Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia
Abstract
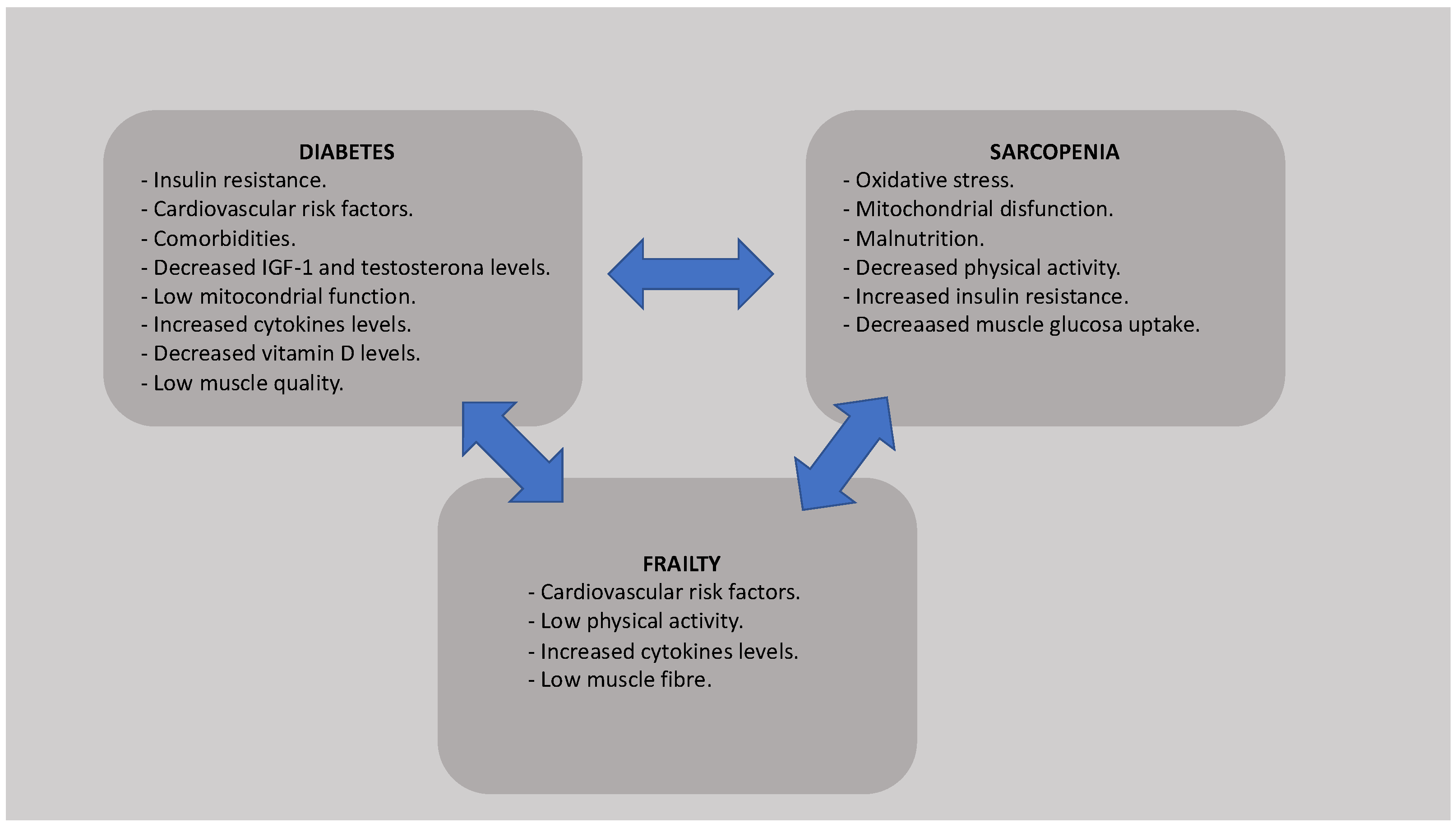
:1. Introduction
2. Sarcopenia and Frailty in Older T2DM Patients
3. Glycemic Control Goals in the Elderly Patient
4. Non-Pharmacological Management
4.1. Physical Activity
4.2. Nutrition
4.3. Other Recommendations
5. Pharmacological Treatment
5.1. Biguanides
5.2. Sulfonylureas
5.3. Meglintinides
5.4. Thiazolidinediones
5.5. Incretins
5.6. Sodium-Glucose Cotransporter 2 (SGLT-2) Inhibitors
5.7. Insulin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.B.; Pinho, C.B.R.P.; Saintrain, M.V.L.; Sodré, A.K.M.B.; Silva, C.A.B.D.; Doucet, J. Characteristics of the clinical treatment of Brazilian and French older adults with diabetes. Diabetes Res. Clin. Pract. 2021, 181, 109088. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Huelgas, R.; Peralta, F.G.; Mañas, L.R.; Formiga, F.; Domingo, M.P.; Bravo, J.M.; Miranda, C.; Ena, J. Tratamiento de la diabetes mellitus tipo 2 en el paciente anciano Treatment of type 2 diabetes mellitus in elderly patients. Rev. Esp. Geriatr. Gerontol. 2018, 53, 89–99. (In Spanish) [Google Scholar] [CrossRef]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in Older Persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef]
- Wu, C.-N.; Tien, K.-J. The Impact of Antidiabetic Agents on Sarcopenia in Type 2 Diabetes: A Literature Review. J. Diabetes Res. 2020, 2020, 9368583. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; McBurnie, M.A. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 146–156. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Rodriguez-Mañas, L. Diabetes and Frailty: Two Converging Conditions? Can. J. Diabetes 2016, 40, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Rolland, Y.; Czerwinski, S.; Abellan Van Kan, G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef] [Green Version]
- Serra-Prat, M.; Palomera, E.; Clave, P.; Puig-Domingo, M. Effect of age and frailty on ghrelin and cholecystokinin responses to a meal test. Am. J. Clin. Nutr. 2009, 89, 1410–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Ohama, H.; Nishiguchi, S.; Higuchi, K. Sarcopenia, frailty and type 2 diabetes mellitus (Review). Mol. Med. Rep. 2021, 24, 854. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Omura, T.; Toyoshima, K.; Araki, A. Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty. Nutrients 2020, 12, 3367. [Google Scholar] [CrossRef] [PubMed]
- Assar, M.E.; Laosa, O.; Mañas, L.R. Diabetes and frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 293–299. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Imataka, K.; Murata, K. Relationship between frailty and mortality, hospitalization, and cardiovascular diseases in diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2019, 18, 81. [Google Scholar] [CrossRef]
- Khalifa, M. Improving Patient Safety by Reducing Falls in Hospitals among the Elderly: A Review of Successful Strategies. ICIMTH 2019, 262, 340–343. [Google Scholar]
- Massimino, E.; Izzo, A.; Riccardi, G.; Della Pepa, G. The Impact of Glucose-Lowering Drugs on Sarcopenia in Type 2 Diabetes: Current Evidence and Underlying Mechanisms. Cells 2021, 10, 1958. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della Pepa, G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.; Dunning, T.; Rodriguez-Mañas, L. Diabetes in older people: New insights and remaining challenges. Lancet Diabetes Endocrinol. 2015, 3, 275–285. [Google Scholar] [CrossRef]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Barbé-Tuana, F.; Funchal, G.; Schmitz, C.R.R.; Maurmann, R.; Bauer, M.E. The interplay between immunosenescence and age-related diseases. Semin. Immunopathol. 2020, 42, 545–557. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Liu, L.; Li, L.; Cui, J.; Wu, S.; Tang, K. Significant abnormal glycemic variability increased the risk for arrhythmias in elderly type 2 diabetic patients. BMC Endocr. Disord. 2021, 21, 83. [Google Scholar] [CrossRef]
- Carrasco-Sánchez, F.; Fernández-Rodríguez, J.; Ena, J.; Gómez-Huelgas, R.; Carretero-Gómez, J. Medical treatment of type 2 diabetes mellitus: Recommendations of the Diabetes, Obesity and Nutrition Group of the Spanish Society of Internal Medicine. Revista Clínica Española 2021, 221, 101–108. [Google Scholar] [CrossRef]
- Xu, W.L.; von Strauss, E.; Qiu, C.X.; Winblad, B.; Fratiglioni, L. Uncontrolled diabetes increases the risk of Alzheimer’s disease: A pop-ulation-based cohort study. Diabetologia 2009, 52, 1031–1039. [Google Scholar] [CrossRef] [Green Version]
- Geller, A.I.; Shehab, N.; Lovegrove, M.C.; Kegler, S.R.; Weidenbach, K.N.; Ryan, G.J.; Budnitz, D.S. National estimates of insulin-related hypo-glycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern. Med. 2014, 174, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Dhaliwal, R.; Weinstock, R.S. Management of Diabetes in the Elderly. Med Clin. N. Am. 2015, 99, 351–377. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 12. Older Adults: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S168–S179. [Google Scholar] [CrossRef]
- Yanase, T.; Yanagita, I.; Muta, K.; Nawata, H. Frailty in elderly diabetes patients. Endocr. J. 2018, 65, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, R.J.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.; Ferrell, R.F.; Hurley, B.F. Strength training for the prevention and treatment of sarcopenia. J. Nutr. Health Aging 2000, 4, 143–155. [Google Scholar]
- Ferriolli, E.; Pessanha, F.P.A.S.; Marchesi, J.C.L.S. Diabetes and Exercise in the Elderly. Med. Sport Sci. 2014, 60, 122–129. [Google Scholar]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement from the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Po-sition Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [Green Version]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Lee, J.Y.; Kim, D.H. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: A systematic review and dose-response meta-analysis. BMC Geriatr. 2018, 18, 206. [Google Scholar] [CrossRef]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W. Vitamin B12 deficiency in the elderly: Is it worth screening? Hong Kong Med. J. 2015, 21, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Balboa-Castillo, T.; Struijk, E.A.; Lopez-Garcia, E.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillon, P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018, 47, 872–879. [Google Scholar] [CrossRef] [Green Version]
- Kwok, T.; Lee, J.; Ma, R.C.; Wong, S.Y.; Kung, K.; Lam, A.; Ho, C.; Lee, V.W.; Harrison, J.; Lam, L. A randomized placebo controlled trial of vitamin B12 supplementation to prevent cognitive decline in older diabetic people with borderline low serum vitamin B12. Clin. Nutr. 2017, 36, 1509–1515. [Google Scholar] [CrossRef]
- Narayana, S.; Dass, A.S.; Venkatarathnamma, P.N. Effect of Vitamin E and omega 3 fatty acids in type 2 diabetes mellitus patients. J. Adv. Pharm. Technol. Res. 2018, 9, 32–36. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O.; Godin, J.; Cahill, L.; Rockwood, K. Association of fatty acid consumption with frailty and mortality among middle-aged and older adults. Nutrition 2020, 70, 110610. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Miki, A.; Kaji, A.; Sakai, R.; Iwai, K.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of KAMOGAWA-DM cohort study. J. Clin. Biochem. Nutr. 2020, 66, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil–derived n−3 PUFA therapy increases muscle mass and function in healthy older adults1. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.; Pizato, N.; Da Mata, F.; Figueiredo, A.; Ito, M.; Pereira, M.G. Mediterranean Diet and Musculoskeletal-Functional Outcomes in Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2018, 22, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Walters, K. Adherence to Mediterranean Diet Reduces Incident Frailty Risk: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2018, 66, 783–788. [Google Scholar] [CrossRef]
- American Geriatrics Society; British Geriatrics Society; American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J. Am. Geriatr. Soc. 2001, 49, 664–672. [Google Scholar] [CrossRef]
- Morley, J.E. Diabetes, sarcopenia, and frailty. Clin. Geriatr. Med. 2008, 24, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.A.; Savva, G.M.; Kenny, R.A. Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res. Rev. 2013, 12, 840–851. [Google Scholar] [CrossRef]
- Lisko, I.; Kulmala, J.; Annetorp, M.; Ngandu, T.; Mangialasche, F.; Kivipelto, M. How can dementia and disability be prevented in older adults: Where are we today and where are we going? J. Intern. Med. 2021, 289, 807–830. [Google Scholar] [CrossRef]
- Ávila-Funes, J.A.; Amieva, H.; Barberger-Gateau, P.; Le Goff, M.; Raoux, N.; Ritchie, K.; Carrière, I.; Tavernier, B.; Tzourio, C.; Gutiérrez-Robledo, L.M.; et al. Cognitive impairment improves the pre-dictive validity of the phenotype of frailty for adverse health outcomes: The three-city study. J. Am. Geriatr. Soc. 2009, 57, 453–461. [Google Scholar] [CrossRef]
- Mehrabi, F.; Béland, F. Frailty as a Moderator of the Relationship between Social Isolation and Health Outcomes in Communi-ty-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 1675. [Google Scholar] [CrossRef]
- Braun, A.K.; Kubiak, T.; Kuntsche, J.; Meier-Höfig, M.; Müller, U.A.; Feucht, I.; Zeyfang, A. SGS: A structured treatment and teaching programme for older patients with diabetes mellitus—A prospective randomised controlled multi-centre trial. Age Ageing 2009, 38, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ohno, Y.; Yamauchi, T.; Kawabata, Y.; Ikegami, H. Efficacy and safety of metformin for treatment of type 2 diabetes in elderly Japanese patients. Geriatr. Gerontol. Int. 2011, 11, 55–62. [Google Scholar] [CrossRef]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Aghili, R.; Malek, M.; Valojerdi, A.E.; Banazadeh, Z.; Najafi, L.; Khamseh, M.E. Body composition in adults with newly diagnosed type 2 diabetes: Effects of metformin. J. Diabetes Metab. Disord. 2014, 13, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskaran, D.; Aparicio-Ugarriza, R.; Ferri-Guerra, J.; Milyani, R.; Florez, H.; Ruiz, J.G. Is There an Association Between Metformin Exposure and Frailty? Gerontol. Geriatr. Med. 2020, 6, 2333721420924956. [Google Scholar] [CrossRef]
- Piskovatska, V.; Stefanyshyn, N.; Storey, K.B.; Vaiserman, A.M.; Lushchak, O. Metformin as a geroprotector: Experimental and clinical evidence. Biogerontology 2019, 20, 33–48. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853, Erratum in Lancet 1999, 354, 602. [Google Scholar]
- Cetrone, M.; Mele, A.; Tricarico, M. Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II. Curr. Diabetes Rev. 2014, 10, 231–237. [Google Scholar] [CrossRef]
- Mele, A.; Calzolaro, S.; Cannone, G.; Cetrone, M.; Conte, D.; Tricarico, D. Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides-induced atrophy in skeletal muscle. Pharmacol. Res. Perspect. 2014, 2, e00028. [Google Scholar] [CrossRef]
- Strain, W.D.; Down, S.; Brown, P.; Puttanna, A.; Sinclair, A. Diabetes and Frailty: An Expert Consensus Statement on the Management of Older Adults with Type 2 Diabetes. Diabetes Ther. 2021, 12, 1227–1247. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Shi, J.; Zhang, Z. Sulfonylureas use and fractures risk in elderly patients with type 2 diabetes mellitus: A meta-analysis study. Aging Clin. Exp. Res. 2021, 33, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Jojima, T.; Aso, Y. Attention to the use of oral anti-diabetic medication in older adults with type 2 diabetes. Nihon Rinsho. Jpn. J. Clin. Med. 2013, 71, 1987–1992. [Google Scholar]
- Abbatecola, A.M.; Olivieri, F.; Corsonello, A.; Strollo, F.; Fumagalli, A.; Lattanzio, F. Frailty and safety: The example of diabetes. Drug Saf. 2012, 35 (Suppl. 1), 63–71. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, E.; Charbonnel, B.; Wilcox, R.G.; Skene, A.M.; Massi-Benedetti, M.; Yates, J.; Tan, M.; Spanheimer, R.; Standl, E.; Dormandy, J.A. PROactive Investigators. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: Data from the PROactive study (PROactive 08). Diabetes Care 2007, 30, 2773–2778. [Google Scholar] [CrossRef] [Green Version]
- Loke, Y.K.; Singh, S.; Furberg, C.D. Long-term use of thiazolidinediones and fractures in type 2 diabetes: A meta-analysis. Can. Med Assoc. J. 2009, 180, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Yokota, T.; Kinugawa, S.; Hirabayashi, K.; Suga, T.; Takada, S.; Omokawa, M.; Kadoguchi, T.; Takahashi, M.; Fukushima, A.; Matsushima, S.; et al. Pioglitazone improves whole-body aerobic capacity and skeletal muscle energy metabolism in patients with metabolic syndrome. J. Diabetes Investig. 2017, 8, 535–541. [Google Scholar] [CrossRef]
- Starner, C.I.; Schafer, J.A.; Heaton, A.H.; Gleason, P.P. Rosiglitazone and Pioglitazone Utilization from January 2007 Through May 2008 Associated with Five Risk-Warning Events. J. Manag. Care Pharm. 2008, 14, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Doucet, J.; Chacra, A.; Maheux, P.; Lu, J.; Harris, S.; Rosenstock, J. Efficacy and safety of saxagliptin in older patients with type 2 diabetes mellitus. Curr. Med Res. Opin. 2011, 27, 863–869. [Google Scholar] [CrossRef]
- Formiga, F.; Vidal, X.; Agustí, A.; Chivite, D.; Rosón, B.; Barbé, J.; López-Soto, A.; Torres, O.H.; Fernández-Moyano, A.; García, J.; et al. Potentially Inappropriate Prescription in Older Patients in Spain (PIPOPS) Investigators’ Project. Inappropriate prescribing in elderly people with diabetes admitted to hospital. Diabet. Med. 2016, 33, 655–662. [Google Scholar] [CrossRef]
- Sposito, A.C.; Berwanger, O.; de Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1Ras in type 2 diabetes: Mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc. Diabetol. 2018, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Nusca, A.; Tuccinardi, D.; Pieralice, S.; Giannone, S.; Carpenito, M.; Monte, L.; Watanabe, M.; Cavallari, I.; Maddaloni, E.; Ussia, G.P.; et al. Platelet Effects of Anti-diabetic Therapies: New Perspectives in the Management of Patients with Diabetes and Cardiovascular Disease. Front. Pharmacol. 2021, 12, 670155. [Google Scholar] [CrossRef] [PubMed]
- Linnebjerg, H.; Kothare, P.A.; Seger, M.; Wolka, A.M.; Mitchell, M.I. Exenatide—Pharmacokinetics, pharmacodynamics, safety and tolerability in patients ≥ 75 years of age with Type 2 diabetes. Int. J. Clin. Pharmacol. Ther. 2011, 49, 99–108. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. 1), S144–S174. [Google Scholar] [CrossRef] [PubMed]
- Palmiero, G.; Cesaro, A.; Vetrano, E.; Pafundi, P.; Galiero, R.; Caturano, A.; Moscarella, E.; Gragnano, F.; Salvatore, T.; Rinaldi, L.; et al. Impact of SGLT2 Inhibitors on Heart Failure: From Pathophysiology to Clinical Effects. Int. J. Mol. Sci. 2021, 22, 5863. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.E.; Rao, P.; Churchwell, M.D.; Lewis, S.J. Novel therapeutic agents for the treatment of diabetic kidney disease. Expert Opin. Investig. Drugs 2020, 29, 1277–1293. [Google Scholar] [CrossRef]
- Custódio, J.S., Jr.; Roriz-Filho, J.; Cavalcanti, C.A.J.; Martins, A.; Salles, J.E.N. Use of SGLT2 Inhibitors in Older Adults: Scientific Evidence and Practical Aspects. Drugs Aging 2020, 37, 399–409. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Bode, B.; Harris, S.; Vijapurkar, U.; Shaw, W.; Desai, M.; Meininger, G. Efficacy and Safety of Canagliflozin in Individuals Aged 75 and Older with Type 2 Diabetes Mellitus: A Pooled Analysis. J. Am. Geriatr. Soc. 2016, 64, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Gannon, J.; Claffey, P.; Laird, E.; Newman, L.; Kenny, R.; Briggs, R. The cross-sectional association between diabetes and orthostatic hypotension in community-dwelling older people. Diabet. Med. 2020, 37, 1299–1307. [Google Scholar] [CrossRef]
- Janka, H.U. Insulin therapy in elderly patients with type 2 diabetes: The role of insulin glargine. Diabetes Obes. Metab. 2008, 10, 35–41. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X.; Zhang, Q.; Zou, R. Diabetes mellitus and risk of falls in older adults: A systematic review and meta-analysis. Age Ageing 2016, 45, 761–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachex-Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, J.M.; Andrés, A.; Ros, M.; Bogónez, E.; Arribas, C.; Fernández-Agulló, T.; De Solis, A.J.; Gallardo, N.; Martínez, C. Development of insulin resistance during aging: Involvement of central processes and role of adipokines. Curr. Protein Pept. Sci. 2011, 12, 305–315. [Google Scholar] [CrossRef] [PubMed]
| Findings |
|---|
| Involuntary weigh loss of 10 lbs or more in the last six months. |
| Reduced grip strength. |
| Difficulty initiation movements, |
| Reduced walking speed. |
| Fatigue. |
| Frequent Causes of Sarcopenia |
|---|
| Nutrition: - Low protein intake - Low energy intake - Micronutrient deficiency - Malabsorption and other gastrointestinal conditions - Anorexia |
| Associated: with inactivity: - Bed rest, immobility deconditioning - Low activity, sedentary lifestyle |
| Diseases: - Cardiorespiratory disorders including chronic heart failure and chronic obstructive pulmonary disease - Metabolic disorders, particularly diabetes mellitus - Endocrine diseases, hormone deficiencies - Neurodegenerative diseases - Cancer - Liver and kidney disorders |
| Others - Hospital admission - Drug-relate - Ageing |
| Main Non-Pharmacological Measures |
|---|
| Physical activity |
| Nutritional counseling |
| Improving mental health |
| Cognitive stimulation |
| Avoid hypoglycemia |
| Fostering social ties |
| Fall prevention |
| Antidiabetic Drugs Recommended in Frail Patients | Antidiabetic Drugs Not Recommended in Frail Patients |
|---|---|
| Biguanides | Sulfonylureas |
| DDP-4 inhibitors | Meglintinides |
| Insulin (individualize) | Thiazolidinediones |
| GLP-1 agonist | |
| SGLT-2 inhibitors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz-Cánovas, J.; López-Sampalo, A.; Cobos-Palacios, L.; Ricci, M.; Hernández-Negrín, H.; Mancebo-Sevilla, J.J.; Álvarez-Recio, E.; López-Carmona, M.D.; Pérez-Belmonte, L.M.; Gómez-Huelgas, R.; et al. Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia. Int. J. Environ. Res. Public Health 2022, 19, 8677. https://doi.org/10.3390/ijerph19148677
Sanz-Cánovas J, López-Sampalo A, Cobos-Palacios L, Ricci M, Hernández-Negrín H, Mancebo-Sevilla JJ, Álvarez-Recio E, López-Carmona MD, Pérez-Belmonte LM, Gómez-Huelgas R, et al. Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia. International Journal of Environmental Research and Public Health. 2022; 19(14):8677. https://doi.org/10.3390/ijerph19148677
Chicago/Turabian StyleSanz-Cánovas, Jaime, Almudena López-Sampalo, Lidia Cobos-Palacios, Michele Ricci, Halbert Hernández-Negrín, Juan José Mancebo-Sevilla, Elena Álvarez-Recio, María Dolores López-Carmona, Luis Miguel Pérez-Belmonte, Ricardo Gómez-Huelgas, and et al. 2022. "Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia" International Journal of Environmental Research and Public Health 19, no. 14: 8677. https://doi.org/10.3390/ijerph19148677
APA StyleSanz-Cánovas, J., López-Sampalo, A., Cobos-Palacios, L., Ricci, M., Hernández-Negrín, H., Mancebo-Sevilla, J. J., Álvarez-Recio, E., López-Carmona, M. D., Pérez-Belmonte, L. M., Gómez-Huelgas, R., & Bernal-López, M. R. (2022). Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia. International Journal of Environmental Research and Public Health, 19(14), 8677. https://doi.org/10.3390/ijerph19148677






