The Effect of Copper and Copper Oxide Nanoparticles on Rainbow Trout (Oncorhynchus mykiss W.) Spermatozoa Motility after Incubation with Contaminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milt Collection
2.2. Chemicals and Storage Condition
2.3. Computer-Assisted Sperm Analysis (CASA)
2.4. Statistical Analysis
3. Results
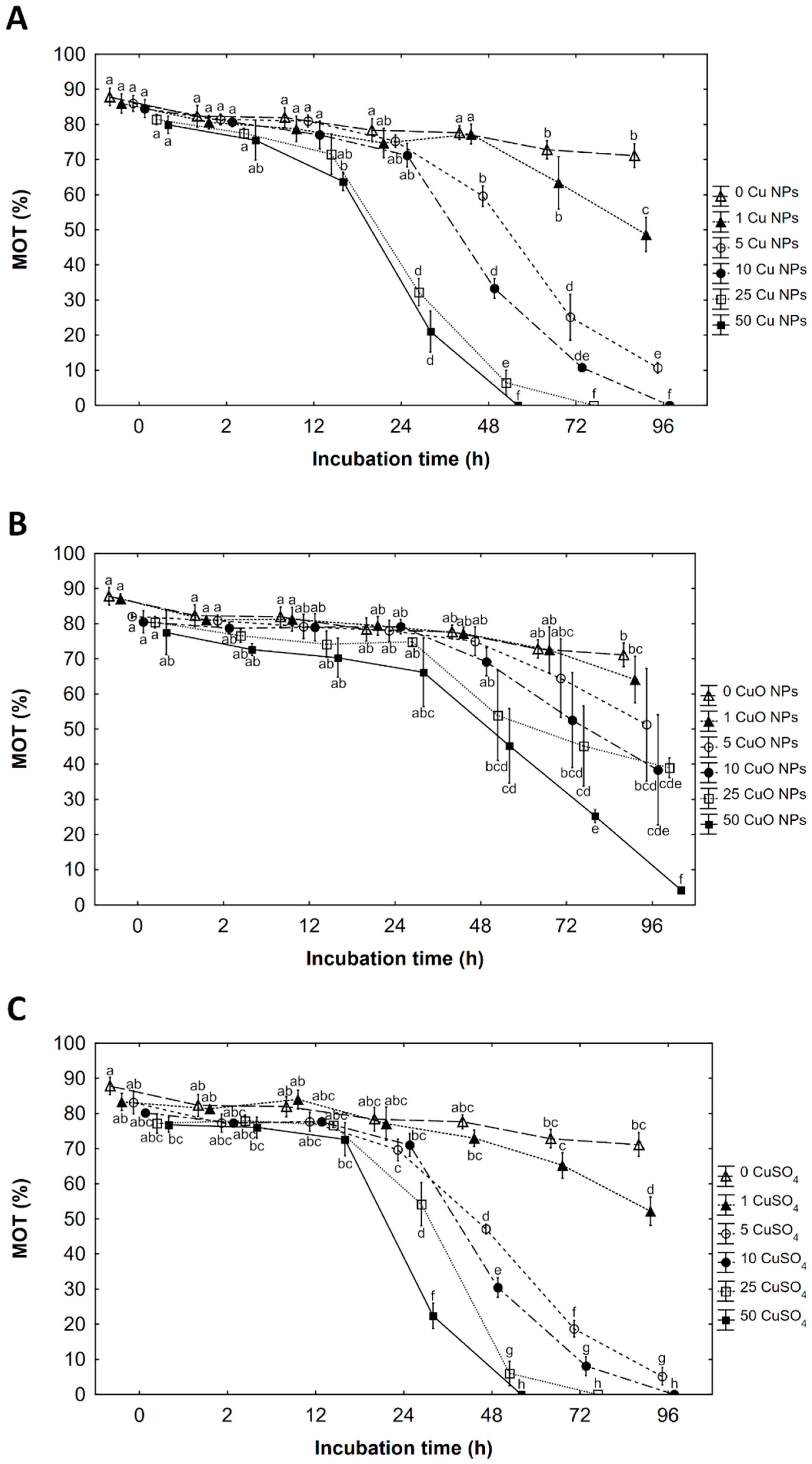
3.1. Percentage of Motile Spermatozoa (MOT)
3.2. Curvilinear Velocity (VCL)
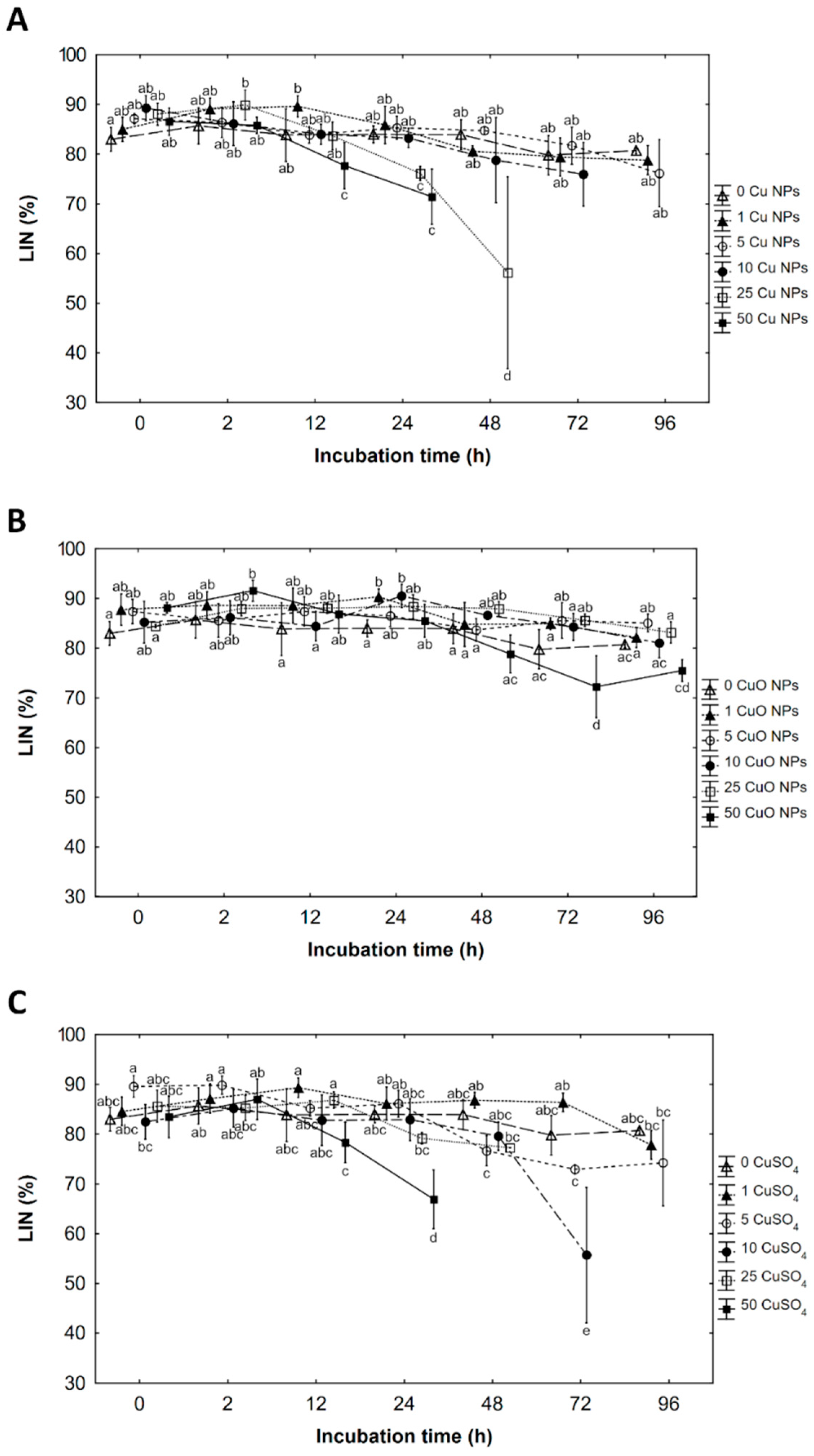
3.3. Linearity (LIN)
3.4. Amplitude of Lateral Head Displacement (ALH)
3.5. Beat Cross Frequency (BCF)
3.6. Motility Duration (MD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arellano, J.; Storch, V.; Sarasquete, C. Histological changes and copper accumulation in liver and gills of the senegales sole, Solea senegalensis. Ecotoxicol. Environ. Saf. 1999, 44, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Taylor, E.; Butler, P. The resting membrane potential of white muscle from brown trout (Salmo trutta) exposed to copper in soft, acidic water. J. Exp. Biol. 2000, 203, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Banta, G.T.; Selck, H.; Berhanu, D.; Valsami-Jones, E.; Forbes, V.E. Toxicity and bioaccumulation of sediment-associated silver nanoparticles in the estuarine polychaete, Nereis (Hediste) diversicolor. Aquat. Toxicol. 2014, 156, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Muna, M.; Knöbel, M.; Kistler, D.; Odzak, N.; Kühnel, D.; Müller, J.; Gupta, G.S.; Kumar, A.; Shanker, R.; et al. Natural water as the test medium for Ag and CuO nanoparticle hazard evaluation: An interlaboratory case study. Environ. Pollut. 2016, 216, 689–699. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Sarkar, A.; Das, J.; Manna, P.; Sil, P.C. Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology 2011, 290, 208–217. [Google Scholar] [CrossRef]
- Hatef, A.; Alavi, S.M.H.; Golshan, M.; Linhart, O. Toxicity of environmental contaminants to fish spermatozoa function in vitro—A review. Aquat. Toxicol. 2013, 140–141, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Góralska, M.; Senze, M.; Polechoński, R.; Dobicki, W.; Pokorny, P.; Skwarka, T. Biocidal properties of silver-nanoparticles in water environments. Pol. J. Environ. Stud. 2015, 24, 1641–1647. [Google Scholar] [CrossRef]
- Kowalska-Góralska, M.; Senze, M.; Łuczyńska, J.; Czyż, K. Effects of the ionic and nanoparticle forms of Cu and Ag on these metals’ bioaccumulation in the eggs and fry of rainbow trout (Oncorhynchus mykiss W.). Int. J. Environ. Res. Public Health 2020, 17, 6392. [Google Scholar] [CrossRef]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Dziewulska, K.; Domagała, J. Effect of pH and cation concentrations on spermatozoan motility of sea trout (Salmo trutta m. trutta L.). Theriogenology 2013, 79, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Gage, M.; Macfarlane, C.; Yeates, S.; Ward, R.; Searle, J.; Parker, G. Spermatozoal traits and sperm competition in Atlantic salmon: Relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 2004, 14, 44–47. [Google Scholar] [CrossRef]
- Gallego, V.; Cavalcante, S.; Fujimoto, R.; Carneiro, P.; Azevedo, H.; Maria, A. Fish sperm subpopulations: Changes after cryopreservation process and relationship with fertilization success in tambaqui (Colossoma macropomum). Theriogenology 2017, 87, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Tuset, V.M.; Dietrich, G.J.; Wojtczak, M.; Słowińska, M.; De Monserrat, J.; Ciereszko, A. Relationships between morphology, motility and fertilization capacity in rainbow trout (Oncorhynchus mykiss) spermatozoa. J. Appl. Ichthyol. 2008, 24, 393–397. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Blinova, I.; Ivask, A.; Heinlaan, M.; Mortimer, M.; Kahru, A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ. Pollut. 2010, 158, 41–47. [Google Scholar] [CrossRef]
- de Oliveira-Filho, E.C.; Lopes, R.M.; Paumgartten, F.J.R. Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 2004, 56, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Harmon, S.M.; Specht, W.L.; Chandler, G.T. A comparison of the daphnids Ceriodaphnia dubia and Daphnia ambigua for their utilization in routine toxicity testing in the Southeastern United States. Arch. Environ. Contam. Toxicol. 2003, 45, 79–85. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Mastin, B.J.; Rodgers, J.H. Toxicity and bioavailability of copper herbicides (Clearigate, Cutrine-Plus, and copper sulfate) to freshwater animals. Arch. Environ. Contam. Toxicol. 2000, 39, 445–451. [Google Scholar] [CrossRef]
- Shaw, B.J.; Handy, R.D. Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environ. Int. 2011, 37, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Garncarek, M.; Kowalska-Góralska, M.; Senze, M.; Czyż, K. The influence of available Cu and Au nanoparticles (NPs) on the survival of water fleas (Daphnia pulex). Int. J. Environ. Res. Public Health 2019, 16, 3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska-Góralska, M.; Dziewulska, K.; Kulasza, M. Effect of copper nanoparticles and ions on spermatozoa motility of sea trout (Salmo trutta m.trutta L.). Aquat. Toxicol. 2019, 211, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef]
- Khoshnood, R.; Jaafarzadeh, N.; Jamili, S.; Farshchi, P.; Taghavi, L. Nanoparticles ecotoxicity on Daphnia magna. Transylv. Rev. Syst. Ecol. Res. 2016, 18, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Sovová, T.; Kocí, V.; Kochánková, L. Ecotoxicity of nano and bulk forms of metal oxides. In Proceedings of the NANOCON Conference, Roznov Pod Radhostem, Czech Republic, 20–22 October 2009; pp. 62–71. [Google Scholar]
- Jo, H.J.; Choi, J.W.; Lee, S.H.; Hong, S.W. Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: The importance of their dissolved fraction varying with preparation methods. J. Hazard. Mater. 2012, 227–228, 301–308. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, G.; Xia, J.; Jin, S. Toxicity of nanoscale CuO and ZnO to Daphnia magna. Chem. Res. Chin. 2012, 28, 209–213. [Google Scholar]
- Liu, J.; Fan, D.; Wang, L.; Shi, L.; Ding, J.; Chen, Y.; Shen, S. Effects of ZnO, CuO, Au, and TiO2 nanoparticles on Daphnia magna and early life stages of zebrafish Danio rerio. Environ. Prot. Eng. 2014, 40, 139–149. [Google Scholar]
- Manusadžianas, L.; Caillet, C.; Fachetti, L.; Gylyte, B.; Grigutyte, R.; Jurkoniene, S.; Karitonas, R.; Sadauskas, K.; Thomas, F.; Vitkus, R.; et al. Toxicity of copper oxide nanoparticle suspensions to aquatic biota. Environ. Toxicol. Chem. 2012, 31, 108–114. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Peng, H.; Huang, W.; Zhou, Y.; Yan, D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J. Colloid Interface Sci. 2008, 325, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Al-Bairuty, G.; Shaw, B.; Handy, R.; Henry, T. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2013, 126, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [Green Version]
- Juganson, K.; Ivask, A.; Blinova, I.; Mortimer, M.; Kahru, A. NanoE-Tox: New and in-depth database concerning ecotoxicity of nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 1788–1804. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Ranville, J.F.; Diamond, S.; Gallego-Urrea, J.A.; Metcalfe, C.; Rose, J.; Horne, N.; Koelmans, A.A.; Klaine, S.J. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012, 31, 50–59. [Google Scholar] [CrossRef]
- Shaw, B.J.; Al-Bairuty, G.; Handy, R.D. Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchus mykiss): Physiol. Accumulation. Aquat. Toxicol. 2012, 116–117, 90–101. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef]
- Ebrahimi, M. Effects of in vivo and in vitro zinc and cadmium treatment on sperm steroidogenesis of the African catfish Clarias gairepinus. Pak. J. Biol. Sci. 2007, 10, 2862–2867. [Google Scholar] [CrossRef]
- Kollár, T.; Kása, E.; Csorbai, B.; Urbányi, B.; Csenki-Bakos, Z.; Horváth, Á. In vitro toxicology test system based on common carp (Cyprinus carpio) sperm analysis. Fish Physiol. Biochem. 2018, 44, 1577–1589. [Google Scholar] [CrossRef]
- Kutluyer, F.; Benzer, F.; Erişir, M.; Öğretmen, F.; Inanan, B.E. The in vitro effect of cypermethrin on quality and oxidative stress indices of rainbow trout Oncorhynchus mykiss spermatozoa. Pestic. Biochem. Physiol. 2016, 128, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, B.; Pietrusewicz, M.; Radziwoniuk, J.; Glogowski, J. The effect of copper, zinc, mercury and cadmium on some sperm enzyme activities in the common carp (Cyprinus carpio L.). Reprod. Biol. 2009, 9, 295–301. [Google Scholar] [CrossRef]
- Shaliutina, O.; Materiienko, A.; Shaliutina-Kolešsová, A.; Gazo, I. Using fish spermatozoa in in vitro toxicity tests: A potential toxicology tool. Aquaculture 2021, 539, 736647. [Google Scholar] [CrossRef]
- Özgür, M.E. The in vitro effect of silica nanoparticles on spermatozoon of rainbow trout (Oncorhynchus mykiss). Fresenius Environ. Bull. 2018, 27, 7433–7437. [Google Scholar]
- Özgür, M.E.; Balcıoğlu, S.; Ulu, A.; Özcan, İ.; Okumuş, F.; Köytepe, S.; Ateş, B. The in vitro toxicity analysis of titanium dioxide (TiO2) nanoparticles on kinematics and biochemical quality of rainbow trout sperm cells. Environ. Toxicol. Pharmacol. 2018, 62, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Özgür, M.E.; Ulu, A.; Balcıoğlu, S.; Özcan, İ.; Köytepe, S.; Ateş, B. The toxicity assessment of iron oxide (Fe3O4) nanoparticles on physical and biochemical quality of rainbow trout spermatozoon. Toxics 2018, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Ozgur, M.E.; Ulu, A.; Balcioglu, S.; Ozcan, I.; Okumuş, F.; Koytepe, S.; Ates, B. Investigation of toxicity properties of flower-like ZnO nanoparticles on Cyprinus carpio sperm cells using computer-assisted sperm analysis (CASA). Turk. J. Fish. Aquat. Sci. 2018, 18, 771–780. [Google Scholar] [CrossRef]
- Abascal, F.; Cosson, J.; Fauvel, C. Characterization of sperm motility in sea bass: The effect of heavy metals and physicochemical variables on sperm motility. J. Fish Biol. 2007, 70, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Chyb, J.; Kime, D.; Mikołajczyk, T.; Szczerbik, P.; Epler, P. The influence of zinc on sperm motility of common carp—A computer assisted studies. Arch. Pol. Fish. 2000, 8, 5–14. [Google Scholar]
- Dietrich, G.J.; Dietrich, M.; Kowalski, R.K.; Dobosz, S.; Karol, H.; Demianowicz, W.; Glogowski, J. Exposure of rainbow trout milt to mercury and cadmium alters sperm motility parameters and reproductive success. Aquat. Toxicol. 2010, 97, 277–284. [Google Scholar] [CrossRef]
- Kime, D.E.; Ebrahimi, M.; Nysten, K.; Roelants, I.; Rurangwa, E.; Moore, H.D.M.; Ollevier, F. Use of computer assisted sperm analysis (CASA) for monitoring the effects of pollution on sperm quality of fish; application to the effects of heavy metals. Aquat. Toxicol. 1996, 36, 223–237. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Mansour, N.; Berger, B. The effect of inorganic and organic pollutants on sperm motility of some freshwater teleosts. J. Fish Biol. 2004, 65, 1283–1297. [Google Scholar] [CrossRef]
- Li, Z.H.; Li, P.; Dzyuba, B.; Randak, T. Influence of environmental related concentrations of heavy metals on motility parameters and antioxidant responses in sturgeon sperm. Chem. Biol. Interact. 2010, 188, 473–477. [Google Scholar] [CrossRef]
- Morisawa, S.; Morisawa, M. Induction of potential for sperm motility by bicarbonate and pH in rainbow trout and chum salmon. J. Exp. Biol. 1988, 136, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Billard, R.; Roubaud, P. The effect of metals and cyanide on fertilization in rainbow trout (Salmo gairdneri). Water Res. 1985, 19, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Chyb, J.; Kime, D.; Szczerbik, P.; Mikołajczyk, T.; Epler, P. Computer-assisted analysis (CASA) of common carp Cyprinus carpio L. Spermatozoa motility in the presence of cadmium. Arch. Pol. Fish. 2001, 9, 173–181. [Google Scholar]
- Chyb, J.; Sokołowska-Mikołajczyk, M.; Kime, D.; Socha, M.; Epler, P. The influence of mercury on computer analyzed sperm motility of common carp, Cyprinus carpio L., in vitro. Arch. Pol. Fish. 2001, 9, 51–60. [Google Scholar]
- Khan, A.T.; Weis, J.S. Toxic effects of mercuric chloride on sperm and egg viability of two populations of mummichog, Fundulus heteroclitus. Environ. Pollut. 1987, 48, 263–273. [Google Scholar] [CrossRef]
- McIntyre, J.D. Toxicity of methyl mercury for steelhead trout sperm. Bull. Environ. Contam. Toxicol. 1973, 9, 98–99. [Google Scholar] [CrossRef]
- Rurangwa, E.; Roelants, I.; Huyskens, G.; Ebrahimi, M.; Kime, D.E.; Ollevier, F. The minimum effective spermatozoa: Egg ratio for artificial insemination and the effects of mercury on sperm motility and fertilization ability in Clarias gariepinus. J. Fish Biol. 1998, 53, 402–413. [Google Scholar] [CrossRef]
- Özgür, M.E.; Ulu, A.; Özcan, İ.; Balcioglu, S.; Ateş, B.; Köytepe, S. Investigation of toxic effects of amorphous SiO2 nanoparticles on motility and oxidative stress markers in rainbow trout sperm cells. Environ. Sci. Pollut. Res. 2019, 26, 15641–15652. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Vijver, M.G.; Ahmad, F.; Richardson, M.K.; Peijnenburg, W.J.G.M. Toxicity of different-sized copper nano- and submicron particles and their shed copper ions to zebrafish embryos. Environ. Toxicol. Chem. 2014, 33, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Long, X.; Cheng, Y.; Liu, Z.; Yan, S. A comparison effect of copper nanoparticles versus copper sulphate on juvenile Epinephelus coioides: Growth parameters, digestive enzymes, body composition, and histology as biomarkers. Int. J. Genom. 2015, 2015, 783021. [Google Scholar] [CrossRef] [Green Version]
- Sovová, T.; Boyle, D.; Sloman, K.A.; Vanegas, C.; Handy, R.D. Impaired behavioural response to alarm substance in rainbow trout exposed to copper nanoparticles. Aquat. Toxicol. 2014, 152, 195–204. [Google Scholar] [CrossRef] [PubMed]

| Organism | Nanoparticle | LC50 | Incubation Time | Source |
|---|---|---|---|---|
| Daphnia pulex | Cu | 0.51 mg·L−1 | 48 h | [22] |
| CuO | 0.06 mg·L−1 | 48 h | [24] | |
| Daphnia magna | CuO | 7.85 mg·L−1 | 24 h | [25] |
| CuO | 3.2 mg·L−1 | 48 h | [19] | |
| CuO | 5.9 mg·L−1 | 48 h | [26] | |
| CuO | 3.3 mg·L−1 | 48 h | [16] | |
| CuO | 2.56 mg·L−1 | 24 h | [27] | |
| CuO | 0.99 mg·L−1 | 48 h | [28] | |
| CuO | 5.66 mg·L−1 | 48 h | [29] | |
| CuO | 6.62 mg·L−1 | 48 h | [25] | |
| Thamnocephalus platyurus | CuO | 9.80 mg·L−1 | 24 h | [30] |
| Dependent Variable | Concentration of Substance | p | Incubation Time | p | Interaction | p |
|---|---|---|---|---|---|---|
| Cu NPs | ||||||
| MOT | F15,75 = 5.90 | p < 0.001 | ||||
| VCL | F5,25 = 0.89 | p > 0.05 | F3,15 = 5.82 | p < 0.01 | F15,75 = 0.59 | p > 0.05 |
| LIN | F15,75 = 2.21 | p < 0.05 | ||||
| ALH | F5,25 = 1.90 | p > 0.05 | F3,15 = 0.88 | p > 0.05 | F15,75 = 1.06 | p > 0.05 |
| BCF | F15,75 = 2.39 | p < 0.01 | ||||
| motility duration | F5,25 = 5.49 | p < 0.01 | F3,15 = 4.00 | p < 0.05 | F15,75 = 1.35 | p > 0.05 |
| CuO NPs | ||||||
| MOT | F30,150 = 2.27 | p < 0.001 | ||||
| VCL | F5,25 = 1.76 | p > 0.05 | F6,30 = 4.04 | p < 0.01 | F30,150 = 0.81 | p > 0.05 |
| LIN | F5,25 = 3.64 | p < 0.05 | F6,30 = 2.88 | p < 0.05 | F30,150 = 1.39 | p > 0.05 |
| ALH | F5,25 = 1.99 | p > 0.05 | F6,30 = 0.39 | p > 0.05 | F30,150 = 1.08 | p > 0.05 |
| BCF | F30,150 = 1.58 | p < 0.05 | ||||
| motility duration | F30,150 = 1.66 | p < 0.05 | ||||
| CuSO4 | ||||||
| MOT | F15,75 = 5.98 | p < 0.001 | ||||
| VCL | F5,25 = 0.81 | p > 0.05 | F3,15 = 5.62 | p < 0.01 | F15,75 = 0.14 | p > 0.05 |
| LIN | F15,75 = 1.96 | p < 0.05 | ||||
| ALH | F5,25 = 2.58 | p > 0.05 | F3,15 = 6.35 | p < 0.01 | F15,75 = 1.44 | p > 0.05 |
| BCF | F5,25 = 1.12 | p > 0.05 | F3,15 = 3.22 | p > 0.05 | F15,75 = 1.51 | p > 0.05 |
| motility duration | F5,25 = 4.47 | p < 0.01 | F3,15 = 3.35 | p < 0.05 | F15,75 = 1.51 | p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garncarek, M.; Dziewulska, K.; Kowalska-Góralska, M. The Effect of Copper and Copper Oxide Nanoparticles on Rainbow Trout (Oncorhynchus mykiss W.) Spermatozoa Motility after Incubation with Contaminants. Int. J. Environ. Res. Public Health 2022, 19, 8486. https://doi.org/10.3390/ijerph19148486
Garncarek M, Dziewulska K, Kowalska-Góralska M. The Effect of Copper and Copper Oxide Nanoparticles on Rainbow Trout (Oncorhynchus mykiss W.) Spermatozoa Motility after Incubation with Contaminants. International Journal of Environmental Research and Public Health. 2022; 19(14):8486. https://doi.org/10.3390/ijerph19148486
Chicago/Turabian StyleGarncarek, Małgorzata, Katarzyna Dziewulska, and Monika Kowalska-Góralska. 2022. "The Effect of Copper and Copper Oxide Nanoparticles on Rainbow Trout (Oncorhynchus mykiss W.) Spermatozoa Motility after Incubation with Contaminants" International Journal of Environmental Research and Public Health 19, no. 14: 8486. https://doi.org/10.3390/ijerph19148486
APA StyleGarncarek, M., Dziewulska, K., & Kowalska-Góralska, M. (2022). The Effect of Copper and Copper Oxide Nanoparticles on Rainbow Trout (Oncorhynchus mykiss W.) Spermatozoa Motility after Incubation with Contaminants. International Journal of Environmental Research and Public Health, 19(14), 8486. https://doi.org/10.3390/ijerph19148486






