Bottled and Well Water Quality in a Small Central Appalachian Community: Household-Level Analysis of Enteric Pathogens, Inorganic Chemicals, and Health Outcomes in Rural Southwest Virginia
Abstract
:1. Introduction
2. Materials & Methods
2.1. Study Setting
2.2. Data Collection
2.3. Water Sample Analyses
2.4. Data Sharing, Ethics, & Statistical Analyses
3. Results
3.1. Household Characteristics
3.2. Water Quality Results
3.3. Health Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO/UNICEF. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017: Special Focus on Inequalities; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- WHO/UNICEF. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020: Five Years into the SDGs; WHO & UNICEF: Geneva, Switzerland, 2021; ISBN 9789240030848. [Google Scholar]
- Lim, S.S.; Allen, K.; Bhutta, Z.A.; Dandona, L.; Forouzanfar, M.H.; Fullman, N.; Gething, P.W.; Goldberg, E.M.; Hay, S.I.; Holmberg, M.; et al. Measuring the Health-Related Sustainable Development Goals in 188 Countries: A Baseline Analysis from the Global Burden of Disease Study 2015. Lancet 2016, 388, 1813–1850. [Google Scholar] [CrossRef] [Green Version]
- Khalil, I.A.; Troeger, C.; Rao, P.C.; Blacker, B.F.; Brown, A.; Brewer, T.G.; Colombara, D.V.; De Hostos, E.L.; Engmann, C.; Guerrant, R.L.; et al. Morbidity, Mortality, and Long-Term Consequences Associated with Diarrhoea from Cryptosporidium Infection in Children Younger than 5 Years: A Meta-Analyses Study. Lancet Glob. Health 2018, 6, e758–e768. [Google Scholar] [CrossRef] [Green Version]
- Prüss-Ustün, A.; Wolf, J.; Bartram, J.; Clasen, T.; Cumming, O.; Freeman, M.C.; Gordon, B.; Hunter, P.R.; Medlicott, K.; Johnston, R. Burden of Disease from Inadequate Water, Sanitation and Hygiene for Selected Adverse Health Outcomes: An Updated Analysis with a Focus on Low- and Middle-Income Countries. Int. J. Hyg. Environ. Health 2019, 222, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-X.; Wang, Z.-H.; Cheng, X.-T.; Li, J.; Sang, Z.-P.; Zhang, X.-D.; Han, L.-L.; Qiao, X.-Y.; Wu, Z.-M.; Wang, Z.-Q. Arsenic and Fluoride Exposure in Drinking Water: Children’s IQ and Growth in Shanyin County, Shanxi Province, China. Environ. Health Perspect. 2007, 115, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, K.A.; Oberoi, S.; Barchowsky, A.; Chen, Y.; Guallar, E.; Nachman, K.E.; Rahman, M.; Sohel, N.; D’Ippoliti, D.; Wade, T.J.; et al. A Dose-Response Meta-Analysis of Chronic Arsenic Exposure and Incident Cardiovascular Disease. Int. J. Epidemiol. 2017, 46, 1924–1939. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental Toxic Metal Contaminants and Risk of Cardiovascular Disease: Systematic Review and Meta-Analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef] [Green Version]
- Greco, S.L.; Belova, A.; Haskell, J.; Backer, L. Estimated Burden of Disease from Arsenic in Drinking Water Supplied by Domestic Wells in the United States. J. Water Health 2019, 17, 801–812. [Google Scholar] [CrossRef]
- Mueller, J.T.; Gasteyer, S. The Widespread and Unjust Drinking Water and Clean Water Crisis in the United States. Nat. Commun. 2021, 12, 3544. [Google Scholar] [CrossRef]
- ARC About the Appalachian Region. Available online: https://www.arc.gov/about-the-appalachian-region/ (accessed on 30 June 2022).
- Krometis, L.-A.; Gohlke, J.; Kolivras, K.; Satterwhite, E.; Marmagas Susan, W.; Marr Linsey, C. Environmental Health Disparities in the Central Appalachian Region of the United States. Rev. Environ. Health 2017, 32, 253. [Google Scholar] [CrossRef]
- McKenna, M.L.; McAtee, S.; Bryan, P.E.; Jeun, R.; Ward, T.; Kraus, J.; Bottazzi, M.E.; Hotez, P.J.; Flowers, C.C.; Mejia, R. Human Intestinal Parasite Burden and Poor Sanitation in Rural Alabama. Am. J. Trop. Med. Hyg. 2017, 97, 1623–1628. [Google Scholar] [CrossRef]
- Cohen, A.; Darling, A.; Patton, H. Drinking Water Contamination, Exposure, and Associated Health Outcomes in Rural Appalachia: A Systematic Review and Meta-Analysis; NIHR-PROSPERO: Online, 2020. [Google Scholar]
- Lee, D.; Murphy, H.M. Private Wells and Rural Health: Groundwater Contaminants of Emerging Concern. Curr. Environ. Health Rep. 2020, 7, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J. Neglected Infections of Poverty in the United States of America. PLoS Negl. Trop. Dis. 2008, 2, e256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotez, P.J. Neglected Parasitic Infections and Poverty in the United States. PLoS Negl. Trop. Dis. 2014, 8, e3012. [Google Scholar] [CrossRef] [Green Version]
- ARC Health Disparities in Appalachia: The First Report in a Series Exploring Health Issues in Appalachia; Appalachian Regional Commission: Washington, DC, USA, 2017.
- Leach, C.R.; Schoenberg, N.E.; Hatcher, J. Factors Associated with Participation in Cancer Prevention and Control Studies among Rural Appalachian Women. Fam. Community Health 2011, 34, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, R.; Theeke, L.A. Strategies for Recruiting a Sample of Adults with Type 2 Diabetes from Primary Care Clinics in Rural Appalachia: Incorporating Cultural Competence. Int. J. Nurs. Sci. 2018, 5, 230–237. [Google Scholar] [CrossRef]
- Thurman, W.A.; Harrison, T.C. Reaching the “Hard-to-Reach”: Recruitment of Rural-Dwelling Adults with Disabilities. J. Transcult. Nurs. 2020, 31, 171–177. [Google Scholar] [CrossRef] [PubMed]
- McSpirit, S.; Reid, C. Residents’ Perceptions of Tap Water and Decisions to Purchase Bottled Water: A Survey Analysis from the Appalachian, Big Sandy Coal Mining Region of West Virginia. Soc. Nat. Resour. 2011, 24, 511–520. [Google Scholar] [CrossRef]
- Krometis, L.-A.; Patton, H.; Wozniak, A.; Sarver, E. Water Scavenging from Roadside Springs in Appalachia. J. Contemp. Water Res. Educ. 2019, 166, 46–56. [Google Scholar] [CrossRef] [Green Version]
- USCB United States Census Bureau. ACS 5-Year Estimates 5-Year Estimates—Public Use Microdata Sample. 2019. Available online: https://data.census.gov/mdat (accessed on 22 February 2022).
- ARC. County Economic Status in Appalachia, FY 2023. Available online: https://www.arc.gov/map/county-economic-status-in-appalachia-fy-2023/ (accessed on 30 June 2022).
- ARC. Distressed Designation and County Economic Status Classification System. Available online: https://www.arc.gov/distressed-designation-and-county-economic-status-classification-system/ (accessed on 30 June 2022).
- USDA. USDA NRCS Web Soil Survey, Wise County, VA. Available online: https://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx (accessed on 2 May 2022).
- Cohen, A.; Sullivan, C.A. Water and Poverty in Rural China: Developing an Instrument to Assess the Multiple Dimensions of Water and Poverty. Ecol. Econ. 2010, 69, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- IFAD; Cohen, A.; Jayne, S. The Multidimensional Poverty Assessment Tool: User’s Guide; United Nations International Fund for Agricultural Development: Rome, Italy, 2014; ISBN 9789290724759.
- Alasdair, C.; Ajay, P.; Qing, L.; Qi, Z.; Hongxing, L.; Gemei, Z.; Gang, Z.; Colford, J.M.; Smith, K.R.; Isha, R.; et al. Boiled or Bottled: Regional and Seasonal Exposures to Drinking Water Contamination and Household Air Pollution in Rural China. Environ. Health Perspect. 2020, 128, 127002. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, and Water Environment Federation: Washington, DC, USA, 2018. [Google Scholar]
- Goddard, F.G.B.; Ban, R.; Barr, D.B.; Brown, J.; Cannon, J.; Colford, J.M.; Eisenberg, J.N.S.; Ercumen, A.; Petach, H.; Freeman, M.C.; et al. Measuring Environmental Exposure to Enteric Pathogens in Low-Income Settings: Review and Recommendations of an Interdisciplinary Working Group. Environ. Sci. Technol. 2020, 54, 11673–11691. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gratz, J.; Amour, C.; Kibiki, G.; Becker, S.; Janaki, L.; Verweij, J.J.; Taniuchi, M.; Sobuz, S.U.; Haque, R.; et al. A Laboratory-Developed TaqMan Array Card for Simultaneous Detection of 19 Enteropathogens. J. Clin. Microbiol. 2013, 51, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of Quantitative Molecular Diagnostic Methods to Identify Causes of Diarrhoea in Children: A Reanalysis of the GEMS Case-Control Study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa de Macena, L.d.G.; Castiglia Feitosa, R.; Vieira, C.B.; Araújo, I.T.; Taniuchi, M.; Miagostovich, M.P. Microbiological Assessment of an Urban Lagoon System in the Coastal Zone of Rio de Janeiro, Brazil. Environ. Sci. Pollut. Res. 2021, 28, 1170–1180. [Google Scholar] [CrossRef]
- Cohen, A. Rural Virginia Public Drinking Water Supply Extension Project: A Prospective Cohort Study—Pre-Specified Study Protocols. Open Sci. Framew. Osfiov 2021, 7685. [Google Scholar]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; for the STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [Green Version]
- EPA US EPA National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 15 April 2022).
- EPA US EPA Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. Available online: https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals (accessed on 15 April 2022).
- Cummings, P. Methods for Estimating Adjusted Risk Ratios. Stata J. 2009, 9, 175–196. [Google Scholar] [CrossRef] [Green Version]
- Farquhar William, B.; Edwards David, G.; Jurkovitz Claudine, T.; Weintraub William, S. Dietary Sodium and Health. J. Am. Coll. Cardiol. 2015, 65, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [Green Version]
- Naser, A.M.; Rahman, M.; Unicomb, L.; Doza, S.; Gazi, M.S.; Alam, G.R.; Karim, M.R.; Uddin, M.N.; Khan, G.K.; Ahmed, K.M.; et al. Drinking Water Salinity, Urinary Macro-Mineral Excretions, and Blood Pressure in the Southwest Coastal Population of Bangladesh. J. Am. Heart Assoc. 2019, 8, e012007. [Google Scholar] [CrossRef] [Green Version]
- EPA. Drinking Water Advisory: Consumer Acceptability Advice and Health Effects Analysis on Sodium; U.S. Environmental Protection Agency Office of Water: Washington, DC, USA, 2003. [Google Scholar]
- WHO. A Global Overview of National Regulations and Standards for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Dai, D.; Rhoads, W.J.; Katner, A.; Strom, L.; Edwards, M.A.; Pruden, A.; Pieper, K.J. Molecular Survey of Legionella and Naegleria Fowleri in Private Well Water and Premise Plumbing Following the 2016 Louisiana Flood. Environ. Sci. Water Res. Technol. 2019, 5, 1464–1477. [Google Scholar] [CrossRef]
- Mapili, K.; Rhoads, W.J.; Coughter, M.; Pieper, K.J.; Edwards, M.A.; Pruden, A. Occurrence of Opportunistic Pathogens in Private Wells after Major Flooding Events: A Four State Molecular Survey. Sci. Total Environ. 2022, 826, 153901. [Google Scholar] [CrossRef]
- Cohen, A.; Zhang, Q.; Luo, Q.; Tao, Y.; Colford, J.M.; Ray, I. Predictors of Drinking Water Boiling and Bottled Water Consumption in Rural China: A Hierarchical Modeling Approach. Environ. Sci. Technol. 2017, 51, 6945–6956. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.; Ray, I. The Global Risks of Increasing Reliance on Bottled Water. Nat. Sustain. 2018, 1, 327–329. [Google Scholar] [CrossRef]
- Stelmach, D.R.; Clasen, T. Household Water Quantity and Health: A Systematic Review. Int. J. Environ. Res. Public. Health 2015, 12, 5954–5974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, I.; Smith, K.R. Towards Safe Drinking Water and Clean Cooking for All. Lancet Glob. Health 2021. [Google Scholar] [CrossRef]
- Olson, E.D.; Poling, D.; Solomon, G. Bottled Water: Pure Drink or Pure Hype? National Resources Defense Council: New York, NY, USA, 1999. [Google Scholar]
- Williams, A.R.; Bain, R.E.S.; Fisher, M.B.; Cronk, R.; Kelly, E.R.; Bartram, J. A Systematic Review and Meta-Analysis of Fecal Contamination and Inadequate Treatment of Packaged Water. PLoS ONE 2015, 10, e0140899. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.; Cui, J.; Song, Q.; Xia, Q.; Huang, J.; Yan, X.; Guo, Y.; Sun, Y.; Colford, J.M.; Ray, I. Bottled Water Quality and Associated Health Outcomes: A Systematic Review and Meta-Analysis of 20 Years of Published Data from China. Environ. Res. Lett. 2022, 17, 013003. [Google Scholar] [CrossRef]
- Felton, R. Looking for Info about Bottled Water Quality? Good Luck. (6 Surprising Things CR Learned While Digging into the Industry); Consumer Reports: Yonkers, NY, USA, 2019. [Google Scholar]
- Fantin, V.; Scalbi, S.; Ottaviano, G.; Masoni, P. A Method for Improving Reliability and Relevance of LCA Reviews: The Case of Life-Cycle Greenhouse Gas Emissions of Tap and Bottled Water. Sci. Total Environ. 2014, 476–477, 228–241. [Google Scholar] [CrossRef]
- Laville, S.; Taylor, M. A Million Bottles a Minute: World’s Plastic Binge “as Dangerous as Climate Change”; Guardian News & Media Limited: London, UK, 2017. [Google Scholar]
- Horowitz, N.; Frago, J.; Mu, D. Life Cycle Assessment of Bottled Water: A Case Study of Green2O Products. Waste Manag. 2018, 76, 734–743. [Google Scholar] [CrossRef]
- Pieper, K.J.; Krometis, L.-A.; Gallagher, D.; Benham, B.; Edwards, M. Profiling Private Water Systems to Identify Patterns of Waterborne Lead Exposure. Environ. Sci. Technol. 2015, 49, 12697–12704. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.; Walker, I.; Lassiter, R.; Lassiter, V.; Gibson, J.M.; Ferguson, P.L.; Deshusses, M.A. Evaluation of Private Well Contaminants in an Underserved North Carolina Community. Sci. Total Environ. 2021, 789, 147823. [Google Scholar] [CrossRef] [PubMed]
- Mulhern, R.; Stallard, M.; Zanib, H.; Stewart, J.; Sozzi, E.; MacDonald Gibson, J. Are Carbon Water Filters Safe for Private Wells? Evaluating the Occurrence of Microbial Indicator Organisms in Private Well Water Treated by Point-of-Use Activated Carbon Block Filters. Int. J. Hyg. Environ. Health 2021, 238, 113852. [Google Scholar] [CrossRef]
- Kostyla, C.; Bain, R.; Cronk, R.; Bartram, J. Seasonal Variation of Fecal Contamination in Drinking Water Sources in Developing Countries: A Systematic Review. Sci. Total Environ. 2015, 514, 333–343. [Google Scholar] [CrossRef]
- Mertens, A.; Balakrishnan, K.; Ramaswamy, P.; Rajkumar, P.; Ramaprabha, P.; Durairaj, N.; Hubbard, A.E.; Khush, R.; Colford, J.M.; Arnold, B.F. Associations between High Temperature, Heavy Rainfall, and Diarrhea among Young Children in Rural Tamil Nadu, India: A Prospective Cohort Study. Environ. Health Perspect. 2019, 127, 047004. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, L.; Huang, T.; Yan, M.; Liu, K.; Miao, Y.; He, H.; Li, S.; Sekar, R. Combined Effects of Seasonality and Stagnation on Tap Water Quality: Changes in Chemical Parameters, Metabolic Activity and Co-Existence in Bacterial Community. J. Hazard. Mater. 2021, 403, 124018. [Google Scholar] [CrossRef] [PubMed]
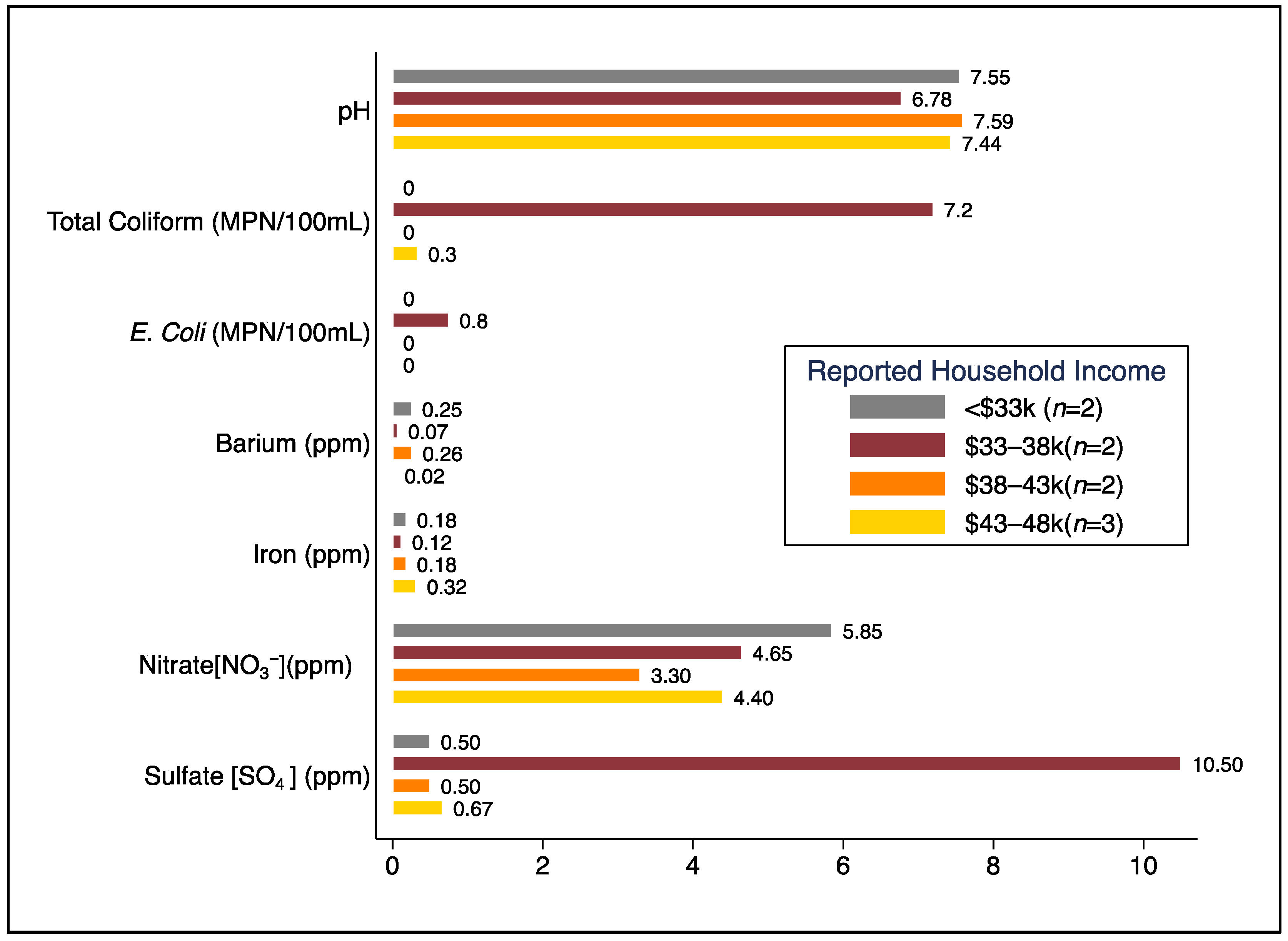
| Private Well (n = 3) | Bottled Water (n = 6) | All Households (n = 9) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| HH Owned or Rented | ||||||
| Own | 2 | 67% | 4 | 80% | 6 | 75% |
| Rent | 1 | 33% | 1 | 20% | 2 | 25% |
| Total a | 3 | 100% | 5 | 100% | 8 | 100% |
| Respondent’s Gender | ||||||
| Male | 2 | 67% | 4 | 67% | 6 | 67% |
| Female | 1 | 33% | 2 | 33% | 3 | 33% |
| Total | 3 | 100% | 6 | 100% | 9 | 100% |
| Respondent’s Race | ||||||
| White/Caucasian | 3 | 100% | 6 | 100% | 9 | 100% |
| HH Annual Income Level b | ||||||
| <33 k | 1 | 33% | 1 | 17% | 2 | 22% |
| 33–38 k | 1 | 33% | 1 | 17% | 2 | 22% |
| 38–43 k | 1 | 33% | 1 | 17% | 2 | 22% |
| 43–48 k | 0 | 0% | 3 | 50% | 3 | 33% |
| Total | 3 | 100% | 6 | 100% | 9 | 100% |
| Working toilet, sink, & tub/shower | ||||||
| Yes | 3 | 100% | 6 | 100% | 9 | 100% |
| Adults (≥18) residing in home | ||||||
| Total | 4 | n/a | 11 | n/a | 15 | n/a |
| Children (<18) residing in home | ||||||
| Total | 0 | n/a | 4 | n/a | 4 | n/a |
| Head of the HH Gender | ||||||
| Male | 2 | 67% | 4 | 67% | 6 | 67% |
| Female | 1 | 33% | 2 | 33% | 3 | 33% |
| Total | 3 | 100% | 6 | 100% | 9 | 100% |
| Head of HH: Age | ||||||
| Mean (standard deviation) | 66.3 | (7.5) | 51.0 | (17.1) | 56.1 | (16.0) |
| Head of HH: Years lived in home | ||||||
| Mean (standard deviation) | 30.7 | (26.1) | 12.6 | (9.9) | 18.6 | (17.7) |
| Bottled Water Samples (n = 6) | Tap Water Samples (n = 9) | Source Water Samples a (n = 9) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Mean | SD | Max | Mean | SD | Max | |
| Physicochemical Parameters | |||||||||
| pH | 6.72 | 0.78 | 7.54 | 7.38 | 0.65 | 7.91 | 7.35 | 0.68 | 7.85 |
| Temperature (Celsius) | 16.4 | 5.2 | 22.1 | 16.3 | 3.4 | 20.9 | 13.3 | 2.0 | 15.6 |
| Total dissolved solids (ppm) [Conductivity/2] | 22.6 | 24.5 | 61.6 | 104.8 | 35.3 | 140.5 | 95.5 | 28.2 | 125.9 |
| Dissolved Oxygen (%) | 80.4 | 3.8 | 86.0 | 44.6 | 14.3 | 72.0 | 43.1 | 13.0 | 69.0 |
| Microbiological Indicators & Pathogens | |||||||||
| Total Coliforms (TC) Detected: % HHs (n) | 16.7% | (n = 1) | 33.3% | (n = 3) | 33.3% | (n = 3) | |||
| MPN/100 mL for HHs with TC b | 1.0 | n/a | 2.0 | 5.5 | 7.3 | 18.3 | 5.1 | 8.0 | 16.9 |
| E. coli (EC) Detected: % HHs (n) | 0% | (n = 0) | 11.1% | (n = 1) | 11.1% | (n = 1) | |||
| MPN/100 mL for HHs with EC b | 0.0 | n/a | 0.0 | 0.5 | n/a | 1.0 | 1.5 | n/a | 3.0 |
| Specific Enteric Pathogens Detected | Not tested | Not tested | 33.3% | (n = 3) | |||||
| Aeromonas bacteria: % HHs (n) | 11.1% | (n = 1) | |||||||
| Campylobacter bacteria: % HHs (n) | 22.2% | (n = 2) | |||||||
| Enterobacter bacteria: % HHs (n) | 22.2% | (n = 2) | |||||||
| Inorganic Chemicals with EPA MCL * | |||||||||
| Arsenic (ppb) | 0.038 | 0.072 | 0.181 | 0.029 | 0.022 | 0.061 | 0.021 | 0.017 | 0.056 |
| Barium (ppm) | 0.005 | 0.008 | 0.021 | 0.137 | 0.120 | 0.272 | 0.135 | 0.119 | 0.274 |
| Cadmium (ppb) | 0.005 | 0.006 | 0.012 | 0.020 | 0.035 | 0.111 | 0.005 | 0.006 | 0.016 |
| Chromium (ppm) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 |
| Copper (ppm) | 0.001 | 0.003 | 0.008 | 0.066 | 0.183 | 0.552 | 0.002 | 0.003 | 0.009 |
| Lead (ppb) | 0.022 | 0.053 | 0.130 | 0.500 | 0.758 | 2.197 | 0.067 | 0.171 | 0.520 |
| Nitrate [NO3−] (ppm) | 4.575 | 1.580 | 6.300 | 4.278 | 3.261 | 10.400 | 4.533 | 1.551 | 7.000 |
| Selenium (ppm) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| % HHs (n) with ≥1 parameter/s: | |||||||||
| Greater than the EPA MCL c | 0% | (n = 0) | 11.1% | (n = 1) | 0% | (n = 0) | |||
| Greater than ½ the EPA MCL c | 50.0% | (n = 3) | 44.4% | (n = 4) | 33.3% | (n = 3) | |||
| Chemicals with EPA SMCL | |||||||||
| Aluminum (ppm) | 0.006 | 0.012 | 0.030 | 0.004 | 0.003 | 0.010 | 0.002 | 0.001 | 0.003 |
| Chloride (ppm) | 2.583 | 3.854 | 10.048 | 9.346 | 7.399 | 23.706 | 9.308 | 7.331 | 23.524 |
| Iron (ppm) | 0.000 | 0.000 | 0.001 | 0.231 | 0.218 | 0.669 | 0.212 | 0.190 | 0.671 |
| Manganese (ppm) | 0.001 | 0.002 | 0.006 | 0.014 | 0.008 | 0.024 | 0.012 | 0.009 | 0.021 |
| Sulfate [SO4] (ppm) | 2.333 | 5.241 | 13.000 | 2.667 | 6.185 | 19.00 | 2.778 | 6.870 | 21.00 |
| Zinc (ppm) | 0.007 | 0.001 | 0.009 | 0.214 | 0.480 | 1.489 | 0.015 | 0.011 | 0.038 |
| % HHs (n) with ≥1 parameter/s: | |||||||||
| Greater than the EPA SMCL d | 0% | (n = 0) | 33.3% | (n = 3) | 11.1% | (n = 1) | |||
| Greater than ½ the EPA SMCL d | 0% | (n = 0) | 55.5% | (n = 5) | 66.6% | (n = 6) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, A.; Rasheduzzaman, M.; Darling, A.; Krometis, L.-A.; Edwards, M.; Brown, T.; Ahmed, T.; Wettstone, E.; Pholwat, S.; Taniuchi, M.; et al. Bottled and Well Water Quality in a Small Central Appalachian Community: Household-Level Analysis of Enteric Pathogens, Inorganic Chemicals, and Health Outcomes in Rural Southwest Virginia. Int. J. Environ. Res. Public Health 2022, 19, 8610. https://doi.org/10.3390/ijerph19148610
Cohen A, Rasheduzzaman M, Darling A, Krometis L-A, Edwards M, Brown T, Ahmed T, Wettstone E, Pholwat S, Taniuchi M, et al. Bottled and Well Water Quality in a Small Central Appalachian Community: Household-Level Analysis of Enteric Pathogens, Inorganic Chemicals, and Health Outcomes in Rural Southwest Virginia. International Journal of Environmental Research and Public Health. 2022; 19(14):8610. https://doi.org/10.3390/ijerph19148610
Chicago/Turabian StyleCohen, Alasdair, Md Rasheduzzaman, Amanda Darling, Leigh-Anne Krometis, Marc Edwards, Teresa Brown, Tahmina Ahmed, Erin Wettstone, Suporn Pholwat, Mami Taniuchi, and et al. 2022. "Bottled and Well Water Quality in a Small Central Appalachian Community: Household-Level Analysis of Enteric Pathogens, Inorganic Chemicals, and Health Outcomes in Rural Southwest Virginia" International Journal of Environmental Research and Public Health 19, no. 14: 8610. https://doi.org/10.3390/ijerph19148610
APA StyleCohen, A., Rasheduzzaman, M., Darling, A., Krometis, L.-A., Edwards, M., Brown, T., Ahmed, T., Wettstone, E., Pholwat, S., Taniuchi, M., & Rogawski McQuade, E. T. (2022). Bottled and Well Water Quality in a Small Central Appalachian Community: Household-Level Analysis of Enteric Pathogens, Inorganic Chemicals, and Health Outcomes in Rural Southwest Virginia. International Journal of Environmental Research and Public Health, 19(14), 8610. https://doi.org/10.3390/ijerph19148610







