Adjustable Parameters and the Effectiveness of Adjunct Robot-Assisted Gait Training in Individuals with Chronic Stroke
Abstract
1. Introduction
2. Methods
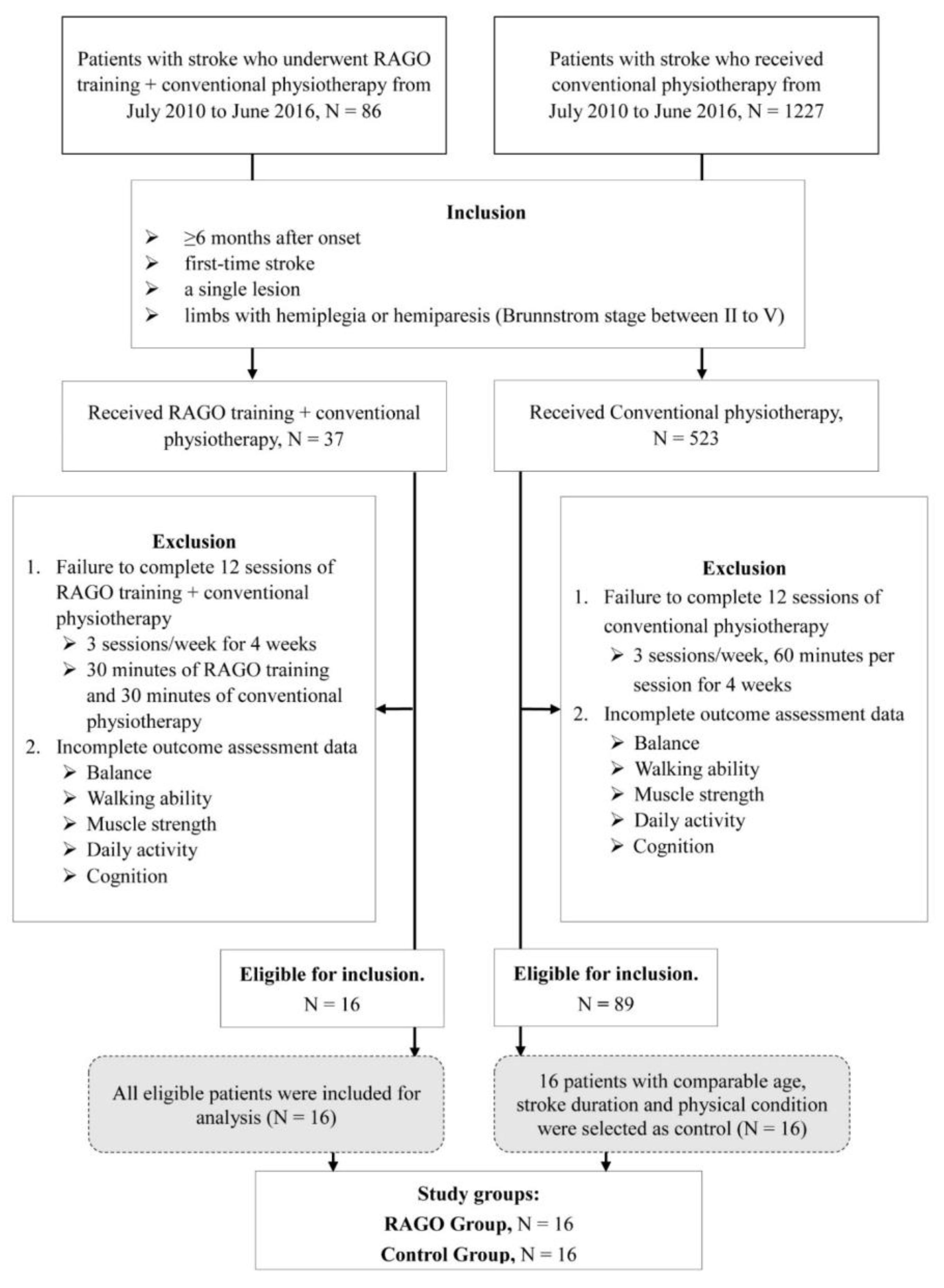
2.1. Patients
2.2. Study Groups
2.3. Treatment
2.4. Outcome Measures
2.4.1. Mini–Mental State Examination
2.4.2. Berg Balance Scale
2.4.3. Motricity Index
2.4.4. Functional Ambulation Category
2.4.5. Barthel Index
2.4.6. Brunnstrom Stage
2.5. Adjustable RAGO Parameters
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Outcome Variables before and after Training
3.3. Robot-Assisted Gait Orthosis (RAGO) Training Adjustable Parameters
3.4. Association between RAGO Parameters and Functional Outcome Variables
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| RAGO | Robot-assisted gait orthosis |
References
- Dettmann, M.A.; Linder, M.T.; Sepic, S.B. Relationships among walking performance, postural stability, and functional assessments of the hemiplegic patient. Am. J. Phys. Med. 1987, 66, 77–90. [Google Scholar] [PubMed]
- Lai, C.H.; Chen, H.C.; Liou, T.H.; Li, W.; Chen, S.C. Exercise interventions for individuals with neurological disorders: A systematic review of systematic reviews. Am. J. Phys. Med. Rehabil. 2019, 98, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Zorowitz, R.; Bates, B.; Choi, J.Y.; Glasberg, J.J.; Graham, G.D.; Katz, R.C.; Lamberty, K.; Reker, D. Management of adult stroke rehabilitation care: A clinical practice guideline. Stroke 2005, 36, e100–e143. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Francisco, G.E.; Zhou, P. Post-stroke hemiplegic gait: New perspective and insights. Front. Physiol. 2018, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef]
- Hesse, S. Treadmill training with partial body weight support after stroke: A review. NeuroRehabilitation 2008, 23, 55–65. [Google Scholar] [CrossRef]
- Hesse, S.A.; Jahnke, M.T.; Schreiner, C.; Mauritz, K.H. Gait symmetry and functional walking performance in hemiparetic patients prior to and after a 4-week rehabilitation programme. Gait Posture 1993, 1, 166–171. [Google Scholar] [CrossRef]
- Srivastava, A.; Taly, A.B.; Gupta, A.; Kumar, S.; Murali, T. Bodyweight-supported treadmill training for retraining gait among chronic stroke survivors: A randomized controlled study. Ann. Phys. Rehabil. Med. 2016, 59, 235–241. [Google Scholar] [CrossRef]
- Drużbicki, M.; Przysada, G.; Guzik, A.; Brzozowska-Magoń, A.; Kołodziej, K.; Wolan-Nieroda, A.; Majewska, J.; Kwolek, A. The efficacy of gait training using a body weight support treadmill and visual biofeedback in patients with subacute stroke: A randomized controlled trial. BioMed Res. Int. 2018, 2018, 3812602. [Google Scholar] [CrossRef]
- Belda-Lois, J.M.; Mena-del Horno, S.; Bermejo-Bosch, I.; Moreno, J.C.; Pons, J.L.; Farina, D.; Iosa, M.; Molinari, M.; Tamburella, F.; Ramos, A.; et al. Rehabilitation of gait after stroke: A review towards a top-down approach. J. Neuroeng. Rehabil. 2011, 8, 66. [Google Scholar] [CrossRef]
- Rodrigues, T.A.; Goroso, D.G.; Westgate, P.M.; Carrico, C.; Batistella, L.R.; Sawaki, L. Slow Versus fast robot-assisted locomotor training after severe stroke: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2017, 96, S165–S170. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Nakata, Y.; Eguchi, K.; Kawamoto, H.; Kamibayashi, K.; Sakane, M.; Sankai, Y.; Ochiai, N. Feasibility of rehabilitation training with a newly developed wearable robot for patients with limited mobility. Arch. Phys. Med. Rehabil. 2013, 94, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Lünenburger, L.; Colombo, G.; Riener, R.; Dietz, V. Biofeedback in gait training with the robotic orthosis Lokomat. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference, San Francisco, CA, USA, 1–5 September 2004; pp. 4888–4891. [Google Scholar]
- Schück, A.; Labruyère, R.; Vallery, H.; Riener, R.; Duschau-Wicke, A. Feasibility and effects of patient-cooperative robot-aided gait training applied in a 4-week pilot trial. J. Neuroeng. Rehabil. 2012, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.E.; Kyeong, S.; Lee, S.H.; Lee, W.J.; Ha, S.W.; Kim, S.M.; Kang, H.; Lee, W.M.; Kang, C.S.; Kim, D.H. Structural and functional improvements due to robot-assisted gait training in the stroke-injured brain. Neurosci. Lett. 2017, 637, 114–119. [Google Scholar] [CrossRef]
- Swinnen, E.; Beckwee, D.; Meeusen, R.; Baeyens, J.P.; Kerckhofs, E. Does robot-assisted gait rehabilitation improve balance in stroke patients? A systematic review. Top. Stroke Rehabil. 2014, 21, 87–100. [Google Scholar] [CrossRef]
- Mayr, A.; Kofler, M.; Quirbach, E.; Matzak, H.; Frohlich, K.; Saltuari, L. Prospective, blinded, randomized crossover study of gait rehabilitation in stroke patients using the Lokomat gait orthosis. Neurorehabilit. Neural Repair 2007, 21, 307–314. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Cheng, Y.H.; Lai, C.H.; Lin, Y.N. Clinical non-superiority of technology-assisted gait training with body weight support in patients with subacute stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2019, 63, 535–542. [Google Scholar] [CrossRef]
- Husemann, B.; Muller, F.; Krewer, C.; Heller, S.; Koenig, E. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: A randomized controlled pilot study. Stroke 2007, 38, 349–354. [Google Scholar] [CrossRef]
- Hidler, J.; Nichols, D.; Pelliccio, M.; Brady, K.; Campbell, D.D.; Kahn, J.H.; Hornby, T.G. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabilit. Neural Repair 2009, 23, 5–13. [Google Scholar] [CrossRef]
- Pohl, M.; Werner, C.; Holzgraefe, M.; Kroczek, G.; Mehrholz, J.; Wingendorf, I.; Hoolig, G.; Koch, R.; Hesse, S. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: A single-blind, randomized multicentre trial (DEutsche GAngtrainerStudie, DEGAS). Clin. Rehabil. 2007, 21, 17–27. [Google Scholar] [CrossRef]
- Schwartz, I.; Sajin, A.; Fisher, I.; Neeb, M.; Shochina, M.; Katz-Leurer, M.; Meiner, Z. The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: A randomized controlled trial. PM R J. Inj. Funct. Rehabil. 2009, 1, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Hornby, T.G.; Campbell, D.D.; Kahn, J.H.; Demott, T.; Moore, J.L.; Roth, H.R. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: A randomized controlled study. Stroke 2008, 39, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Aprile, I.; Iacovelli, C.; Padua, L.; Galafate, D.; Criscuolo, S.; Gabbani, D.; Cruciani, A.; Germanotta, M.; Di Sipio, E.; De Pisi, F.; et al. Efficacy of Robotic-Assisted Gait Training in chronic stroke patients: Preliminary results of an Italian bi-centre study. NeuroRehabilitation 2017, 41, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Westlake, K.P.; Patten, C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J. Neuroeng. Rehabil. 2009, 6, 18. [Google Scholar] [CrossRef]
- Bruni, M.F.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabro, R.S. What does best evidence tell us about robotic gait rehabilitation in stroke patients: A systematic review and meta-analysis. J. Clin.Neurosci. Off. J. Neurosurg. Soc. Australas. 2018, 48, 11–17. [Google Scholar] [CrossRef]
- Bang, D.H.; Shin, W.S. Effects of robot-assisted gait training on spatiotemporal gait parameters and balance in patients with chronic stroke: A randomized controlled pilot trial. NeuroRehabilitation 2016, 38, 343–349. [Google Scholar] [CrossRef]
- Knaepen, K.; Mierau, A.; Swinnen, E.; Fernandez Tellez, H.; Michielsen, M.; Kerckhofs, E.; Lefeber, D.; Meeusen, R. Human-robot interaction: Does robotic guidance force affect gait-related brain dynamics during robot-assisted treadmill walking? PLoS ONE 2015, 10, e0140626. [Google Scholar] [CrossRef]
- Khaw, J.; Subramaniam, P.; Abd Aziz, N.A.; Ali Raymond, A.; Wan Zaidi, W.A.; Ghazali, S.E. Current update on the clinical utility of MMSE and MoCA for stroke patients in Asia: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 8962. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health Rev. Can. De Sante Publique 1992, 83 (Suppl. 2), S7–S11. [Google Scholar]
- Wang, T.Y.; Chen, S.C.; Peng, C.W.; Kang, C.W.; Chen, Y.L.; Chen, C.L.; Chou, Y.L.; Lai, C.H. Relevance of nerve conduction velocity in the assessment of balance performance in older adults with diabetes mellitus. Disabil. Rehabil. 2017, 39, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.Y.; Chen, S.C.; Peng, C.W.; Lin, Y.N.; Chang, Y.T.; Lai, C.H. Effects of interactive video-game-based exercise on balance in older adults with mild-to-moderate Parkinson’s disease. J. Neuroeng. Rehabil. 2020, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The balance scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar] [PubMed]
- Cameron, D.; Bohannon, R.W. Criterion validity of lower extremity motricity index scores. Clin. Rehabil. 2000, 14, 208–211. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Quinn, T.J.; Langhorne, P.; Stott, D.J. Barthel index for stroke trials: Development, properties, and application. Stroke 2011, 42, 1146–1151. [Google Scholar] [CrossRef]
- Shah, S.K.; Harasymiw, S.J.; Stahl, P.L. Stroke rehabilitation: Outcome based on brunnstrom recovery stages. OTJR Occup. Particip. Health 1986, 6, 365–376. [Google Scholar] [CrossRef]
- Kelley, C.P.; Childress, J.; Boake, C.; Noser, E.A. Over-ground and robotic-assisted locomotor training in adults with chronic stroke: A blinded randomized clinical trial. Disabil. Rehabil. Assist. Technol. 2013, 8, 161–168. [Google Scholar] [CrossRef]
- Chisari, C.; Bertolucci, F.; Monaco, V.; Venturi, M.; Simonella, C.; Micera, S.; Rossi, B. Robot-assisted gait training improves motor performances and modifies Motor Unit firing in poststroke patients. Eur. J. Phys. Rehabil. Med. 2015, 51, 59–69. [Google Scholar]
- Harris, N.K.; Cronin, J.B.; Hopkins, W.G.; Hansen, K.T. Relationship between sprint times and the strength/power outputs of a machine squat jump. J. Strength Cond. Res. 2008, 22, 691–698. [Google Scholar] [CrossRef]
- Hidler, J.; Sainburg, R. Role of Robotics in Neurorehabilitation. Top. Spinal Cord Inj. Rehabil. 2011, 17, 42–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Kammen, K.; Boonstra, A.M.; van der Woude, L.H.; Reinders-Messelink, H.A.; den Otter, R. The combined effects of guidance force, bodyweight support and gait speed on muscle activity during able-bodied walking in the Lokomat. Clin. Biomech. 2016, 36, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.; Kotsapouikis, D.; Dhaher, Y.Y.; Rymer, W.Z. Reducing robotic guidance during robot-assisted gait training improves gait function: A case report on a stroke survivor. Arch. Phys. Med. Rehabil. 2013, 94, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Conditt, M.A.; Gandolfo, F.; Mussa-Ivaldi, F.A. The motor system does not learn the dynamics of the arm by rote memorization of past experience. J. Neurophysiol. 1997, 78, 554–560. [Google Scholar] [CrossRef]
- Tyson, S.F.; Woodward-Nutt, K.; Plant, S. How are balance and mobility problems after stroke treated in England? An observational study of the content, dose and context of physiotherapy. Clin. Rehabil. 2018, 32, 1145–1152. [Google Scholar] [CrossRef]
- Lee, S.Y.; Han, E.Y.; Kim, B.R.; Chun, M.H.; Lee, Y.K. Can lowering the guidance force of robot-assisted gait training induce a sufficient metabolic demand in subacute dependent ambulatory patients with stroke? Arch. Phys. Med. Rehabil. 2017, 98, 695–700. [Google Scholar] [CrossRef] [PubMed]
- van Kammen, K.; Boonstra, A.M.; van der Woude, L.H.V.; Visscher, C.; Reinders-Messelink, H.A.; den Otter, R. Lokomat guided gait in hemiparetic stroke patients: The effects of training parameters on muscle activity and temporal symmetry. Disabil. Rehabil. 2020, 42, 2977–2985. [Google Scholar] [CrossRef]
- McGrath, R.L.; Ziegler, M.L.; Pires-Fernandes, M.; Knarr, B.A.; Higginson, J.S.; Sergi, F. The effect of stride length on lower extremity joint kinetics at various gait speeds. PLoS ONE 2019, 14, e0200862. [Google Scholar] [CrossRef]


| Control | RAGO | p-Value | |
|---|---|---|---|
| N = 16 | N = 16 | ||
| Sex | |||
| Male | 9 (56.25%) | 8 (50.0%) | 0.72 a |
| Female | 7 (43.75%) | 8 (50.0%) | |
| Age, years | 58.0 (16.1) | 59.8 (14.1) | 0.59 † |
| ≥65 | 5 (31.25%) | 6 (37.5%) | 0.71 a |
| Side of stroke | |||
| Left | 6 (37.5%) | 10 (62.5%) | 0.15 a |
| Right | 10 (62.5%) | 6 (37.5%) | |
| Stroke type | |||
| hemorrhagic | 6 (37.5%) | 10 (62.5%) | 0.15 a |
| ischemic | 10 (62.5%) | 6 (37.5%) | |
| Stroke duration, months | 18.9 (26.1) | 19.3 (13.3) | 0.26 † |
| Brunnstrom stage | |||
| Upper extremity | 3.4 (1.21) | 3.7 (1.34) | 0.53 † |
| Lower extremity | 3.5 (0.89) | 3.8 (0.84) | 0.37 † |
| Mobility on level surface | |||
| 0 | 5 (31.25%) | 3 (18.75%) | 0.58 † |
| 5 | 3 (18.75%) | 3 (18.75%) | |
| 10 | 3 (18.75%) | 5 (31.25%) | |
| 15 | 5 (31.25%) | 5 (31.25%) |
| Control | RAGO | Mean Difference (95% CI) ¶ | p-Value † | |
|---|---|---|---|---|
| N = 16 | N = 16 | |||
| Muscle Strength (Motricity Index of lower extremity) | ||||
| Before training | 17.2 (9.50) | 19.1 (5.42) | −1.87 (−7.52, 3.77) | 0.62 |
| After training | 18.2 (9.60) | 21.8 (4.89) | −3.62 (−9.20, 1.95) | 0.31 |
| p-value ‡ | 0.36 | 0.04 * | ||
| Difference | 1.0 (4.15) | 2.7 (4.42) | −1.75 (−4.84, 1.34) | 0.21 |
| Balance (Berg Balance Scale) | ||||
| Before training | 25.8 (21.88) | 22.3 (16.44) | 3.50 (−10.47, 17.47) | 0.74 |
| After training | 29.4 (22.84) | 28.3 (16.89) | 1.12 (−13.43, 15.68) | 0.91 |
| p-value ‡ | 0.02 * | <0.0001 * | ||
| Difference | 3.6 (5.82) | 5.9 (4.43) | −2.37 (−6.11, 1.36) | 0.03 * |
| Daily Activity Independence (Barthel Index) | ||||
| Before training | 56.9 (38.38) | 57.5 (28.58) | −0.62 (−25.05, 23.80) | 0.95 |
| After training | 59.7 (36.76) | 63.4 (25.61) | −3.75 (−26.74, 19.24) | 0.88 |
| p-value ‡ | 0.42 | 0.02 * | ||
| Difference | 2.8 (9.99) | 5.9 (8.21) | −3.12 (−9.72, 3.47) | 0.29 |
| Mobility on level surfaces | ||||
| Before training | 7.5 (6.33) | 8.8 (5.63) | −1.25 (−5.57, 3.07) | 0.58 |
| After training | 8.1 (6.55) | 10.9 (5.23) | −2.81 (−7.09, 1.46) | 0.19 |
| p-value ‡ | 0.16 | 0.04 * | ||
| Difference | 0.6 (1.70) | 2.2 (4.07) | −1.56 (−3.86, 0.73) | 0.19 |
| Cognition Function (Mini–Mental State Examination) | ||||
| Before training | 21.3 (8.96) | 21.6 (8.07) | −0.25 (−6.40, 5.90) | 0.91 |
| After training | 22.7 (8.07) | 23.6 (7.92) | −0.87 (−6.64, 4.89) | 0.39 |
| p-value ‡ | 0.03 * | 0.02 * | ||
| Difference | 1.4 (2.28) | 2.0 (3.31) | −0.62 (−2.67, 1.42) | 0.21 |
| Walking Ability (Functional Ambulation Category) | ||||
| Before training | 2.7 (1.85) | 2.2 (1.60) | 0.50 (−0.75, 1.75) | 0.44 |
| After training | 3.1 (2.00) | 2.5 (1.71) | 0.62 (−0.71, 1.96) | 0.29 |
| p-value ‡ | 0.06 | 0.06 | ||
| Difference | 0.4 (0.89) | 0.3 (0.48) | 0.12 (−0.39, 0.64) | 0.82 |
| F Statistics | p-Value | |
|---|---|---|
| Muscle strength (lower extremity) | ||
| Group effect | 1.11 | 0.3 |
| Time effect | 6.13 | 0.02 |
| Time x Group effect | 1.33 | 0.26 |
| Balance | ||
| Group effect | 0.11 | 0.74 |
| Time effect | 26.98 | 0.00001 |
| Time x Group effect | 1.69 | 0.2 |
| Daily activity independence | ||
| Group effect | 0.04 | 0.85 |
| Time effect | 7.32 | 0.01 |
| Time x Group effect | 9.34 | 0.34 |
| Mobility on level surfaces | ||
| Group effect | 4.83 | 0.05 |
| Time effect | 3.00 | 0.10 |
| Time x Group effect | 0.002 | 0.97 |
| Cognition | ||
| Group effect | 0.04 | 0.84 |
| Time effect | 11.31 | 0.002 |
| Time x Group effect | 0.39 | 0.54 |
| Walking ability | ||
| Group effect | 0.82 | 0.37 |
| Time effect | 8.78 | 0.01 |
| Time x Group effect | 0.24 | 0.63 |
| Brunnstrom Stage | Control N = 16 | RAGO N = 16 | Mean Difference (95% CI) ¶ | p-Value † |
|---|---|---|---|---|
| Upper extremity | ||||
| Before training | 3.4 (1.21) | 3.7 (1.34) | −0.31 (−1.23, 0.61) | 0.53 |
| After training | 3.7 (1.24) | 4.0 (1.28) | −0.31 (−1.22, 0.60) | 0.49 |
| p-value ‡ | 0.06 | 0.03 * | ||
| Difference | 0.3 (0.60) | 0.3 (0.54) | 0.05 (−0.39, 0.49) | 0.79 |
| Lower extremity | ||||
| Before training | 3.5 (0.89) | 3.8 (0.84) | −0.31 (−0.93, 0.31) | 0.37 |
| After training | 3.9 (0.81) | 4.0 (0.99) | −0.18 (−0.84, 0.46) | 0.52 |
| p-value ‡ | 0.08 | 0.06 | ||
| Difference | 0.4 (0.80) | 0.3 (0.51) | 0.12 (−0.41, 0.66) | 0.52 |
| F Statistics | p-Value | |
|---|---|---|
| Guidance force—Paretic side | 10.36 | <0.0001 |
| Guidance force—Non-paretic side | 10.36 | <0.0001 |
| Walking speed | 2.95 | 0.02 |
| Body-weight support | 7.11 | <0.0001 |
| Guidance Force (Paretic Side) | Guidance Force (Non-Paretic Side) | Speed | Body-Weight Support | |
|---|---|---|---|---|
| Muscle strength (Motricity Index of lower extremity) | 0.199 | 0.192 | 0.092 | −0.231 |
| Walking ability (Functional Ambulation Category) | 0.099 | 0.099 | 0.284 | −0.168 |
| Daily activity independence (Barthel Index) | −0.644 ** | −0.613 * | −0.172 | −0.634 ** |
| Cognition (Mini–Mental State Examination) | 0.247 | 0.175 | 0.181 | 0.054 |
| Balance (Berg Balance Scale) | −0.008 | −0.006 | 0.601 * | −0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-C.; Kang, J.-H.; Peng, C.-W.; Hsu, C.-C.; Lin, Y.-N.; Lai, C.-H. Adjustable Parameters and the Effectiveness of Adjunct Robot-Assisted Gait Training in Individuals with Chronic Stroke. Int. J. Environ. Res. Public Health 2022, 19, 8186. https://doi.org/10.3390/ijerph19138186
Chen S-C, Kang J-H, Peng C-W, Hsu C-C, Lin Y-N, Lai C-H. Adjustable Parameters and the Effectiveness of Adjunct Robot-Assisted Gait Training in Individuals with Chronic Stroke. International Journal of Environmental Research and Public Health. 2022; 19(13):8186. https://doi.org/10.3390/ijerph19138186
Chicago/Turabian StyleChen, Shih-Ching, Jiunn-Horng Kang, Chih-Wei Peng, Chih-Chao Hsu, Yen-Nung Lin, and Chien-Hung Lai. 2022. "Adjustable Parameters and the Effectiveness of Adjunct Robot-Assisted Gait Training in Individuals with Chronic Stroke" International Journal of Environmental Research and Public Health 19, no. 13: 8186. https://doi.org/10.3390/ijerph19138186
APA StyleChen, S.-C., Kang, J.-H., Peng, C.-W., Hsu, C.-C., Lin, Y.-N., & Lai, C.-H. (2022). Adjustable Parameters and the Effectiveness of Adjunct Robot-Assisted Gait Training in Individuals with Chronic Stroke. International Journal of Environmental Research and Public Health, 19(13), 8186. https://doi.org/10.3390/ijerph19138186








