CrossRef]
- Lakshmanan, M.R.; Campbell, B.S.; Chirtel, S.J.; Ekarohita, N.; Ezekiel, M. Studies on the mechanism of absorption and distribution of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat. J. Pharm. Exp. Ther. 1986, 239, 673–677. Available online: https://www.ncbi.nlm.nih.gov/pubmed/3795035 (accessed on 1 July 2020).
- Birnbaum, L.S. The mechanism of dioxin toxicity: Relationship to risk assessment. Environ. Health Perspect. 1994, 102 (Suppl. 9), 157–167. [Google Scholar] [CrossRef] [Green Version]
- WHO. Persistent Organic Pollutants (POPs). Retrieved 2020.11. 2020. Available online: https://www.who.int/foodsafety/areas_work/chemical-risks/pops/en/ (accessed on 1 July 2020).
- Bertazzi, P.A.; Consonni, D.; Bachetti, S.; Rubagotti, M.; Baccarelli, A.; Zocchetti, C.; Pesatori, A.C. Health effects of dioxin exposure: A 20-year mortality study. Am. J. Epidemiol. 2001, 153, 1031–1044. [Google Scholar] [CrossRef]
- Calvert, G.M.; Sweeney, M.H.; Deddens, J.; Wall, D.K. Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup. Environ. Med. 1999, 56, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, G.L.; Ketchum, N.S.; Michalek, J.E.; Swaby, J.A. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology 1997, 8, 252–258. [Google Scholar] [CrossRef]
- Steenland, K.; Calvert, G.; Ketchum, N.; Michalek, J. Dioxin and diabetes mellitus: An analysis of the combined NIOSH and Ranch Hand data. Occup Environ. Med. 2001, 58, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Bertazzi, P.A.; Bernucci, I.; Brambilla, G.; Consonni, D.; Pesatori, A.C. The Seveso studies on early and long-term effects of dioxin exposure: A review. Environ. Health Perspect 1998, 106 (Suppl. 2), 625–633. [Google Scholar] [CrossRef] [Green Version]
- Steenland, K.; Piacitelli, L.; Deddens, J.; Fingerhut, M.; Chang, L.I. Cancer, Heart Disease, and Diabetes in Workers Exposed to 2,3,7,8-Tetrachlorodibenzo-p-dioxin. JNCI J. Natl. Cancer Inst. 1999, 91, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukropec, J.; Radikova, Z.; Huckova, M.; Koska, J.; Kocan, A.; Sebokova, E.; Drobna, B.; Trnovec, T.; Susienkova, K.; Labudova, V.; et al. High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia 2010, 53, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vena, J.; Boffetta, P.; Becher, H.; Benn, T.; Bueno-de-Mesquita, H.B.; Coggon, D.; Colin, D.; Flesch-Janys, D.; Green, L.; Kauppinen, T.; et al. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environ. Health Perspect. 1998, 106 (Suppl. 2), 645–653. [Google Scholar] [CrossRef] [Green Version]
- Hoyeck, M.P.; Blair, H.; Ibrahim, M.; Solanki, S.; Elsawy, M.; Prakash, A.; Rick, K.R.C.; Matteo, G.; O’Dwyer, S.; Bruin, J.E. Long-term metabolic consequences of acute dioxin exposure differ between male and female mice. Sci. Rep. 2020, 10, 1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, K.W.; Novak, R.F.; Anderson, H.A.; Birnbaum, L.S.; Blystone, C.; Devito, M.; Jacobs, D.; Köhrle, J.; Lee, D.-H.; Rylander, L.; et al. Evaluation of the Association between Persistent Organic Pollutants (POPs) and Diabetes in Epidemiological Studies: A National Toxicology Program Workshop Review. Environ. Health Perspect. 2013, 121, 774–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polychlorinated Biphenyls and Polybrominated Biphenyls: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: 107. 2016. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/08/mono107.pdf (accessed on 1 July 2020).
- Birnbaum, L.S. Developmental effects of dioxins and related endocrine disrupting chemicals. Toxicol. Lett. 1995, 82–83, 743–750. [Google Scholar] [CrossRef]
- Cole, P.; Trichopoulos, D.; Pastides, H.; Starr, T.; Mandel, J.S. Dioxin and cancer: A critical review. Regul. Toxicol. Pharmacol. 2003, 38, 378–388. [Google Scholar] [CrossRef]
- Collins, J.J.; Strauss, M.E.; Levinskas, G.J.; Conner, P.R. The mortality experience of workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin in a trichlorophenol process accident. Epidemiology 1993, 4, 7–13. [Google Scholar] [CrossRef]
- Kogevinas, M.; Becher, H.; Benn, T.; Bertazzi, P.A.; Boffetta, P.; Bueno-de-Mesquita, H.B.; Coggon, D.; Colin, D.; Flesch-Janys, D.; Fingerhut, M.; et al. Cancer mortality in workers exposed to phenoxy herbicides, chlorophenols, and dioxins. An expanded and updated international cohort study. Am. J. Epidemiol. 1997, 145, 1061–1075. [Google Scholar] [CrossRef]
- Kogevinas, M.; Saracci, R.; Winkelmann, R.; Johnson, E.S.; Bertazzi, P.A.; Bueno de Mesquita, B.H.; Kauppinen, T.; Littorin, M.; Lynge, E.; Neuberger, M.; et al. Cancer incidence and mortality in women occupationally exposed to chlorophenoxy herbicides, chlorophenols, and dioxins. Cancer Causes Control. 1993, 4, 547–553. [Google Scholar] [CrossRef]
- Manz, A.; Flesch-Janys, D.; Waltsgott, H.; Berger, J.; Nagel, S.; Dwyer, J.H. Cancer mortality among workers in chemical plant contaminated with dioxin. Lancet 1991, 338, 959–964. [Google Scholar] [CrossRef]
- Pavuk, M.; Cerhan, J.R.; Lynch, C.F.; Schecter, A.; Petrik, J.; Chovancova, J.; Kocan, A. Environmental exposure to PCBs and cancer incidence in eastern Slovakia. Chemosphere 2004, 54, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Pesatori, A.C.; Consonni, D.; Rubagotti, M.; Grillo, P.; Bertazzi, P.A. Cancer incidence in the population exposed to dioxin after the “Seveso accident”: Twenty years of follow-up. Environ. Health 2009, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Revich, B.; Aksel, E.; Ushakova, T.; Ivanova, I.; Zhuchenko, N.; Klyuev, N.; Brodsky, B.; Sotskov, Y. Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere 2001, 43, 951–966. [Google Scholar] [CrossRef]
- Reynolds, P.; Hurley, S.E.; Petreas, M.; Goldberg, D.E.; Smith, D.; Gilliss, D.; Mahoney, M.E.; Jeffrey, S.S. Adipose levels of dioxins and risk of breast cancer. Cancer Causes Control. 2005, 16, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, J.T.; Pekkanen, J.; Kiviranta, H.; Tukiainen, E.; Vartiainen, T.; Tuomisto, J. Soft-tissue sarcoma and dioxin: A case-control study. Int. J. Cancer 2004, 108, 893–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villeneuve, S.; Cyr, D.; Lynge, E.; Orsi, L.; Sabroe, S.; Merletti, F.; Gorini, G.; Morales-Suarez-Varela, M.; Ahrens, W.; Baumgardt-Elms, C.; et al. Occupation and occupational exposure to endocrine disrupting chemicals in male breast cancer: A case-control study in Europe. Occup. Environ. Med. 2010, 67, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Warner, M.; Eskenazi, B.; Mocarelli, P.; Gerthoux, P.M.; Samuels, S.; Needham, L.; Patterson, D.; Brambilla, P. Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study. Environ. Health Perspect. 2002, 110, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Zambon, P.; Ricci, P.; Bovo, E.; Casula, A.; Gattolin, M.; Fiore, A.R.; Chiosi, F.; Guzzinati, S. Sarcoma risk and dioxin emissions from incinerators and industrial plants: A population-based case-control study (Italy). Environ. Health 2007, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Zober, A.; Messerer, P.; Huber, P. Thirty-four-year mortality follow-up of BASF employees exposed to 2,3,7,8-TCDD after the 1953 accident. Int. Arch. Occup. Environ. Health 1990, 62, 139–157. [Google Scholar] [CrossRef]
- Xu, J.; Ye, Y.; Huang, F.; Chen, H.; Wu, H.; Huang, J.; Hu, J.; Xia, D.; Wu, Y. Association between dioxin and cancer incidence and mortality: A meta-analysis. Sci. Rep. 2016, 6, 38012. [Google Scholar] [CrossRef] [PubMed]
- Ball, M. Dioxins, Furans and WHO PCB in Whole Blood [Biomonitoring Methods, 2003]; Wiley Online Library: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Jee, Y.H.; Emberson, J.; Jung, K.J.; Lee, S.J.; Lee, S.; Back, J.H.; Hong, S.; Kimm, H.; Sherliker, P.; Jee, S.H.; et al. Cohort Profile: The Korean Cancer Prevention Study-II (KCPS-II) Biobank. Int. J. Epidemiol. 2018, 47, 385–386f. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srogi, K. Levels and congener distributions of PCDDs, PCDFs and dioxin-like PCBs in environmental and human samples: A review. Environ. Chem. Lett. 2008, 6, 1–28. [Google Scholar] [CrossRef] [Green Version]
- CDC. Laboratory Procedure Manual Method 6501.04, Centers for Disease Control and Prevention. 2016. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/DOXPOL_F_MET.pdf (accessed on 1 July 2020).
- Lee, D.-H.; Lind, P.M.; Jacobs, D.R.; Salihovic, S.; Van Bavel, B.; Lind, L. Polychlorinated Biphenyls and Organochlorine Pesticides in Plasma Predict Development of Type 2 Diabetes in the Elderly. Diabetes Care 2011, 34, 1778–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernert, J.T.; Turner, W.E.; Patterson, D.G.; Needham, L.L. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere 2007, 68, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Loomis, D.; Grosse, Y.; Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013, 14, 287–288. [Google Scholar] [CrossRef] [Green Version]
- Boffetta, P.; Catalani, S.; Tomasi, C.; Pira, E.; Apostoli, P. Occupational exposure to polychlorinated biphenyls and risk of cutaneous melanoma: A meta-analysis. Eur. J. Cancer Prev. 2018, 27, 62–69. [Google Scholar] [CrossRef]
- Zani, C.; Toninelli, G.; Filisetti, B.; Donato, F. Polychlorinated biphenyls and cancer: An epidemiological assessment. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2013, 31, 99–144. [Google Scholar] [CrossRef]
- Silverstone, A.E.; Rosenbaum, P.F.; Weinstock, R.S.; Bartell, S.M.; Foushee, H.R.; Shelton, C.; Pavuk, M. Polychlorinated Biphenyl (PCB) Exposure and Diabetes: Results from the Anniston Community Health Survey. Environ. Health Perspect. 2012, 120, 727–732. [Google Scholar] [CrossRef]
- Wang, S.L.; Tsai, P.C.; Yang, C.Y.; Leon Guo, Y. Increased Risk of Diabetes and Polychlorinated Biphenyls and Dioxins: A 24-year follow-up study of the Yucheng cohort. Diabetes Care 2008, 31, 1574–1579. [Google Scholar] [CrossRef] [Green Version]
- Reale, C.; Russo, F.; Credendino, S.; Cuomo, D.; De Vita, G.; Mallardo, M.; Pennino, F.; Porreca, I.; Triassi, M.; De Felice, M.; et al. A Toxicogenomic Approach Reveals a Novel Gene Regulatory Network Active in In Vitro and In Vivo Models of Thyroid Carcinogenesis. Int. J. Environ. Res. Public Health 2019, 16, 122. [Google Scholar] [CrossRef] [Green Version]
- Lans, M.C.; Spiertz, C.; Brouwer, A.; Koeman, J.H. Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs, PCDDs and PCDFs. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1994, 270, 129–136. [Google Scholar] [CrossRef]
- Brouwer, A.; Morse, D.C.; Lans, M.C.; Gerlienke Schuur, A.; Murk, A.J.; Klasson-Wehler, E.; Bergman, Å.; Visser, T.J. Interactions of Persistent Environmental Organohalogens With the Thyroid Hormone System: Mechanisms and Possible Consequences for Animal and Human Health. Toxicol. Ind. Health 1998, 14, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Pavuk, M.; Schecter, A.J.; Akhtar, F.Z.; Michalek, J.E. Serum 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) Levels and Thyroid Function in Air Force Veterans of the Vietnam War. Ann. Epidemiol. 2003, 13, 335–343. [Google Scholar] [CrossRef]
- Haymart, M.R.; Repplinger, D.J.; Leverson, G.E.; Elson, D.F.; Sippel, R.S.; Jaume, J.C.; Chen, H. Higher Serum Thyroid Stimulating Hormone Level in Thyroid Nodule Patients Is Associated with Greater Risks of Differentiated Thyroid Cancer and Advanced Tumor Stage. J. Clin. Endocrinol. Metab. 2008, 93, 809–814. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Yoon, J.H.; Kim, S.J.; Cho, J.S.; Kweon, S.-S.; Kang, H.-C. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin. Endocrinol. 2012, 78, 472–477. [Google Scholar] [CrossRef]
- Kerger, B.D.; Scott, P.K.; Pavuk, M.; Gough, M.; Paustenbach, D.J. Re-analysis of Ranch Hand study supports reverse causation hypothesis between dioxin and diabetes. Crit. Rev. Toxicol. 2012, 42, 669–687. [Google Scholar] [CrossRef]
- Safe, S.H. Development validation and problems with the toxic equivalency factor approach for risk assessment of dioxins and related compounds. J. Anim. Sci. 1998, 76, 134. [Google Scholar] [CrossRef]
- Ceriello, A. Possible Role of Oxidative Stress in the Pathogenesis of Hypertension. Diabetes Care 2008, 31 (Suppl. 2), S181–S184. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-L.; Chang, Y.-C.; Chao, H.-R.; Li, C.-M.; Li, L.-A.; Lin, L.-Y.; Päpke, O. Body Burdens of Polychlorinated Dibenzo- p -dioxins, Dibenzofurans, and Biphenyls and Their Relations to Estrogen Metabolism in Pregnant Women. Environ. Health Perspect. 2006, 114, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Michalek, J.E.; Pavuk, M. Diabetes and Cancer in Veterans of Operation Ranch Hand After Adjustment for Calendar Period, Days of Spraying, and Time Spent in Southeast Asia. J. Occup. Environ. Med. 2008, 50, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bichteler, A.; Wikoff, D.S.; Loko, F.; Harris, M.A. Estimating serum concentrations of dioxin-like compounds in the US population effective 2005–2006 and 2007–2008: A multiple imputation and trending approach incorporating NHANES pooled sample data. Environ. Int. 2017, 105, 112–125. [Google Scholar] [CrossRef] [PubMed]
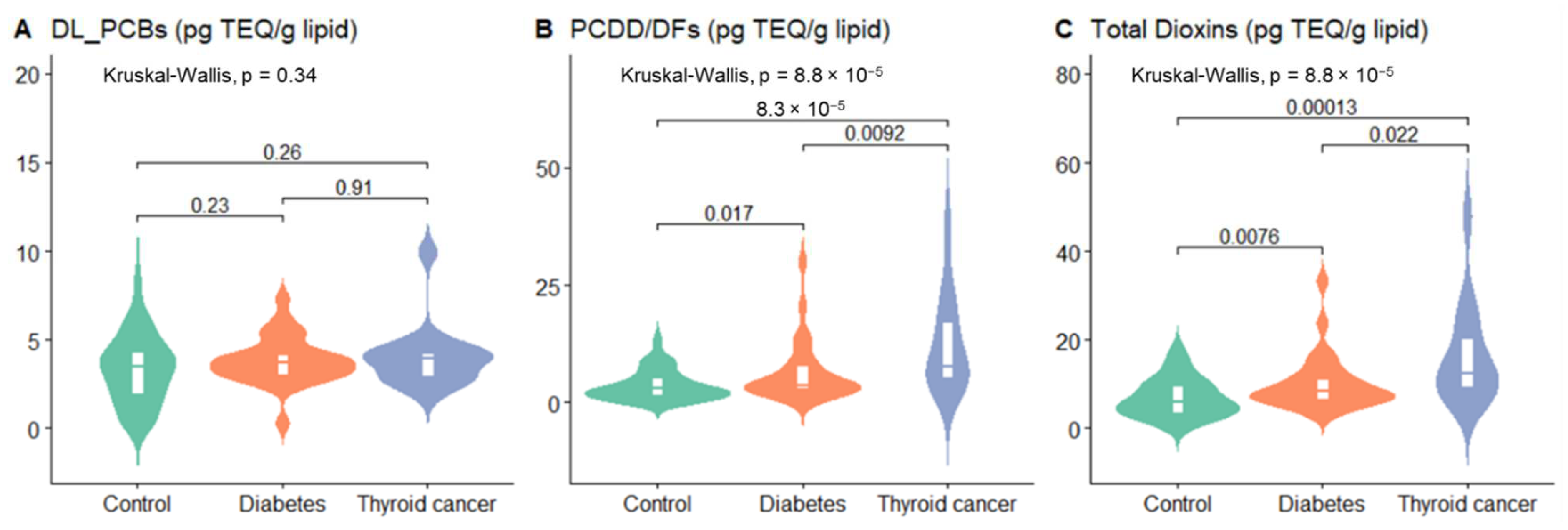
| Characteristic | Normal Range | Overall | Control (n = 55) | Type 2 Diabetes (n = 30) | Thyroid Cancer (n = 15) | p-Value |
|---|---|---|---|---|---|---|
| Sex | n (%) | 0.795 | ||||
| Male | 48 (48.00) | 27 (49.09) | 15 (50.00) | 6 (40.00) | ||
| Female | 52 (52.00) | 28 (50.91) | 15 (50.00) | 9 (60.00) | ||
| Median (IQR) | ||||||
| Age, years | 54.06 (21.04) | 54.25 (20.57) | 54.04 (22.14) | 52.60 (19.20) | 0.813 | |
| BMI, kg/m2 | 25–29.9 | 24.28 (2.15) | 24.31 (1.94) | 24.36 (2.21) | 24.20 (1.83) | 0.619 |
| FBS, mg/dL | 70–100 | 92.88 (14.23) | 89.42 (6.73) | 135.73 (40.13) | 92.88 (7.59) | ≤0.001 |
| HDL-C, mg/dL | 40 or higher | 52.89 (9.57) | 55.20 (12.23) | 51.56 (6.70) | 50.00 (5.23) | 0.004 |
| LDL-C, mg/dL | Less than 110 | 117.32 (26.32) | 112.35 (27.29) | 121.61 (22.17) | 115.51 (18.99) | 0.046 |
| SBP, mmHg | Less than 120 | 123.71 (14.35) | 121.81 (10.95) | 129.50 (12.44) | 116.25 (16.32) | 0.005 |
| TG, mg/dL | Less than 150 | 135.95 (66.38) | 131.56 (61.10) | 163.28 (67.74) | 125.05 (45.32) | 0.009 |
| TSH, uIU/mL | 0.35–5.5 | 1.66 (0.83) | 1.66 (0.82) | 1.64 (0.79) | 1.69 (0.92) | 0.659 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lim, Y.; Kang, Y.; Jung, K.; Jee, S. The Association between Blood Concentrations of PCDD/DFs, DL-PCBs and the Risk of Type 2 Diabetes Mellitus and Thyroid Cancer in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 8745. https://doi.org/10.3390/ijerph19148745
Lee S, Lim Y, Kang Y, Jung K, Jee S. The Association between Blood Concentrations of PCDD/DFs, DL-PCBs and the Risk of Type 2 Diabetes Mellitus and Thyroid Cancer in South Korea. International Journal of Environmental Research and Public Health. 2022; 19(14):8745. https://doi.org/10.3390/ijerph19148745
Chicago/Turabian StyleLee, SuHyun, YoungWook Lim, YounSeok Kang, KeumJi Jung, and SunHa Jee. 2022. "The Association between Blood Concentrations of PCDD/DFs, DL-PCBs and the Risk of Type 2 Diabetes Mellitus and Thyroid Cancer in South Korea" International Journal of Environmental Research and Public Health 19, no. 14: 8745. https://doi.org/10.3390/ijerph19148745
APA StyleLee, S., Lim, Y., Kang, Y., Jung, K., & Jee, S. (2022). The Association between Blood Concentrations of PCDD/DFs, DL-PCBs and the Risk of Type 2 Diabetes Mellitus and Thyroid Cancer in South Korea. International Journal of Environmental Research and Public Health, 19(14), 8745. https://doi.org/10.3390/ijerph19148745






