Development and Evaluation of the Clinical Trial HEalth Knowledge and Beliefs Scale (CHEKS)
Abstract
:1. Introduction
History of Mistrust and Barriers to Participation
2. Materials and Methods
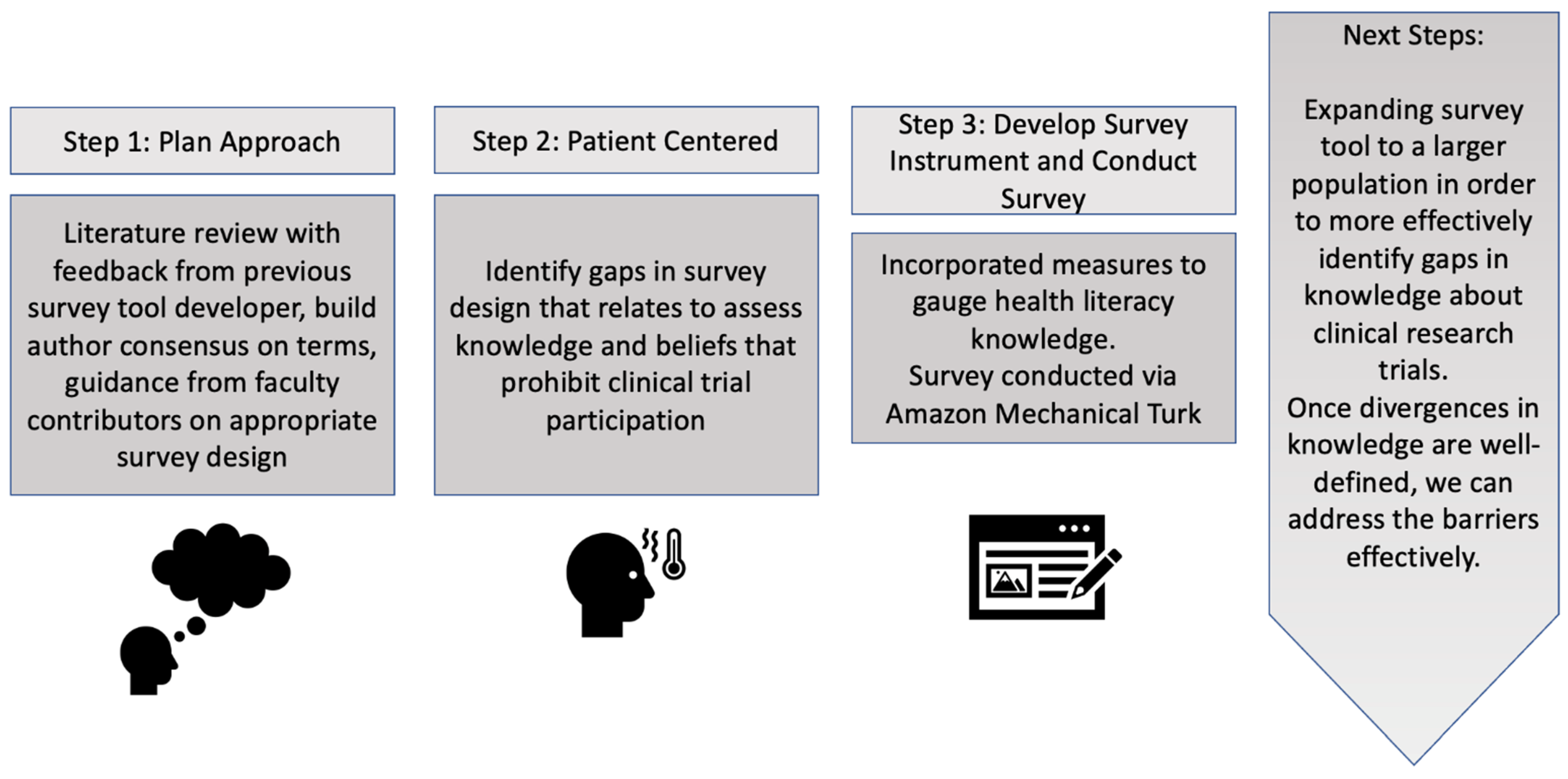
2.1. Survey Development Phase
2.2. Patient Centered Approach
2.3. Participants
2.4. Analysis
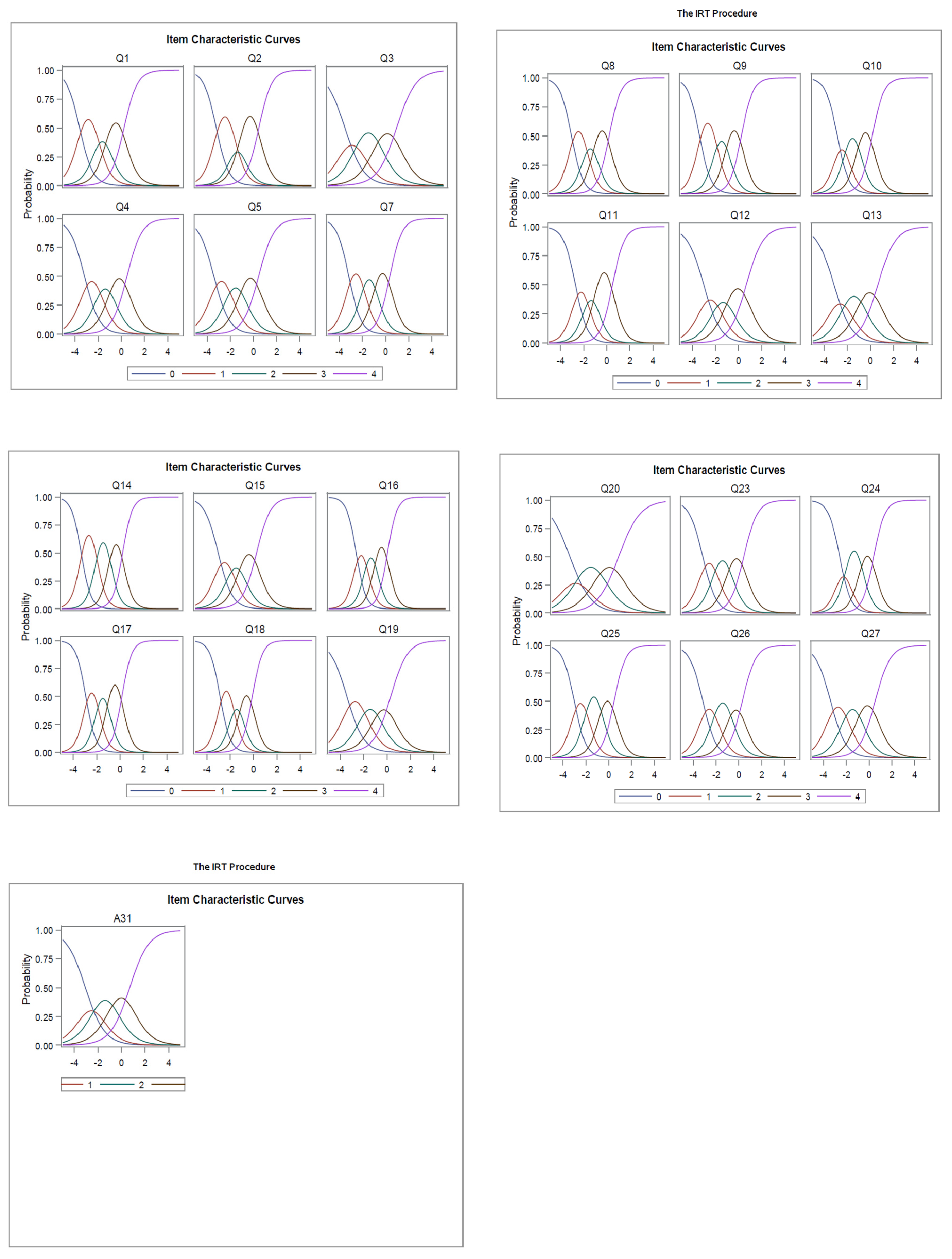
3. Results
3.1. Sample and Evaluation of Survey Items
3.2. Associations of Clinical Trial Knowledge with Demographic Variables
4. Discussion
4.1. Implications for Future Research and Practice
4.2. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Variable Name | Item Stem |
|---|---|
| Q1 | A clinical trial is a research study that involves people. |
| Q2 | Clinical trials test strategies designed to improve health. |
| Q3 | An intervention is a new treatment or strategy that is being tested by the research team. |
| Q4 | The goal of a Clinical Trial is to find out if an intervention works. |
| Q5 | The goal of a clinical trial is to find out if an intervention is safe. |
| Q6 | Clinical trials always involve medications. |
| Q7 | A research team is led by a principal investigator. |
| Q8 | Clinical trials can be funded by universities. |
| Q9 | Clinical can be funded by private companies. |
| Q10 | Clinical Trials can be funded by the government. |
| Q11 | Clinical Trials can take place at a hospital or doctor’s office. |
| Q12 | Clinical Trials can take place in a doctor’s office. |
| Q13 | Clinical Trials can take place in my community. |
| Q14 | Clinical trials follow a research plan called a protocol. |
| Q15 | The research plan is explained to the participants before the start of the study. |
| Q16 | The risks of research are explained to the volunteers before they agree to take part in the research study. |
| Q17 | The potential benefits are explained to the volunteers before they agree to take part in the research study. |
| Q18 | Participation in a clinical trial is voluntary. |
| Q19 | You can choose to leave the research study at any time. |
| Q20 | Research participants are kept updated as the study goes on. |
| Q21 | If other participants experience side effects while in the study, I will not know until the study is over. |
| Q22 | If you choose to leave the research study, you will not get good care from your doctor. |
| Q23 | Patient medical information is kept private during a clinical trial. |
| Q24 | The institutional review board exists to protect the patient’s rights during a clinical trial. |
| Q25 | The institutional review board is an ethics group that reviews the research plan before the start of a clinical trial. |
| Q26 | If a clinical trial is found to be unsafe, the research study will stop. |
| Q27 | If an intervention is found to be unsafe during a clinical trial the intervention will be discontinued. |
| Q28 | If an intervention is found to work much better than the control, then the control group will be discontinued, even if the study is not completed. |
| Q29 | People of color have low representation in clinical trials compared to Whites. |
| Q30 | Not enough health information on people of color is available through research. |
| A31 | Research is important to improve the health of people of color. |
| Characteristic | Percent |
|---|---|
| Age (mean and range) in years | 34 (18–73) |
| Median | 29 |
| Female gender | 48% |
| Race | |
| White | 35% |
| Black or African American | 11% |
| American Indian or Alaska Native | 5% |
| Asian | 44% |
| Native Hawaiian or Pacific Islander | <1% |
| Other | 4% |
| Education | |
| <High School | 2% |
| High School or GED | 7% |
| Some College, But No Degree | 11% |
| Bachelor’s Degree | 56% |
| Graduate or Professional Degree | 17% |
| Employed | 79% |
| Income: | |
| Less than $10,000 | 21% |
| $10,000–$19,999 | 15% |
| $20,000–$39,000 | 26% |
| $40,000–$59,999 | 20% |
| $60,000–$100,000 | 13% |
| Greater than $100,000 | 5% |
| Variable | Item Stem | Mean | SD | Item-Scale Correlations (Corrected for Item Overlap with Total) | p-Value |
|---|---|---|---|---|---|
| Q1 | A clinical trial is a research study that involves people. | 3.14 | 0.93 | 0.56 | p < 0.0001 |
| Q2 | Clinical trials test strategies designed to improve health. | 3.01 | 0.98 | 0.61 | p < 0.0001 |
| Q3 | An intervention is a new treatment or strategy that is being tested by the research team. | 2.78 | 1.01 | 0.45 | p < 0.0001 |
| Q4 | The goal of a clinical trial is to find out if an intervention works. | 2.94 | 1.02 | 0.53 | p < 0.0001 |
| Q5 | The goal of a clinical trial is to find out if an intervention is safe. | 2.99 | 0.99 | 0.54 | p < 0.0001 |
| Q7 | A research team is led by a principal investigator. | 3.03 | 0.96 | 0.64 | p < 0.0001 |
| Q8 | Clinical trials can be funded by universities. | 3.11 | 0.96 | 0.61 | p < 0.0001 |
| Q9 | Clinical can be funded by private companies. | 3.11 | 0.93 | 0.62 | p < 0.0001 |
| Q10 | Clinical Trials can be funded by the government. | 3.13 | 0.94 | 0.61 | p < 0.0001 |
| Q11 | Clinical Trials can take place at a hospital or doctor’s office. | 2.97 | 1.01 | 0.63 | p < 0.0001 |
| Q12 | Clinical Trials can take place in a doctor’s office. | 2.85 | 1.08 | 0.52 | p < 0.0001 |
| Q13 | Clinical Trials can take place in my community. | 2.85 | 1.07 | 0.49 | p < 0.0001 |
| Q14 | Clinical trials follow a research plan called a protocol. | 3.12 | 0.90 | 0.68 | p < 0.0001 |
| Q15 | The research plan is explained to the participants before the start of the study. | 3.06 | 1.02 | 0.57 | p < 0.0001 |
| Q16 | The risks of research are explained to the volunteers before they agree to take part in the research study in the consent form. | 3.18 | 0.97 | 0.68 | p < 0.0001 |
| Q17 | The potential benefits are explained to the volunteers before they agree to take part in the research study. | 3.17 | 0.91 | 0.68 | p < 0.0001 |
| Q18 | Participation in a clinical trial is voluntary. | 3.24 | 0.96 | 0.64 | p < 0.0001 |
| Q19 | You can choose to leave the research study at any time. | 2.77 | 1.06 | 0.52 | p < 0.0001 |
| Q20 | Research participants are kept updated as the study goes on. | 2.81 | 1.07 | 0.44 | p < 0.0001 |
| Q23 | Patient medical information is kept private during a clinical trial. | 2.99 | 0.99 | 0.59 | p < 0.0001 |
| Q24 | The institutional review board exists to protect the patient’s rights during a clinical trial. | 2.96 | 1.00 | 0.68 | p < 0.0001 |
| Q25 | The institutional review board is an ethics group that reviews the research plan before the start of a clinical trial. | 2.94 | 0.97 | 0.64 | p < 0.0001 |
| Q26 | If a clinical trial is found to be unsafe, the research study will be stopped. | 3.02 | 1.01 | 0.59 | p < 0.0001 |
| Q27 | If an intervention is found to be unsafe during a clinical trial the intervention will be discontinued. | 2.95 | 1.00 | 0.54 | p < 0.0001 |
| A31 | Research is important to improve the health of people of color. | 2.80 | 1.11 | 0.46 | p < 0.0001 |
References
- Winter, S.S.; Page-Reeves, J.M.; Page, K.A.; Haozous, E.; Solares, A.; Cordova, C.N.; Larson, R.S. Inclusion of special populations in clinical research: Important considerations and guidelines. J. Clin. Transl. Res. 2018, 4, 56–69. [Google Scholar]
- Miller, S.M.; Hudson, S.; Egleston, B.; Manne, S.L.; Buzaglo, J.; Devarajan, K.; Fleisher, L.D.; Millard, J.; Solarino, N.; Trinastic, J.; et al. The relationships among knowledge, self-efficacy, preparedness, decisional conflict, and decisions to participate in a cancer clinical trial. Psycho-Oncology 2013, 22, 481–489. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services. Health Literacy—Fact Sheet: Health Literacy Basics. Available online: https://health.gov/communication/literacy/quickguide/factsbasic.htm (accessed on 1 December 2021).
- Evans, K.R.; Lewis, M.J.; Hudson, S.V. The role of health literacy on African American and Hispanic/Latino perspectives on cancer clinical trials. J. Cancer Educ. 2012, 27, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Cutilli, C.C.; Bennett, I.M. Understanding the health literacy of America results of the national assessment of adult literacy. Orthop. Nurs./Natl. Assoc. Orthop. Nurses 2009, 28, 27. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization & Regional Office for Europe. Health Literacy: The Solid Facts. 2013. Available online: http://www.euro.who.int/pubrequest (accessed on 1 December 2021).
- Abdullah, A.; Liew, S.M.; Salim, H.; Ng, C.J.; Chinna, K. Prevalence of limited health literacy among patients with type 2 diabetes mellitus: A systematic review. PLoS ONE 2019, 14, e0216402. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.D.; Stucky, B.D.; Lee, J.Y.; Rozier, R.G.; Bender, D.E. Short assessment of health literacy—Spanish and English: A comparable test of health literacy for Spanish and English speakers. Health Serv. Res. 2010, 45, 1105–1120. [Google Scholar] [CrossRef]
- Arozullah, A.M.; Yarnold, P.R.; Bennett, C.L.; Soltysik, R.C.; Wolf, M.S.; Ferreira, R.M.; Lee, S.Y.D.; Costello, S.; Shakir, A.; Denwood, C.; et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med. Care 2007, 45, 1026–1033. [Google Scholar] [CrossRef]
- Lee, S.Y.D.; Bender, D.E.; Ruiz, R.E.; Cho, Y.I. Development of an easy-to-use Spanish health literacy test. Health Serv. Res. 2006, 41, 1392–1412. [Google Scholar] [CrossRef] [Green Version]
- Chung, A.; Seixas, A.; Williams, N. Development of “Advancing people of color in clinical trials now!”: Web-based randomized controlled trial protocol. JMIR Res. Protoc. 2020, 9, e17589. [Google Scholar] [CrossRef]
- Choi, Y.J.; Beck, S.-H.; Kang, W.Y.; Yoo, S.; Kim, S.-Y.; Lee, J.S.; Burt, T.; Kim, T.W. Knowledge and perception about clinical research shapes behavior: Face to face survey in Korean General public. J. Korean Med. Sci. 2016, 31, 674–681. [Google Scholar]
- Balasubramanian, J.; Rukmani, A.; Rajesh, R.; Purushothaman, K. A questionnaire based survey on awareness of clinical trials among general population. Int. J. Allied Med. Sci. Clin. Res. 2013, 1, 8–17. [Google Scholar]
- Cameron, P.; Pond, G.R.; Xu, R.Y.; Ellis, P.M.; Goffin, J.R. A comparison of patient knowledge of clinical trials and trial list priorities. Curr. Oncol. 2013, 20, e193. [Google Scholar] [CrossRef] [Green Version]
- Hersh, L.; Brooke, S.; Snyderman, D. Health literacy in primary care practice. Am. Fam. Physician 2015, 92, 118–124. [Google Scholar]
- Schnitzler, L.; Smith, S.K.; Shepherd, H.; Shaw, J.; Dong, S.; Carpenter, D.; Nguyen, F.; Dhillon, H. Communication during radiation therapy education sessions: The role of medical jargon and emotional support in clarifying patient confusion. Patient Educ. Couns. 2017, 100, 112–120. [Google Scholar] [CrossRef]
- Van Der Heide, I.; Wang, J.; Droomers, M.; Spreeuwenberg, P.; Rademakers, J.; Uiters, E. The relationship between health, education, and health literacy: Results from the Dutch Adult Literacy and Life Skills Survey. J. Health Commun. 2013, 18 (Suppl. 1), 172–184. [Google Scholar] [CrossRef] [Green Version]
- Berkman, N.D.; Sheridan, S.L.; Donahue, K.E. Health literacy interventions and outcomes: An updated systematic review. Evid. Rep. Technol. Assess. 2011, 199, 941. [Google Scholar]
- Chandler, J.; Danielle, S. Conducting clinical research using crowdsourced convenience samples. Ann. Rev. Clin. Psychol. 2016, 12, 53–81. [Google Scholar] [CrossRef] [Green Version]
- Bardos, J.; Friendenthal, J.; Spiegelman, J.; Williams, Z. Cloud based surveys to assess patient perceptions of health care: 1000 respondents in 3 days for US $300. JMIR Res. Protoc. 2016, 5, e166. [Google Scholar] [CrossRef]
- Georgsson, M.; Kushniruk, A. Mediating the cognitive walkthrough with patient groups to achieve personalized health in chronic disease self-management system evaluation. In Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef] [Green Version]
- Krieger, J.L.; Neil, J.M.; Strekalova, Y.A.; Sarge, M.A. Linguistic strategies for improving informed consent in clinical trials among low health literacy patients. J. Natl. Cancer Inst. 2017, 109, djw233. [Google Scholar] [CrossRef] [Green Version]
- Batterham, R.W.; Hawkins, M.; Collins, P.A.; Buchbinder, R.; Osborne, R.H. Health literacy: Applying current concepts to improve health services and reduce health inequalities. Public Health 2016, 132, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.; Geoghegan, C.; Lin, L.; McGuire, F.H.; Nido, V.; Grabert, B.; Morin, S.L.; Hallinan, Z.P.; Corneli, A. Patient preferences for using mobile technologies in clinical trials. Contemp. Clin. Trials Commun. 2019, 15, 100399. [Google Scholar] [CrossRef] [PubMed]
- Joffe, S.; Francis, E.; Paul, D.; Cleary, J.W.; Clark, J.C. Quality of informed consent: A new measure of understanding among research subjects. J. Natl. Cancer Inst. 2001, 93, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robb, K.A.; Lauren, G.; Jane, W. What impact do questionnaire length and monetary incentives have on mailed health psychology survey response? Br. J. Health Psychol. 2017, 22, 671–685. [Google Scholar] [CrossRef]
- Bolt, E.E.; Agnes van der, H.; Bregje, D.O.-P. Reducing questionnaire length did not improve physician response rate: A randomized trial. J. Clin. Epidemiol. 2014, 67, 477–481. [Google Scholar] [CrossRef]
- Follmer, D.J.; Sperling, R.A.; Suen, H.K. The role of MTurk in education research: Advantages, issues, and future directions. Educ. Res. 2017, 46, 329–334. [Google Scholar] [CrossRef]
- Swavely, D.A.; Maldonado, E.; Eid, S.; Etchason, J. Implementation and evaluation of a low health literacy and culturally sensitive diabetes education program. J. Healthc. Qual. 2014, 36, 16–23. [Google Scholar] [CrossRef]
- Abraham, M.B.; Nicholus, J.A.; Crone, M.; Ly, T.T.; Davis, E.A.; Jones, T.W. The importance of the Hawthorne effect on psychological outcomes unveiled in a randomized controlled trial of diabetes technology. J. Diabetes Sci. Technol. 2018, 12, 735–736. [Google Scholar] [CrossRef] [Green Version]
- Hays, R.D.; Liu, L.; Arie, K. Use of Internet panels to conduct surveys. Behav. Res. Methods 2015, 47, 685–690. [Google Scholar] [CrossRef]
| Variable Name | Item Stem |
|---|---|
| Q1 | A clinical trial is a research study that involves people. |
| Q2 | Clinical trials test strategies designed to improve health. |
| Q3 | An intervention is a new treatment or strategy that is being tested by the research team. |
| Q4 | The goal of a Clinical Trial is to find out if an intervention works. |
| Q5 | The goal of a clinical trial is to find out if an intervention is safe. |
| Q7 | A research team is led by a principal investigator. |
| Q8 | Clinical trials can be funded by universities. |
| Q9 | Clinical can be funded by private companies. |
| Q10 | Clinical Trials can be funded by the government. |
| Q11 | Clinical Trials can take place at a hospital or doctor’s office. |
| Q12 | Clinical Trials can take place in a doctor’s office. |
| Q13 | Clinical Trials can take place in my community. |
| Q14 | Clinical trials follow a research plan called a protocol. |
| Q15 | The research plan is explained to the participants before the start of the study. |
| Q16 | The risks of research are explained to the volunteers before they agree to take part in the research study. |
| Q17 | The potential benefits are explained to the volunteers before they agree to take part in the research study. |
| Q18 | Participation in a clinical trial is voluntary. |
| Q19 | You can choose to leave the research study at any time. |
| Q20 | Research participants are kept updated as the study goes on. |
| Q23 | Patient medical information is kept private during a clinical trial. |
| Q24 | The institutional review board exists to protect the patient’s rights during a clinical trial. |
| Q25 | The institutional review board is an ethics group that reviews the research plan before the start of a clinical trial. |
| Q26 | If a clinical trial is found to be unsafe, the research study will stop. |
| Q27 | If an intervention is found to be unsafe during a clinical trial the intervention will be discontinued. |
| A31 | Research is important to improve the health of people of color. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, A.; Donley, T.; Hays, R.D.; Robbins, R.; Seixas, A.; Jean-Louis, G. Development and Evaluation of the Clinical Trial HEalth Knowledge and Beliefs Scale (CHEKS). Int. J. Environ. Res. Public Health 2022, 19, 8660. https://doi.org/10.3390/ijerph19148660
Chung A, Donley T, Hays RD, Robbins R, Seixas A, Jean-Louis G. Development and Evaluation of the Clinical Trial HEalth Knowledge and Beliefs Scale (CHEKS). International Journal of Environmental Research and Public Health. 2022; 19(14):8660. https://doi.org/10.3390/ijerph19148660
Chicago/Turabian StyleChung, Alicia, Tiffany Donley, Ron D. Hays, Rebecca Robbins, Azizi Seixas, and Girardin Jean-Louis. 2022. "Development and Evaluation of the Clinical Trial HEalth Knowledge and Beliefs Scale (CHEKS)" International Journal of Environmental Research and Public Health 19, no. 14: 8660. https://doi.org/10.3390/ijerph19148660
APA StyleChung, A., Donley, T., Hays, R. D., Robbins, R., Seixas, A., & Jean-Louis, G. (2022). Development and Evaluation of the Clinical Trial HEalth Knowledge and Beliefs Scale (CHEKS). International Journal of Environmental Research and Public Health, 19(14), 8660. https://doi.org/10.3390/ijerph19148660






