Multi-Level System to Assess Toxicity in Water Distribution Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sampling and Sites
2.2. Determination of the Main Physicochemical Water Parameters
2.3. Metals Detection
2.4. Viral and Bacterial Pathogens in Water
2.5. Determination of Cyanotoxins
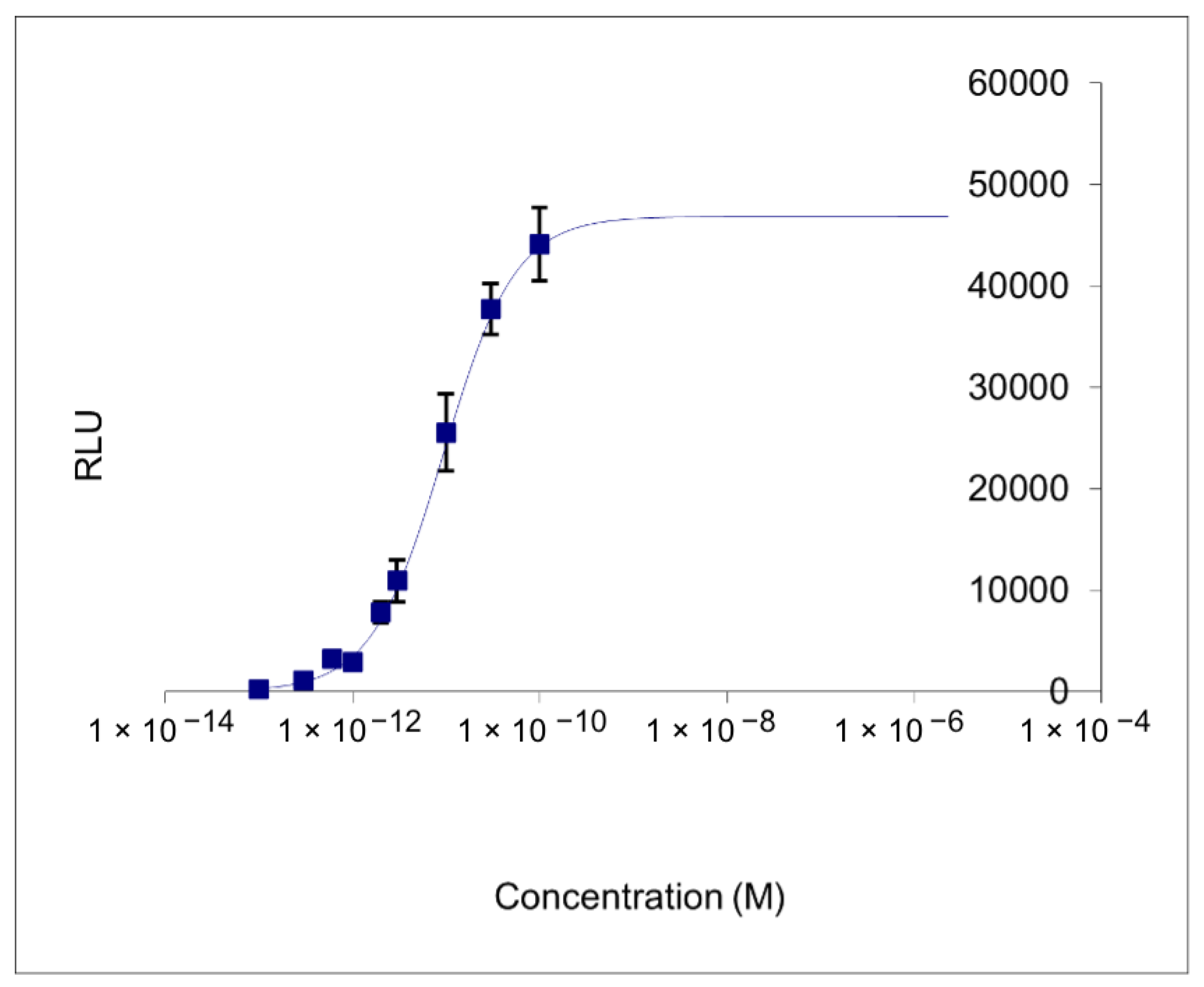
2.6. Determination of Endocrine Interference Activity with CALUX Method
2.7. Solid-Phase Extraction of Water Samples
2.8. Toxicity Assay on EPC Cell Line Monolayers
2.9. Statistical Analyses
3. Results
3.1. Water Physicochemical Parameters
3.2. Metals in Water
3.3. Viral and Bacterial Pathogens in Water Samples
3.4. Determination of Cyanotoxins in Water Samples
3.5. Determination of Endocrine Interference Activity
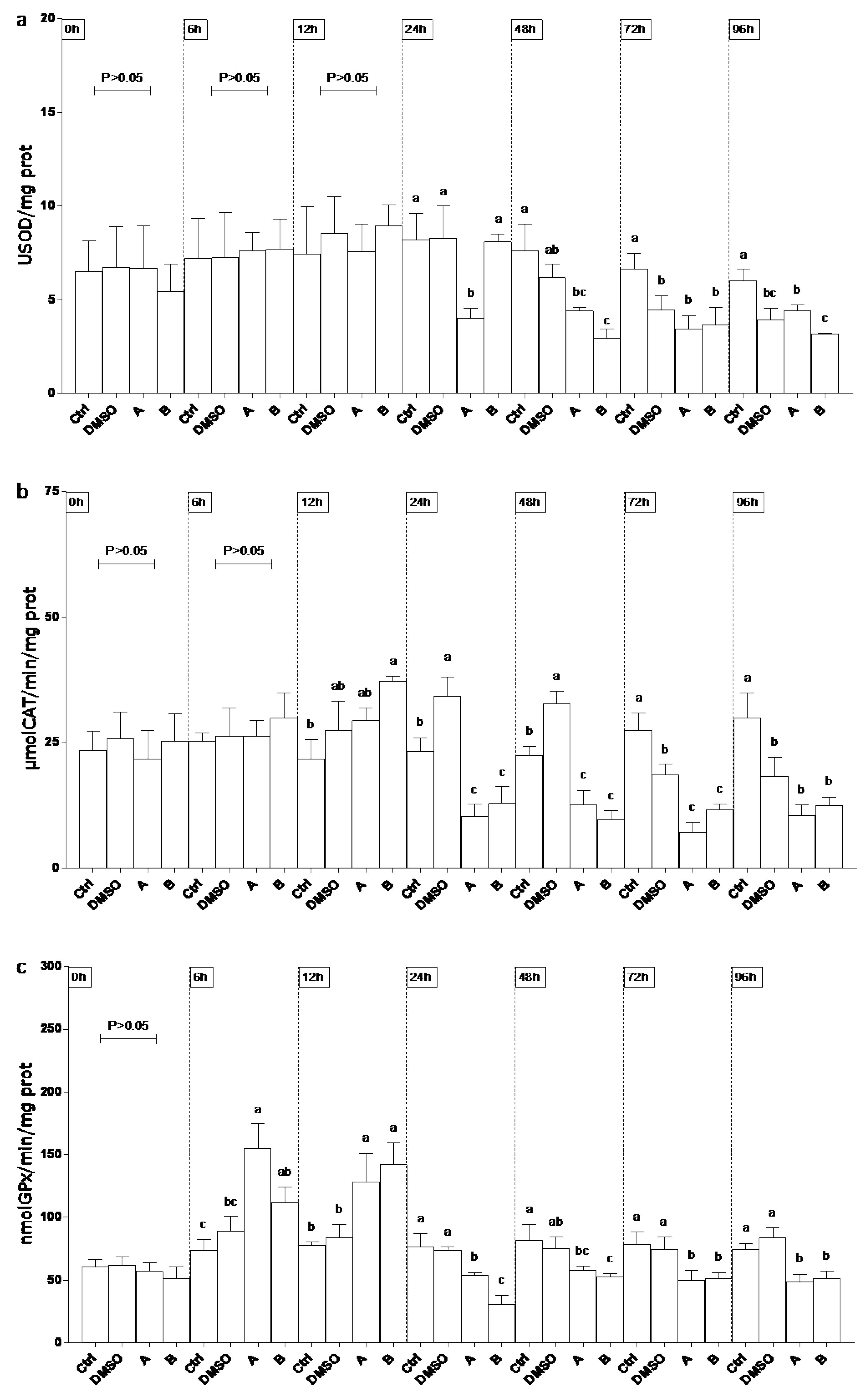
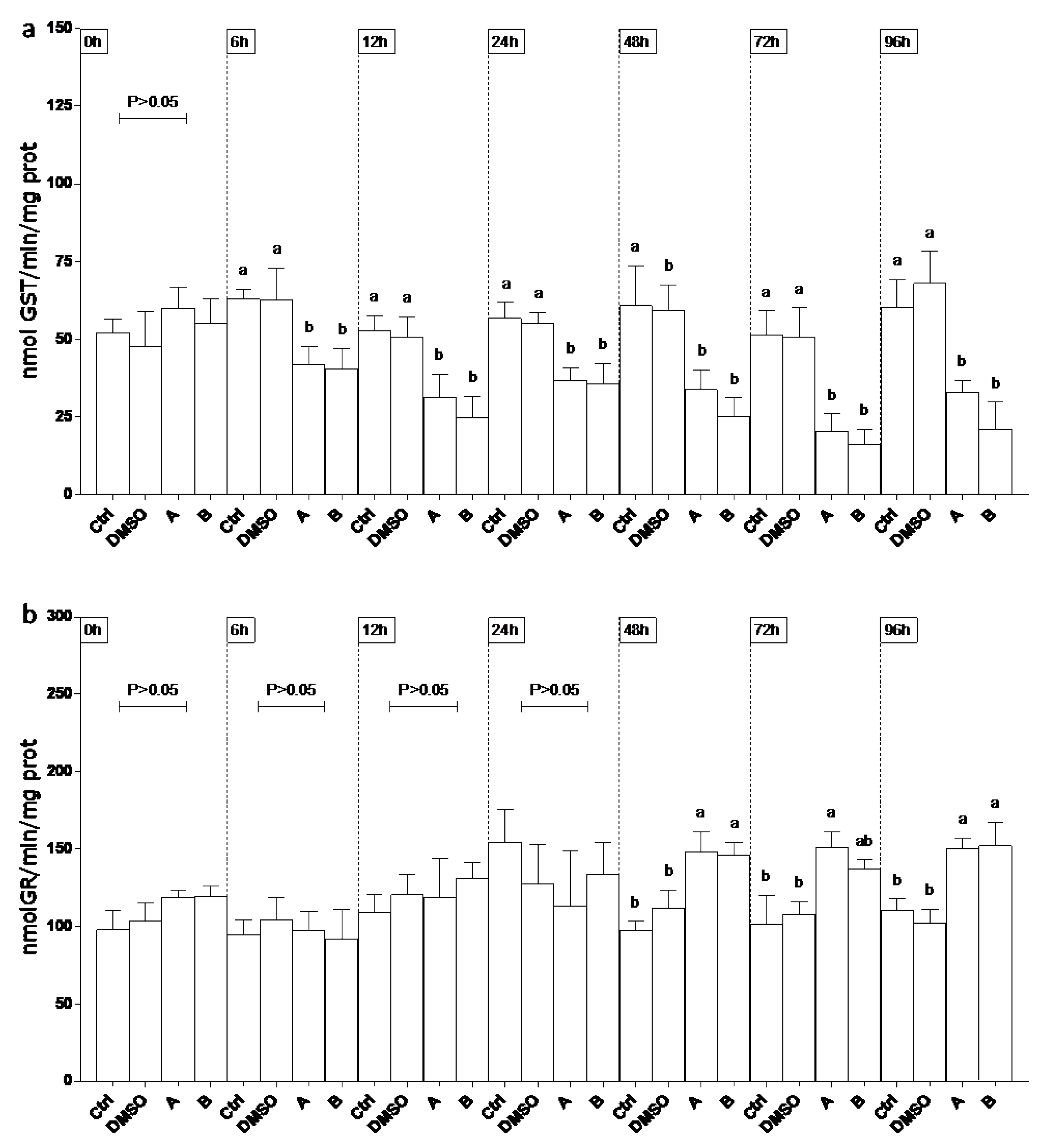
3.6. Oxidative Stress Responses in EPC Cell Line
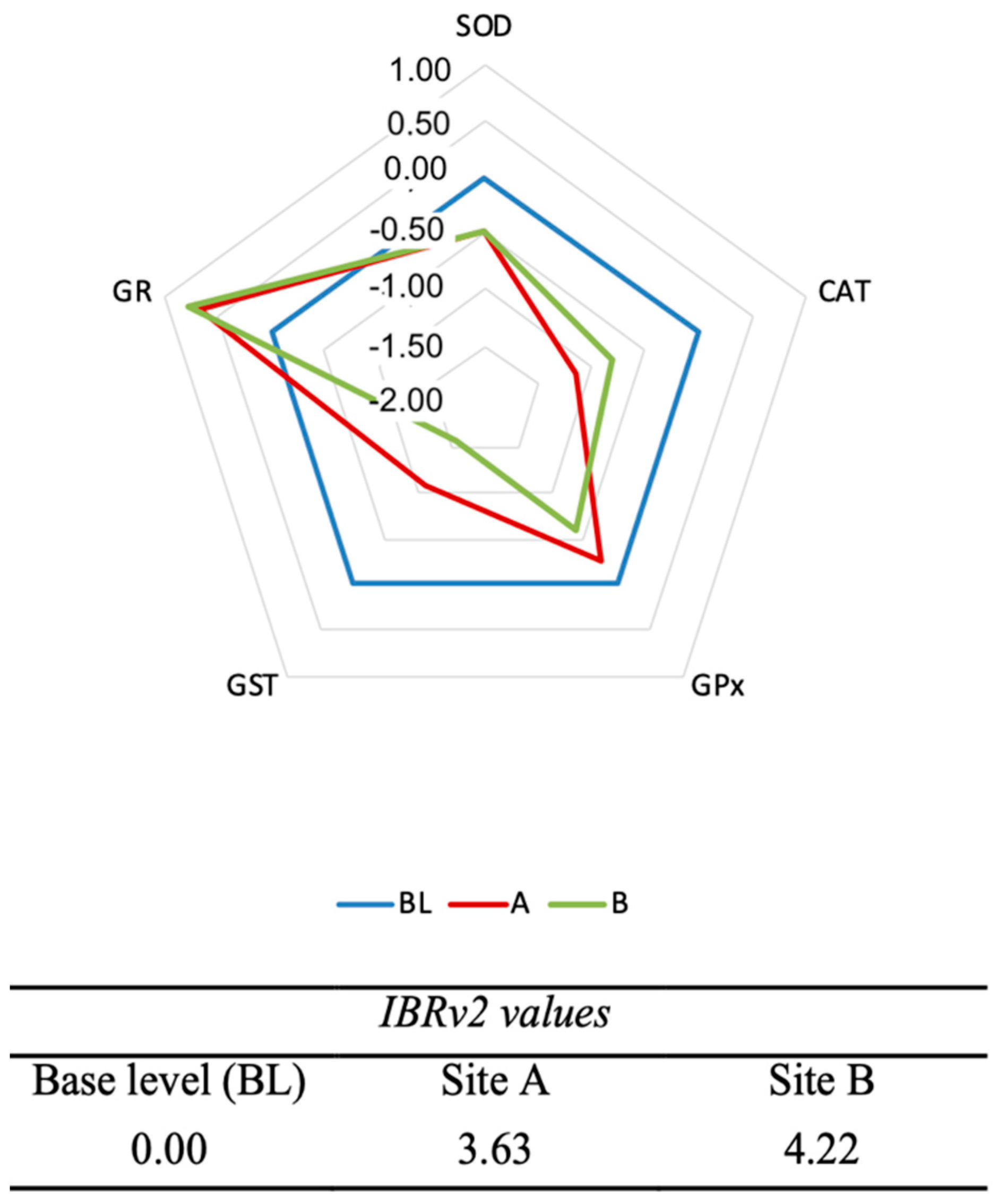
3.7. Integrated Biomarker Responses Version 2 (IBRv2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosgrove, W.J.; Loucks, D.P. Water Management: Current and Future Challenges and Research Directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef]
- Elia, A.C.; Fanetti, A.; Dörr, A.J.M.; Taticchi, M.I. Effects of Concentrated Drinking Water Injection on Glutathione and Glutathione-Dependent Enzymes in Liver of Cyprinus carpio L. Chemosphere 2008, 72, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, J.P.; Moore, D.R.; Teed, R.S. An Ecological Risk Assessment of Inorganic Chloramines in Surface Water. Hum. Ecol. Risk Assess. 2003, 9, 453–482. [Google Scholar] [CrossRef]
- Pandey, G.; Madhuri, S. Heavy Metals Causing Toxicity in Animals and Fishes. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Sharma, S.; Bhattacharya, A. Drinking Water Contamination and Treatment Techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Nozaic, D.J. Chlorine: Is It Really so Bad and What Are the Alternatives? Water SA 2004, 30, 18–24. [Google Scholar] [CrossRef][Green Version]
- Warton, B.; Heitz, A.; Joll, C.; Kagi, R. A New Method for Calculation of the Chlorine Demand of Natural and Treated Waters. Water Res. 2006, 40, 2877–2884. [Google Scholar] [CrossRef]
- Jeong, C.H.; Wagner, E.D.; Siebert, V.R.; Anduri, S.; Richardson, S.D.; Daiber, E.J.; McKague, A.B.; Kogevinas, M.; Villanueva, C.M.; Goslan, E.H. Occurrence and Toxicity of Disinfection Byproducts in European Drinking Waters in Relation with the HIWATE Epidemiology Study. Environ. Sci. Technol. 2012, 46, 12120–12128. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, Genotoxicity, and Carcinogenicity of Regulated and Emerging Disinfection by-Products in Drinking Water: A Review and Roadmap for Research. Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- An, D.; Chen, Y.; Gu, B.; Westerhoff, P.; Hanigan, D.; Herckes, P.; Fischer, N.; Donovan, S.; Croue, J.P.; Atkinson, A. Lower Molecular Weight Fractions of PolyDADMAC Coagulants Disproportionately Contribute to N-Nitrosodimethylamine Formation during Water Treatment. Water Res. 2019, 150, 466–472. [Google Scholar] [CrossRef]
- Christman, R.F.; Norwood, D.L.; Millington, D.S.; Johnson, J.D.; Stevens, A.A. Identity and Yields of Major Halogenated Products of Aquatic Fulvic Acid Chlorination. Environ. Sci. Technol. 1983, 17, 625–628. [Google Scholar] [CrossRef]
- Krasner, S.W.; McGuire, M.J.; Jacangelo, J.G.; Patania, N.L.; Reagan, K.M.; Aieta, E.M. The Occurrence of Disinfection By products in US Drinking Water. J. Am. Water Work. Assoc. 1989, 81, 41–53. [Google Scholar] [CrossRef]
- Norwood, D.L.; Johnson, J.D.; Christman, R.F.; Millington, D.S. Chlorination Products from Aquatic Humic Material at Neutral PH. Water Chlorination Environ. Impact Health Eff. 1983, 4, 191–199. [Google Scholar]
- Qian, Y.; Chen, Y.; Hu, Y.; Hanigan, D.; Westerhoff, P.; An, D. Formation and Control of C-and N-DBPs during Disinfection of Filter Backwash and Sedimentation Sludge Water in Drinking Water Treatment. Water Res. 2021, 194, 116964. [Google Scholar] [CrossRef]
- Rook, J.J. Formation of Haloforms during Chlorination of Natural Waters. J. Soc. Water Treat Exam. 1974, 23, 234–243. [Google Scholar]
- Elia, A.C.; Anastasi, V.; Dörr, A.J.M. Hepatic Antioxidant Enzymes and Total Glutathione of Cyprinus Carpio Exposed to Three Disinfectants, Chlorine Dioxide, Sodium Hypochlorite and Peracetic Acid, for Superficial Water Potabilization. Chemosphere 2006, 64, 1633–1641. [Google Scholar] [CrossRef]
- Luan, X.; Liu, X.; Fang, C.; Chu, W.; Xu, Z. Ecotoxicological Effects of Disinfected Wastewater Effluents: A Short Review of in Vivo Toxicity Bioassays on Aquatic Organisms. Environ. Sci. Water Res. Technol. 2020, 6, 2275–2286. [Google Scholar] [CrossRef]
- Boorman, G.A. Drinking Water Disinfection Byproducts: Review and Approach to Toxicity Evaluation. Environ. Health Perspect. 1999, 107, 207–217. [Google Scholar]
- Kar, S.; Senthilkumaran, B. Water Disinfection By-Products Cause Acute Toxicity in Teleosts: A Review. In Disinfection By-Products in Drinking Water; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–411. [Google Scholar]
- Ji, L.L. Exercise and Oxidative Stress: Role of the Cellular Antioxidant Systems. Exerc. Sport Sci. Rev. 1995, 23, 135–166. [Google Scholar] [CrossRef]
- Elia, A.C.; Magara, G.; Caruso, C.; Masoero, L.; Prearo, M.; Arsieni, P.; Caldaroni, B.; Dörr, A.J.M.; Scoparo, M.; Salvati, S. A Comparative Study on Subacute Toxicity of Arsenic Trioxide and Dimethylarsinic Acid on Antioxidant Status in Crandell Rees Feline Kidney (CRFK), Human Hepatocellular Carcinoma (PLC/PRF/5), and Epithelioma Papulosum Cyprini (EPC) Cell Lines. J. Toxicol. Environ. Health Part A 2018, 81, 333–348. [Google Scholar] [CrossRef]
- Elia, A.C.; Magara, G.; Righetti, M.; Dörr, A.J.M.; Scanzio, T.; Pacini, N.; Abete, M.C.; Prearo, M. Oxidative Stress and Related Biomarkers in Cupric and Cuprous Chloride-Treated Rainbow Trout. Environ. Sci. Pollut. Res. 2017, 24, 10205–10219. [Google Scholar] [CrossRef]
- Elia, A.C.; Prearo, M.; Dörr, A.J.M.; Pacini, N.; Magara, G.; Brizio, P.; Gasco, L.; Abete, M.C. Effects of Astaxanthin and Canthaxanthin on Oxidative Stress Biomarkers in Rainbow Trout. J. Toxicol. Environ. Health Part A 2019, 82, 760–768. [Google Scholar] [CrossRef]
- Magara, G.; Elia, A.C.; Dörr, A.J.M.; Abete, M.C.; Brizio, P.; Caldaroni, B.; Righetti, M.; Pastorino, P.; Scoparo, M.; Prearo, M. Metal Load and Oxidative Stress Driven by Organotin Compounds on Rainbow Trout. Environ. Sci. Pollut. Res. 2021, 28, 35012–35022. [Google Scholar] [CrossRef]
- Cravo, A.; Pereira, C.; Gomes, T.; Cardoso, C.; Serafim, A.; Almeida, C.; Rocha, T.; Lopes, B.; Company, R.; Medeiros, A. A Multibiomarker Approach in the Clam Ruditapes Decussatus to Assess the Impact of Pollution in the Ria Formosa Lagoon, South Coast of Portugal. Mar. Environ. Res. 2012, 75, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, W.; Burgeot, T.; Porcher, J.-M. A Novel “Integrated Biomarker Response” Calculation Based on Reference Deviation Concept. Environ. Sci. Pollut. Res. 2013, 20, 2721–2725. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, M.; Chiesara, E.; Radice, S.; Giovara, A.; Frigerio, S.; Fumagalli, R.; Marabini, L. Study of Potential Toxic Effects on Rainbow Trout Hepatocytes of Surface Water Treated with Chlorine or Alternative Disinfectants. Chemosphere 2005, 60, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Gustavino, B.; Monfrinotti, M.; Canistro, D.; Broccoli, M.; Pozzetti, L.; Affatato, A.; Valgimigli, L.; Forti, G.C.; Pedulli, G.F. Perturbation of Cytochrome P450, Generation of Oxidative Stress and Induction of DNA Damage in Cyprinus Carpio Exposed in Situ to Potable Surface Water. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2007, 626, 143–154. [Google Scholar] [CrossRef]
- Ueno, H.; Sayato, Y.; Nakamuro, K. Hematological Effects of Chlorine Dioxide on in Vitro Exposure in Mouse, Rat and Human Blood and on Subchronic Exposure in Mice. J. Health Sci. 2000, 46, 110–116. [Google Scholar] [CrossRef]
- Velma, V.; Vutukuru, S.S.; Tchounwou, P.B. Ecotoxicology of Hexavalent Chromium in Freshwater Fish: A Critical Review. Rev. Environ. Health 2009, 24, 129–146. [Google Scholar] [CrossRef]
- Winton, J.; Batts, W.; DeKinkelin, P.; LeBerre, M.; Bremont, M.; Fijan, N. Current Lineages of the Epithelioma Papulosum Cyprini (EPC) Cell Line Are Contaminated with Fathead Minnow, Pimephales Promelas, Cells. J. Fish Dis. 2010, 33, 701–704. [Google Scholar] [CrossRef]
- ASTM. Manual of Water and Environmental Technology, D1426-92 Standard: Test Methods for Ammonia Nitrogen in Water; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Costafreda, M.I.; Bosch, A.; Pintó, R.M. Development, Evaluation, and Standardization of a Real-Time TaqMan Reverse Transcription-PCR Assay for Quantification of Hepatitis A Virus in Clinical and Shellfish Samples. Appl. Environ. Microbiol. 2006, 72, 3846–3855. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A Broadly Reactive One-Step Real-Time RT-PCR Assay for Rapid and Sensitive Detection of Hepatitis E Virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef]
- da Silva, A.K.; Le Saux, J.-C.; Parnaudeau, S.; Pommepuy, M.; Elimelech, M.; Le Guyader, F.S. Evaluation of Removal of Noroviruses during Wastewater Treatment, Using Real-Time Reverse Transcription-PCR: Different Behaviors of Genogroups I and II. Appl. Environ. Microbiol. 2007, 73, 7891–7897. [Google Scholar] [CrossRef]
- Guzzella, L.; Di Caterino, F.; Monarca, S.; Zani, C.; Feretti, D.; Zerbini, I.; Nardi, G.; Buschini, A.; Poli, P.; Rossi, C. Detection of Mutagens in Water-Distribution Systems after Disinfection. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2006, 608, 72–81. [Google Scholar] [CrossRef]
- Monarca, S.; Zanardini, A.; Feretti, D.; Dalmiglio, A.; Falistocco, E.; Manica, P.; Nardi, G. Mutagenicity of Extracts of Lake Drinking Water Treated with Different Disinfectants in Bacterial and Plant Tests. Water Res. 1998, 32, 2689–2695. [Google Scholar] [CrossRef]
- Elia, A.C.; Burioli, E.; Magara, G.; Pastorino, P.; Caldaroni, B.; Menconi, V.; Dörr, A.J.M.; Colombero, G.; Abete, M.C.; Prearo, M. Oxidative Stress Ecology on Pacific Oyster Crassostrea Gigas from Lagoon and Offshore Italian Sites. Sci. Total Environ. 2020, 739, 139886. [Google Scholar] [CrossRef]
- Magara, G.; Sangsawang, A.; Pastorino, P.; Oddon, S.B.; Caldaroni, B.; Menconi, V.; Kovitvadhi, U.; Gasco, L.; Meloni, D.; Dörr, A.J.M. First Insights into Oxidative Stress and Theoretical Environmental Risk of Bronopol and Detarox® AP, Two Biocides Claimed to Be Ecofriendly for a Sustainable Aquaculture. Sci. Total Environ. 2021, 778, 146375. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Mustapha, M.K. Assessment of the Water Quality of Oyun Reservoir, Offa, Nigeria, Using Selected Physico-Chemical Parameters. Turk. J. Fish. Aquat. Sci. 2008, 8, 309–319. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Ali, Z.; Malik, R.N.; Qadir, A. Heavy Metals Distribution and Risk Assessment in Soils Affected by Tannery Effluents. Chem. Ecol. 2013, 29, 676–692. [Google Scholar] [CrossRef]
- Segura, R.; Arancibia, V.; Zúñiga, M.C.; Pastén, P. Distribution of Copper, Zinc, Lead and Cadmium Concentrations in Stream Sediments from the Mapocho River in Santiago, Chile. J. Geochem. Explor. 2006, 91, 71–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, F.; Meng, W.; Wang, X.-Q. Water Quality Assessment and Source Identification of Daliao River Basin Using Multivariate Statistical Methods. Environ. Monit. Assess. 2009, 152, 105–121. [Google Scholar] [CrossRef]
- Macler, B.A.; Merkle, J.C. Current Knowledge on Groundwater Microbial Pathogens and Their Control. Hydrogeol. J. 2000, 8, 29–40. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Al Shehri, A.M. Microcystins in Groundwater Wells and Their Accumulation in Vegetable Plants Irrigated with Contaminated Waters in Saudi Arabia. J. Hazard. Mater. 2009, 172, 310–315. [Google Scholar] [CrossRef]
- Yang, Z.; Kong, F.; Zhang, M. Groundwater Contamination by Microcystin from Toxic Cyanobacteria Blooms in Lake Chaohu, China. Environ. Monit. Assess. 2016, 188, 280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Husk, B.R.; Duy, S.V.; Dinh, Q.T.; Sanchez, J.S.; Sauvé, S.; Whalen, J.K. Quantitative Screening for Cyanotoxins in Soil and Groundwater of Agricultural Watersheds in Quebec, Canada. Chemosphere 2021, 274, 129781. [Google Scholar] [CrossRef]
- Escher, B.I.; Baumgartner, R.; Koller, M.; Treyer, K.; Lienert, J.; McArdell, C.S. Environmental Toxicology and Risk Assessment of Pharmaceuticals from Hospital Wastewater. Water Res. 2011, 45, 75–92. [Google Scholar] [CrossRef]
- Kidd, K.A.; Paterson, M.J.; Rennie, M.D.; Podemski, C.L.; Findlay, D.L.; Blanchfield, P.J.; Liber, K. Direct and Indirect Responses of a Freshwater Food Web to a Potent Synthetic Oestrogen. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130578. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Simon, E.; Creusot, N.; Jayasinghe, B.S.; Kienle, C.; Maletz, S.; Schifferli, A.; Schönlau, C.; Aït-Aïssa, S.; Denslow, N.D. Effect-Based Tools for Monitoring Estrogenic Mixtures: Evaluation of Five in Vitro Bioassays. Water Res. 2017, 110, 378–388. [Google Scholar] [CrossRef]
- Blasco, J.; Corsi, I. Ecotoxicology of Nanoparticles in Aquatic Systems; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-1-351-65755-6. [Google Scholar]
- Wang, C.; Yang, X.; Zheng, Q.; Moe, B.; Li, X.-F. Halobenzoquinone-Induced Developmental Toxicity, Oxidative Stress, and Apoptosis in Zebrafish Embryos. Environ. Sci. Technol. 2018, 52, 10590–10598. [Google Scholar] [CrossRef]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Nencioni, L.; Garaci, E.; Palamara, A.T.; Magnani, M. GSH and Analogs in Antiviral Therapy. Mol. Asp. Med. 2009, 30, 99–110. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.; Zhou, L.-H.; Zou, Y.-L.; Lu, W.-Q. Oxidative Stress and DNA Damage Induced by a Drinking-Water Chlorination Disinfection Byproduct 3-Chloro-4-(Dichloromethyl)-5-Hydroxy-2(5H)-Furanone (MX) in Mice. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2006, 609, 129–136. [Google Scholar] [CrossRef]
- TONG, Z.; BOARD, P.G.; Anders, M.W. Glutathione Transferase Zeta Catalyses the Oxygenation of the Carcinogen Dichloroacetic Acid to Glyoxylic Acid. Biochem. J. 1998, 331, 371–374. [Google Scholar] [CrossRef]
| Parameter | Site A | Site B |
|---|---|---|
| pH (unit) | 8.32 ± 0.12 | 8.02 ± 0.09 |
| Conductivity (μS cm−1) | 132 ± 3.14 | 226 ± 2.34 |
| NH4+ (mg L−1) | 0.04 ± 0.001 | 0.03 ± 0.002 |
| NO3− (mg L−1) | 8.45 ± 1.24 | 9.23 ± 2.15 |
| PO43− (mg L−1) | 0.01 ± 0.01 | 0.02 ± 0.01 |
| Extraction Information | Bioassay Calculation | Summary Results | |||||
|---|---|---|---|---|---|---|---|
| Sample | Extracted | Unit | DMSO (uL) | Dilution | M | SD (%) | 17ß-Estradiol Eq. |
| A | 250 | mL | 40.00 | 1 | <Min | 1.5 | <Min |
| A | 250 | mL | 40.00 | 3 | <Min | 1.2 | <Min |
| A | 250 | mL | 40.00 | 10 | <Min | 1.5 | <Min |
| B | 250 | mL | 40.00 | 1 | <Min | 2.7 | <Min |
| B | 250 | mL | 40.00 | 3 | <Min | 2.3 | <Min |
| B | 250 | mL | 40.00 | 10 | <Min | 0.0 | <Min |
| DMSO | 2.00 | mL | 40.00 | 1 | <Min | 0.5 | <Min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magara, G.; Varello, K.; Pastorino, P.; Francese, D.R.; Arsieni, P.; Pezzolato, M.; Masoero, L.; Messana, E.; Caldaroni, B.; Abete, M.C.; et al. Multi-Level System to Assess Toxicity in Water Distribution Plants. Int. J. Environ. Res. Public Health 2022, 19, 8469. https://doi.org/10.3390/ijerph19148469
Magara G, Varello K, Pastorino P, Francese DR, Arsieni P, Pezzolato M, Masoero L, Messana E, Caldaroni B, Abete MC, et al. Multi-Level System to Assess Toxicity in Water Distribution Plants. International Journal of Environmental Research and Public Health. 2022; 19(14):8469. https://doi.org/10.3390/ijerph19148469
Chicago/Turabian StyleMagara, Gabriele, Katia Varello, Paolo Pastorino, Danila Raffaella Francese, Paola Arsieni, Marzia Pezzolato, Loretta Masoero, Erika Messana, Barbara Caldaroni, Maria Cesarina Abete, and et al. 2022. "Multi-Level System to Assess Toxicity in Water Distribution Plants" International Journal of Environmental Research and Public Health 19, no. 14: 8469. https://doi.org/10.3390/ijerph19148469
APA StyleMagara, G., Varello, K., Pastorino, P., Francese, D. R., Arsieni, P., Pezzolato, M., Masoero, L., Messana, E., Caldaroni, B., Abete, M. C., Pederiva, S., Squadrone, S., Elia, A. C., Prearo, M., & Bozzetta, E. (2022). Multi-Level System to Assess Toxicity in Water Distribution Plants. International Journal of Environmental Research and Public Health, 19(14), 8469. https://doi.org/10.3390/ijerph19148469









