Study on the Enhanced Remediation of Petroleum-Contaminated Soil by Biochar/g-C3N4 Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Materials
2.3. Characterization Analysis of BC and BC/g-C3N4
2.4. Adsorption and Photodegradation Experiments
2.5. Free Radical Trapping Experiments
2.6. Analysis of n-Alkanes and PAHs in Soil
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization Analysis
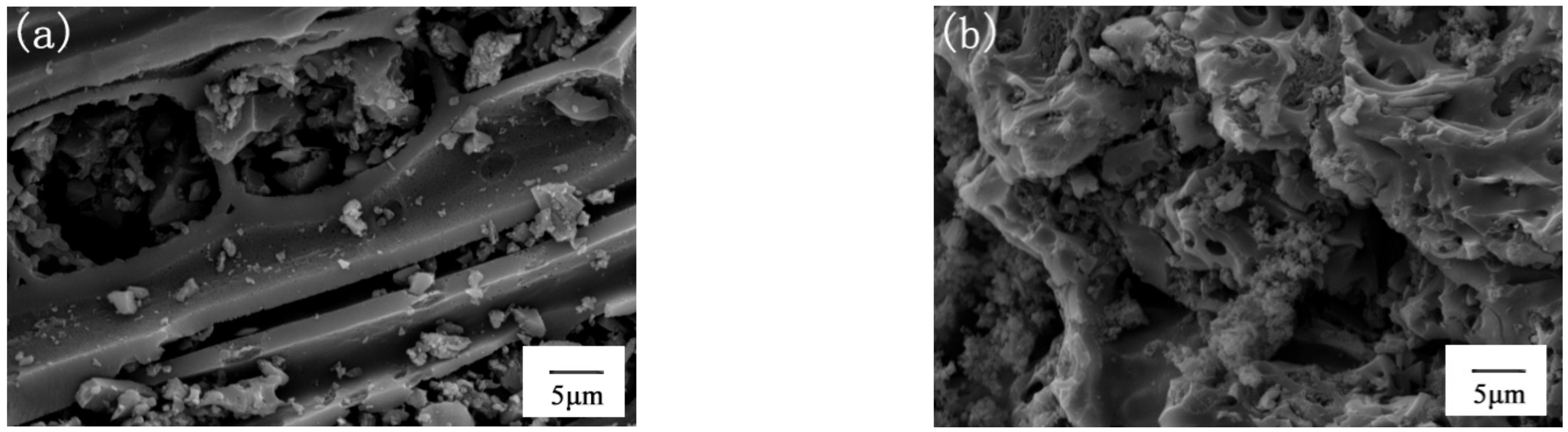
3.1.1. SEM Analysis
3.1.2. BET Analysis
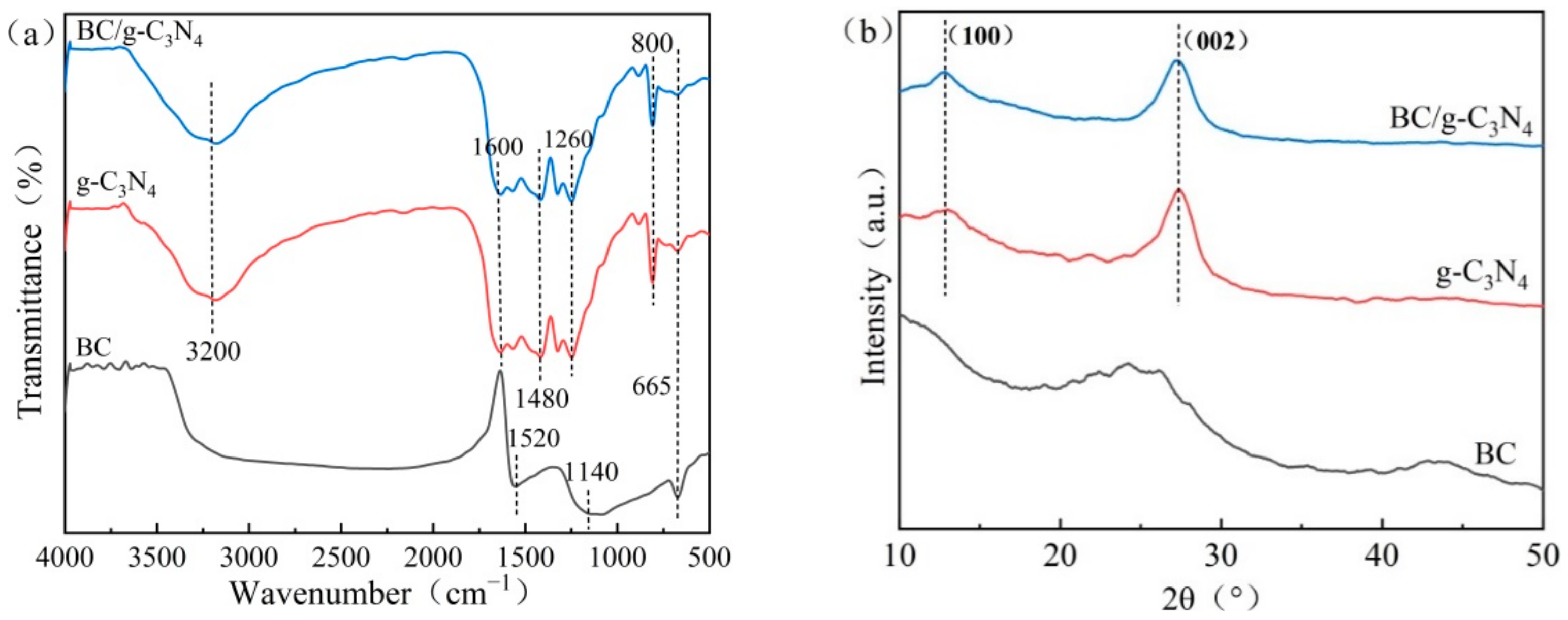
3.1.3. FTIR and XRD Analysis
3.2. Analysis of Adsorption and Photocatalytic Capacity
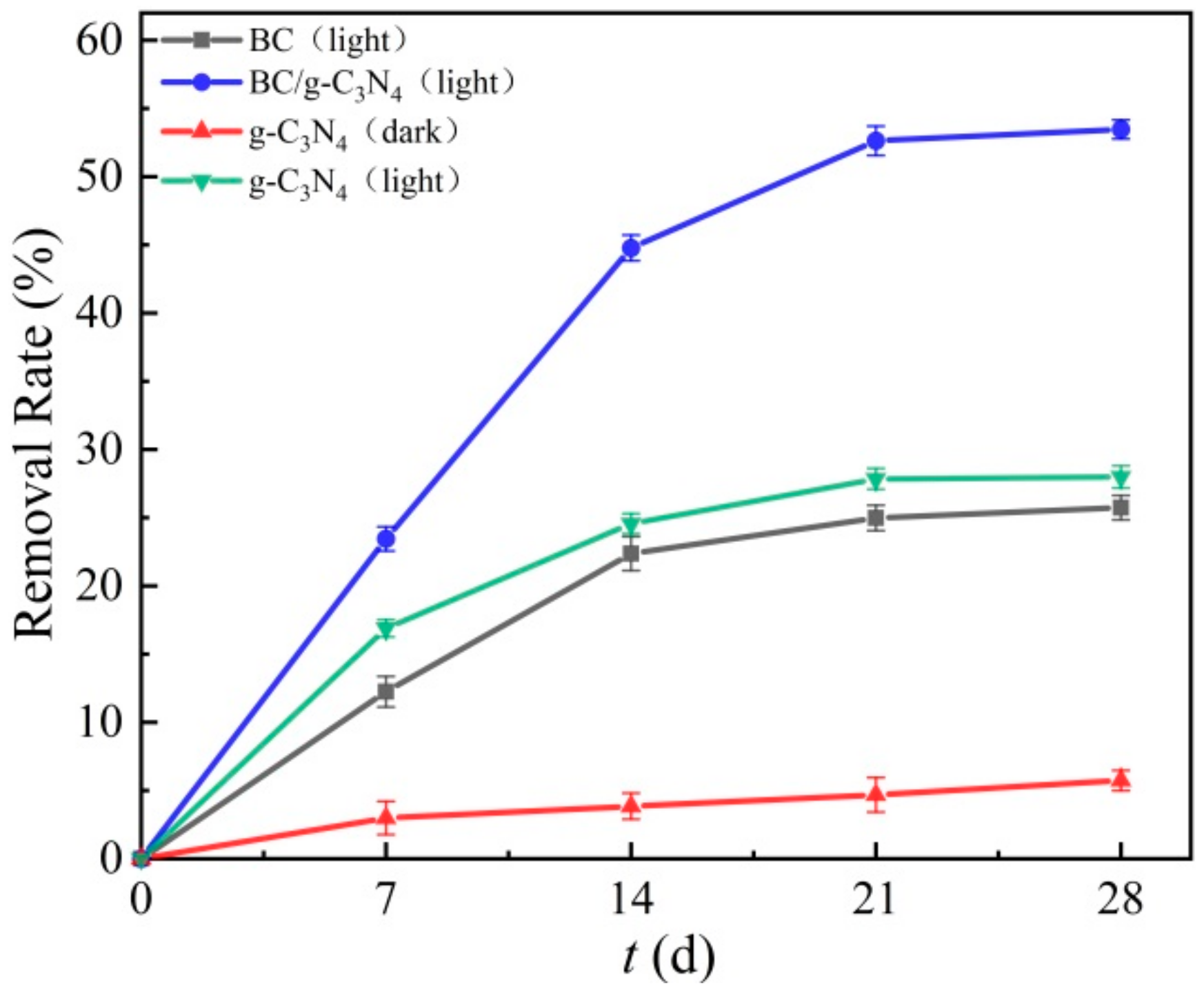
3.3. Removal of TPH in Soil by BC/g-C3N4
3.3.1. Effect of the BC to g-C3N4 Doping Ratio on TPH Removal Efficiency
3.3.2. Effect of Soil Acidity and Alkalinity on TPH Removal Efficiency
3.3.3. Effect of Moisture Content on TPH Removal Efficiency
3.3.4. Effect of BC/g-C3N4 Dosage on TPH Removal Efficiency
3.4. Kinetic Analysis
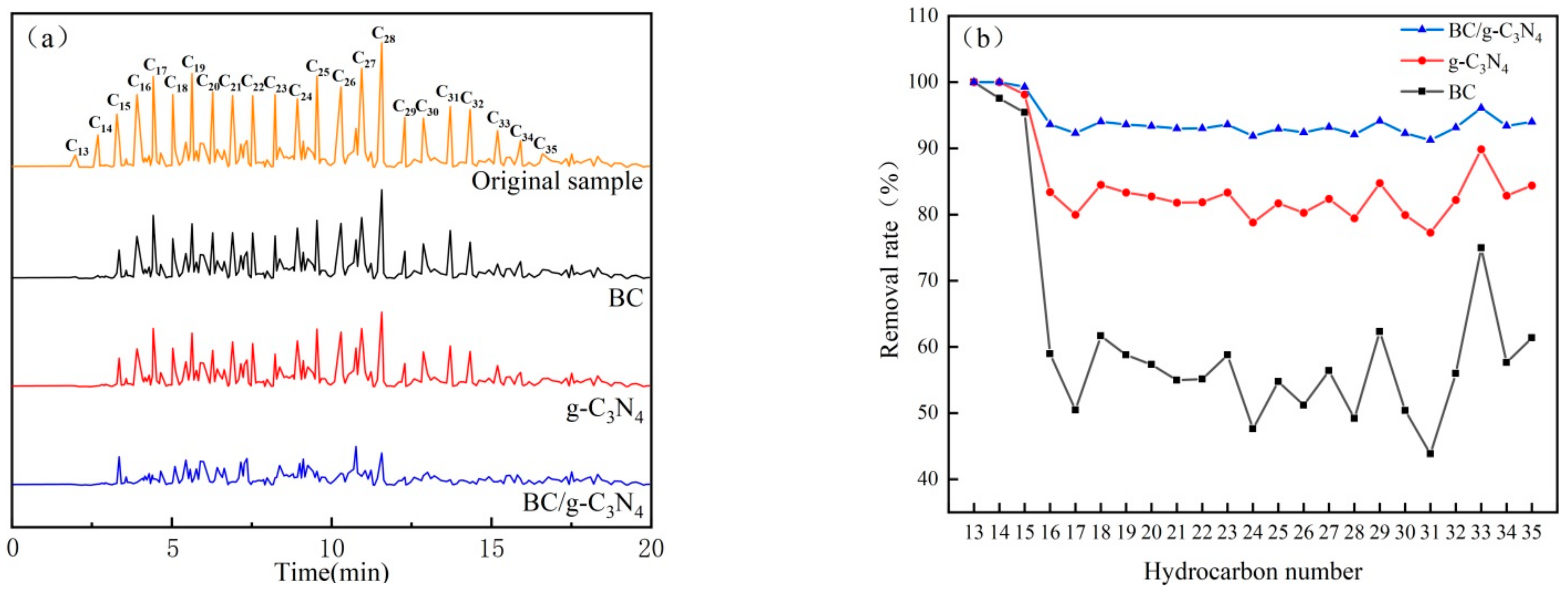
3.5. Gas Chromatography Analysis
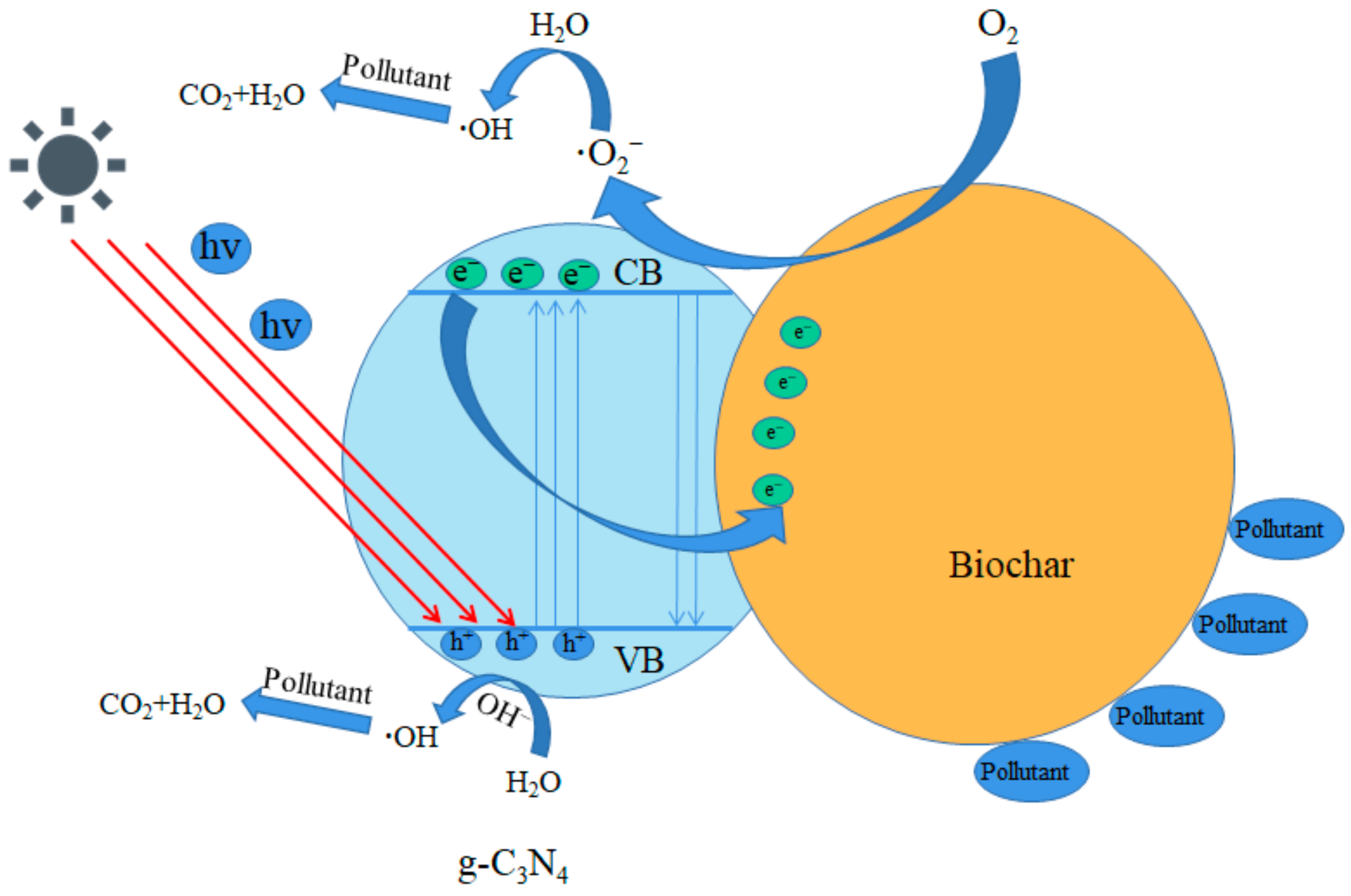
3.6. Photocatalysis Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Varjani, S.J.; Upasani, V.N. Core Flood study for enhanced oil recovery through ex-situ bioaugmentation with thermo- and halo-tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Bioresour. Technol. 2016, 220, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Seo, Y.; Ha, M.; Lee, J.; Yang, H.; Cho, K.S. Evaluation of rhizoremediation and methane emission in diesel-contaminated soil cultivated with tall fescue (Festuca arundinacea). Environ. Res. 2021, 194, 110606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Z.-H.; Wang, H.; Wang, X.-M.; Zhang, X.-H.; Xie, Y.F. Synergistic effects of combining ozonation, ceramic membrane filtration and biologically active carbon filtration for wastewater reclamation. J. Hazard. Mater. 2020, 382, 121091. [Google Scholar] [CrossRef] [PubMed]
- Bulai, I.S.; Adamu, H.; Umar, Y.A.; Sabo, A. Biocatalytic remediation of used motor oil-contaminated soil by fruit garbage enzymes. J. Environ. Chem. Eng. 2021, 9, 105465. [Google Scholar] [CrossRef]
- Guo, H.; Yao, J.; Cai, M.; Qian, Y.; Guo, Y.; Richnow, H.H.; Blake, R.E.; Doni, S.; Ceccanti, B. Effects of petroleum contamination on soil microbial numbers, metabolic activity and urease activity. Chemosphere 2012, 87, 1273–1280. [Google Scholar] [CrossRef]
- Kwon, M.J.; O’Loughlin, E.J.; Ham, B.; Hwang, Y.; Shim, M.; Lee, S. Application of an in-situ soil sampler for assessing subsurface biogeochemical dynamics in a diesel-contaminated coastal site during soil flushing operations. J. Environ. Manag. 2018, 206, 938–948. [Google Scholar] [CrossRef]
- Pinedo, J.; Ibañez, R.; Lijzen, J.P.; Irabien, A. Assessment of soil pollution based on total petroleum hydrocarbons and individual oil substances. J. Environ. Manag. 2013, 130, 72–79. [Google Scholar] [CrossRef]
- Zahed, M.A.; Salehi, S.; Madadi, R.; Hejabi, F. Biochar as a sustainable product for remediation of petroleum contaminated soil. Curr. Res. Green Sustain. Chem. 2021, 4, 100055. [Google Scholar] [CrossRef]
- Samolada, M.C.; Zabaniotou, A.A. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014, 34, 411–420. [Google Scholar] [CrossRef]
- Desalegn, B.; Megharaj, M.; Chen, Z.L.; Naidu, R. Green mango peel-nanozerovalent iron activated persulfate oxidation of petroleum hydrocarbons in oil sludge contaminated soil. Environ. Technol. Innov. 2018, 11, 142–152. [Google Scholar] [CrossRef]
- Chen, S.L.; Yi, Z.Y.; Wang, J.; Pan, C.Y.; Chang, S.; Guo, Q.W.; Zhou, J.G.; Sun, L. Case study on remediation of diesel contaminated soil and groundwater by eluent-extraction technology. Environ. Eng. 2020, 38, 178–182. [Google Scholar] [CrossRef]
- Steliga, T.; Kluk, D. Application of Festuca arundinacea in phytoremediation of soils contaminated with Pb, Ni, Cd and petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2020, 194, 110409. [Google Scholar] [CrossRef]
- Chen, C.-H.; Liu, P.G.; Whang, L.-M. Effects of natural organic matters on bioavailability of petroleum hydrocarbons in soil-water environments. Chemosphere 2019, 233, 843–851. [Google Scholar] [CrossRef]
- Dickson, U.J.; Coffey, M.; Mortimer, R.J.G.; Smith, B.; Ray, N.; Di Bonito, M. Investigating the potential of sunflower species, fermented palm wine and Pleurotus ostreatus for treatment of petroleum-contaminated soil. Chemosphere 2020, 240, 124881. [Google Scholar] [CrossRef]
- Jasmine, J.; Mukherji, S. Impact of bioremediation strategies on slurry phase treatment of aged oily sludge from a refinery. J. Environ. Manag. 2019, 246, 625–635. [Google Scholar] [CrossRef]
- Yuan, M.J.; Tong, S.T.; Zhao, S.Q.; Jia, C.Q. Adsorption of polycyclic aromatic hydrocarbons from water using petroleum coke-derived porous carbon. J. Hazard. Mater. 2010, 181, 1115–1120. [Google Scholar] [CrossRef]
- Esmaeili, A.; Saremnia, B. Comparison study of adsorption and nanofiltration methods for removal of total petroleum hydrocarbons from oil-field wastewater. J. Pet. Sci. Eng. 2018, 171, 403–413. [Google Scholar] [CrossRef]
- Sajjad, A.; Jabeen, F.; Farid, M.; Fatima, Q.; Akbar, A.; Ali, Q.; Hussain, I.; Iftikhar, U.; Farid, S.; Ishaq, H.K. Biochar: A Sustainable Product for Remediation of Contaminated Soils. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 787–799. [Google Scholar] [CrossRef]
- Aziz, S.; Ali, M.I.; Farooq, U.; Jamal, A.; Liu, F.-J.; He, H.; Guo, H.; Urynowicz, M.; Huang, Z. Enhanced bioremediation of diesel range hydrocarbons in soil using biochar made from organic wastes. Environ. Monit. Assess. 2020, 192, 569. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Choi, Y.-K.; Kim, H.J.; Song, H.-S.; Park, S.L.; Lee, H.S.; Lee, S.M.; Choi, K.-Y.; et al. Adsorptive removal of crude petroleum oil from water using floating pinewood biochar decorated with coconut oil-derived fatty acids. Sci. Total Environ. 2021, 781, 146636. [Google Scholar] [CrossRef]
- Sun, Y.W.; Zeng, B.Y.; Dai, Y.T.; Liang, X.J.; Zhang, L.J.; Ahmad, R.; Su, X.T. Modification of sludge-based biochar using air roasting-oxidation and its performance in adsorption of uraniu m(VI) from aqueous solutions. J. Colloid Interface Sci. 2022, 614, 547–555. [Google Scholar] [CrossRef]
- Leichtweis, J.; Silvestri, S.; Carissimi, E. New composite of pecan nutshells biochar-ZnO for sequential removal of acid red 97 by adsorption and photocatalysis. Biomass-Bioenergy 2020, 140, 105648. [Google Scholar] [CrossRef]
- Wang, H.X.; Teng, H.W.; Wang, X.Y.; Xu, J.L.; Sheng, L.X. Physicochemical modification of corn straw biochar to improve performance and its application of constructed wetland substrate to treat city tail water. J. Environ. Manag. 2022, 310, 114758. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Binh, Q.A.; Nguyen, X.C.; Nguyen, T.T.H.; Vo, Q.N.; Nguyen, T.D.; Tran, T.C.P.; Kim, S.Y.; Nguyen, T.P.; Bae, J.; et al. Metal salt-modified biochars derived from agro-waste for effective congo red dye removal. Environ. Res. 2021, 200, 111492. [Google Scholar] [CrossRef]
- Liu, Y.N.; Li, F.M.; Deng, J.Q.; Wu, Z.Q.; Lei, T.Z.; Tan, M.J.; Wu, Z.J.; Qin, X.L.; Li, H. Mechanism of sulfamic acid modified biochar for highly efficient removal of tetracycline. J. Anal. Appl. Pyrolysis 2021, 158, 105247. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Z.J.; Song, Y.Y.; Zheng, X.R.; Qu, L.L.; Qian, J.C.; Wu, Y.W.; Wu, X.Y.; Wu, Z.R. Remediation of phenanthrene contaminated soil by g-C3N4/Fe3O4 composites and its phytotoxicity evaluation. Chemosphere 2019, 221, 554–562. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, T.; Wang, R.T.; Feng, J.R.; Chen, D.M.; Yao, W.Q. A separation-free polyacrylamide/bentonite/graphitic carbon nitride hydrogel with excellent performance in water treatment. J. Clean. Prod. 2018, 197, 1222–1230. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Barathi, D.; Meyvel, S.; Sathya, P. S-scheme Ag2CrO4/g-C3N4 photocatalyst for effective degradation of organic pollutants under visible light. Inorg. Chem. Commun. 2021, 132, 108849. [Google Scholar] [CrossRef]
- Sharma, A.; Kanth, S.K.; Xu, S.S.; Han, N.; Zhu, L.; Fan, L.L.; Liu, C.; Zhang, Q.F. Visible light driven g-C3N4/Bi4NbO8X (X=Cl, Br) heterojunction photocatalyst for the degradation of organic pollutants. J. Alloys Compd. 2022, 895, 162576. [Google Scholar] [CrossRef]
- Fan, G.; Ma, Z.Y.; Li, X.B.; Deng, L.J. Coupling of Bi2O3 nanoparticles with g-C3N4 for enhanced photocatalytic degradation of methylene blue. Ceram. Int. 2021, 47, 5758–5766. [Google Scholar] [CrossRef]
- Luo, Z.J.; Song, Y.Y.; Wang, M.J.; Zheng, X.R.; Qu, L.L.; Wang, J.; Wu, X.Y.; Wu, Z.R. Comparison of g-C3N4 synthesized by different precursors in remediation of phenanthrene contaminated soil and ecotoxicity. J. Photochem. Photobiol. A Chem. 2020, 389, 112241. [Google Scholar] [CrossRef]
- Luo, S.Y.; Li, S.P.; Zhang, S.; Cheng, Z.Y.; Nguyen, T.T.; Guo, M.H. Visible-light-driven Z-scheme protonated g-C3N4/wood flour biochar/BiVO4 photocatalyst with biochar as charge-transfer channel for enhanced RhB degradation and Cr(VI) reduction. Sci. Total Environ. 2022, 806, 150662. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.D.; Gong, D.X.; Deng, Y.C.; Xiong, S.; Zheng, J.F.; Li, L.; Zhou, Z.P.; Su, L.; Zhao, J. π-π stacking derived from graphene-like biochar/g-C3N4 with tunable band structure for photocatalytic antibiotics degradation via peroxymonosulfate activation. J. Hazard. Mater. 2021, 423, 126944. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Lin, M.X. Synthesis of Biochar-Supported K-doped g-C3N4 Photocatalyst for Enhancing the Polycyclic Aromatic Hydrocarbon Degradation Activity. Int. J. Environ. Res. Public Health 2020, 17, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Liang, L.; Ma, J.; Wang, F.; Sun, J. Remarkably enhanced photocatalytic activity of ordered mesoporous carbon/g-C3N4 composite photocatalysts under visible light. Dalton Trans. 2014, 43, 7236–7244. [Google Scholar] [CrossRef]
- Muttakin, M.; Mitra, S.; Thu, K.; Ito, K.; Saha, B.B. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int. J. Heat Mass Transf. 2018, 122, 795–805. [Google Scholar] [CrossRef]
- Ramli, A.Z.; Sabudin, S.; Batcha, M.F.M.; Waehahyee, M. Analysis of Tar Properties Produced During Co-Gasification of Empty Fruit Bunch Pellet and Oil Palm Shell. Int. J. Eng. Sci. 2020, 12, 52–59. Available online: https://publisher.uthm.edu.my/ojs/index.php/ijie/article/view/2930 (accessed on 15 May 2022).
- Shekardasht, M.B.; Givianrad, M.H.; Gharbani, P.; Mirjafary, Z.; Mehrizad, A. Preparation of a novel Z-scheme g-C3N4/RGO/Bi2Fe4O9 nanophotocatalyst for degradation of Congo Red dye under visible light. Diam. Relat. Mater. 2020, 109, 108008. [Google Scholar] [CrossRef]
- Li, H.; Mahyoub, S.A.A.; Liao, W.J.; Xia, S.Q.; Zhao, H.C.; Guo, M.Y.; Ma, P.S. Effect of pyrolysis temperature on characteristics and aromatic contaminants adsorption behavior of magnetic biochar derived from pyrolysis oil distillation residue. Bioresour. Technol. 2017, 223, 20–26. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Naushad, M.; Stadler, F.J.; Ghfar, A.A.; Dhiman, P.; Saini, R.V. Sustainable nano-hybrids of magnetic biochar supported g-C3N4/FeVO4 for solar powered degradation of noxious pollutants—Synergism of adsorption, photocatalysis & photo-ozonation. J. Clean. Prod. 2017, 165, 431–451. [Google Scholar] [CrossRef]
- Lin, M.X. Photocatalysis and Microwave Degradation of Petroleum Hydrocarbons in Soil by Biochar-Supported Modified g-C3N4. Master’s Thesis, Liaoning Petrochemical University, Fushun, China, 2019. [Google Scholar] [CrossRef]
- Fan, S.S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X.D. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Pradubmook, T.; O’Haver, J.H.; Malakul, P.; Harwell, J.H. Effect of pH on adsolubilization of toluene and acetophenone into adsorbed surfactant on precipitated silica. Colloids Surf. A Physicochem. Eng. Asp. 2003, 224, 93–98. [Google Scholar] [CrossRef]
- Bulut, E.; Özacar, M.; Şengil, I.A. Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design. Microporous Mesoporous Mater. 2008, 115, 234–246. [Google Scholar] [CrossRef]
- Ji, X.Q.; Lv, L.; Chen, F.; Yang, C.P. Sorption properties and mechanisms of organic dyes by straw biochar. Acta Sci. Circumstantiae 2016, 36, 1648–1654. [Google Scholar] [CrossRef]
- Li, Y.-T.; Zhang, J.-J.; Li, Y.-H.; Chen, J.-L.; Du, W.-Y. Treatment of soil contaminated with petroleum hydrocarbons using activated persulfate oxidation, ultrasound, and heat: A kinetic and thermodynamic study. Chem. Eng. J. 2021, 428, 131336. [Google Scholar] [CrossRef]
- Li, Y.; Xing, X.Y.; Pei, J.Z.; Li, R.; Wen, Y.; Cui, S.C.; Liu, T. Automobile exhaust gas purification material based on physical adsorption of tourmaline powder and visible light catalytic decomposition of g-C3N4/BiVO4. Ceram. Int. 2020, 46, 12637–12647. [Google Scholar] [CrossRef]
- Ma, Z.-P.; Zhang, L.N.; Ma, X.; Zhang, Y.-H.; Shi, F.-N. Design of Z-scheme g-C3N4/BC/Bi25FeO40 photocatalyst with unique electron transfer channels for efficient degradation of tetracycline hydrochloride waste. Chemosphere 2022, 289, 133262. [Google Scholar] [CrossRef]
- Yin, H.F.; Cao, Y.; Fan, T.L.; Li, P.F.; Liu, X.H. Construction of AgBr/β-Ag2WO4/g-C3N4 ternary composites with dual Z-scheme band alignment for efficient organic pollutants removal. Sep. Purif. Technol. 2021, 272, 118251. [Google Scholar] [CrossRef]


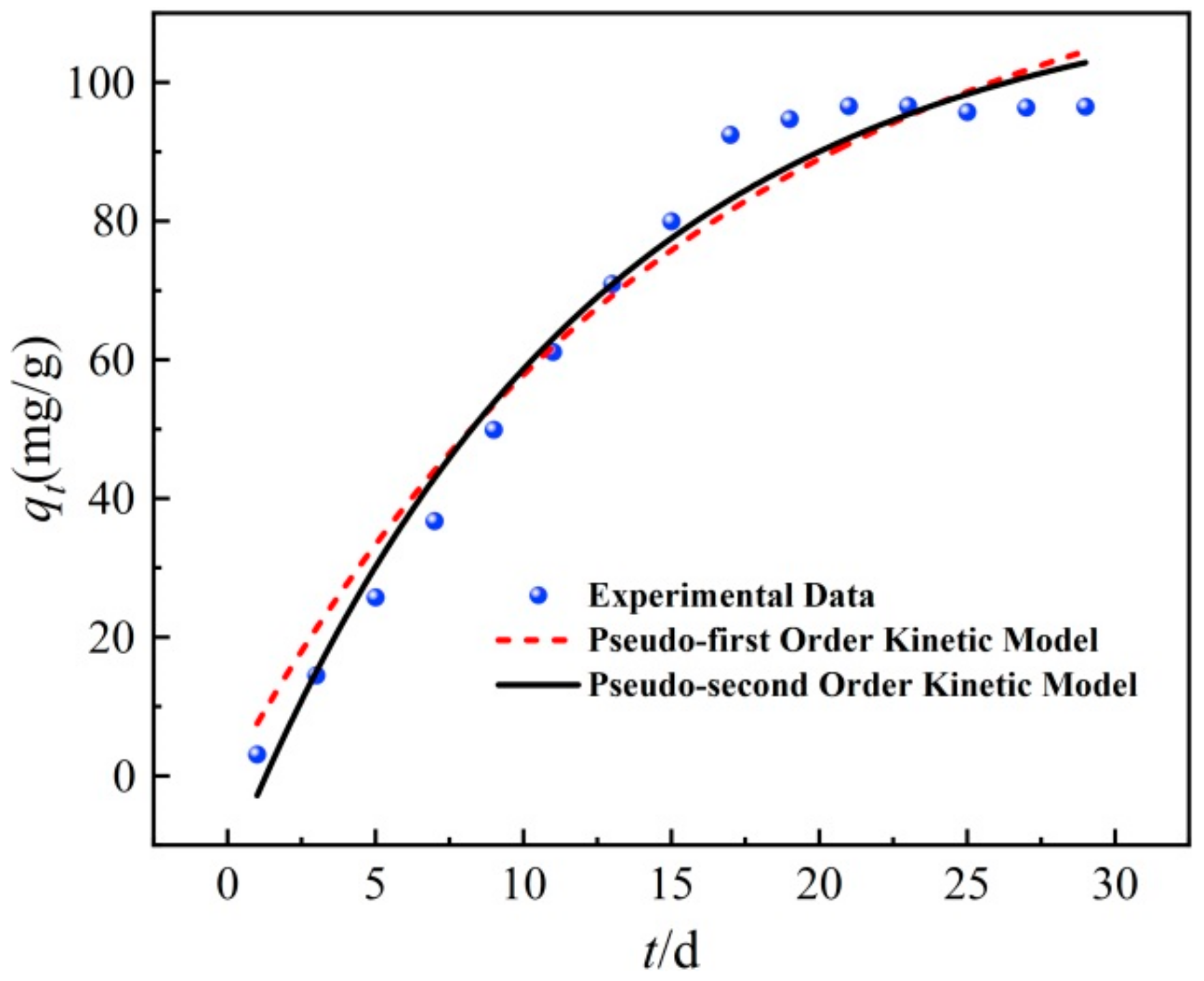

| Pseudo-First-Order Kinetics Model | Pseudo-Second-Order Kinetics Model | ||||
|---|---|---|---|---|---|
| qe/(mg·g−1) | K1/d−1 | R2 | qe/(mg·g−1) | K2/(mg·g−1·d−1) | R2 |
| 102.51 | 0.0620 | 0.959 | 101.72 | 0.0161 | 0.981 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Yang, Y.; Shang, Z.; Li, Q.; Niu, X.; Ma, Y.; Liu, A. Study on the Enhanced Remediation of Petroleum-Contaminated Soil by Biochar/g-C3N4 Composites. Int. J. Environ. Res. Public Health 2022, 19, 8290. https://doi.org/10.3390/ijerph19148290
Lin H, Yang Y, Shang Z, Li Q, Niu X, Ma Y, Liu A. Study on the Enhanced Remediation of Petroleum-Contaminated Soil by Biochar/g-C3N4 Composites. International Journal of Environmental Research and Public Health. 2022; 19(14):8290. https://doi.org/10.3390/ijerph19148290
Chicago/Turabian StyleLin, Hongyang, Yang Yang, Zhenxiao Shang, Qiuhong Li, Xiaoyin Niu, Yanfei Ma, and Aiju Liu. 2022. "Study on the Enhanced Remediation of Petroleum-Contaminated Soil by Biochar/g-C3N4 Composites" International Journal of Environmental Research and Public Health 19, no. 14: 8290. https://doi.org/10.3390/ijerph19148290
APA StyleLin, H., Yang, Y., Shang, Z., Li, Q., Niu, X., Ma, Y., & Liu, A. (2022). Study on the Enhanced Remediation of Petroleum-Contaminated Soil by Biochar/g-C3N4 Composites. International Journal of Environmental Research and Public Health, 19(14), 8290. https://doi.org/10.3390/ijerph19148290






