The Occurrence of Microplastics and the Formation of Biofilms by Pathogenic and Opportunistic Bacteria as Threats in Aquaculture
Abstract
:1. Introduction
2. Occurrence of Microplastics in Aquaculture
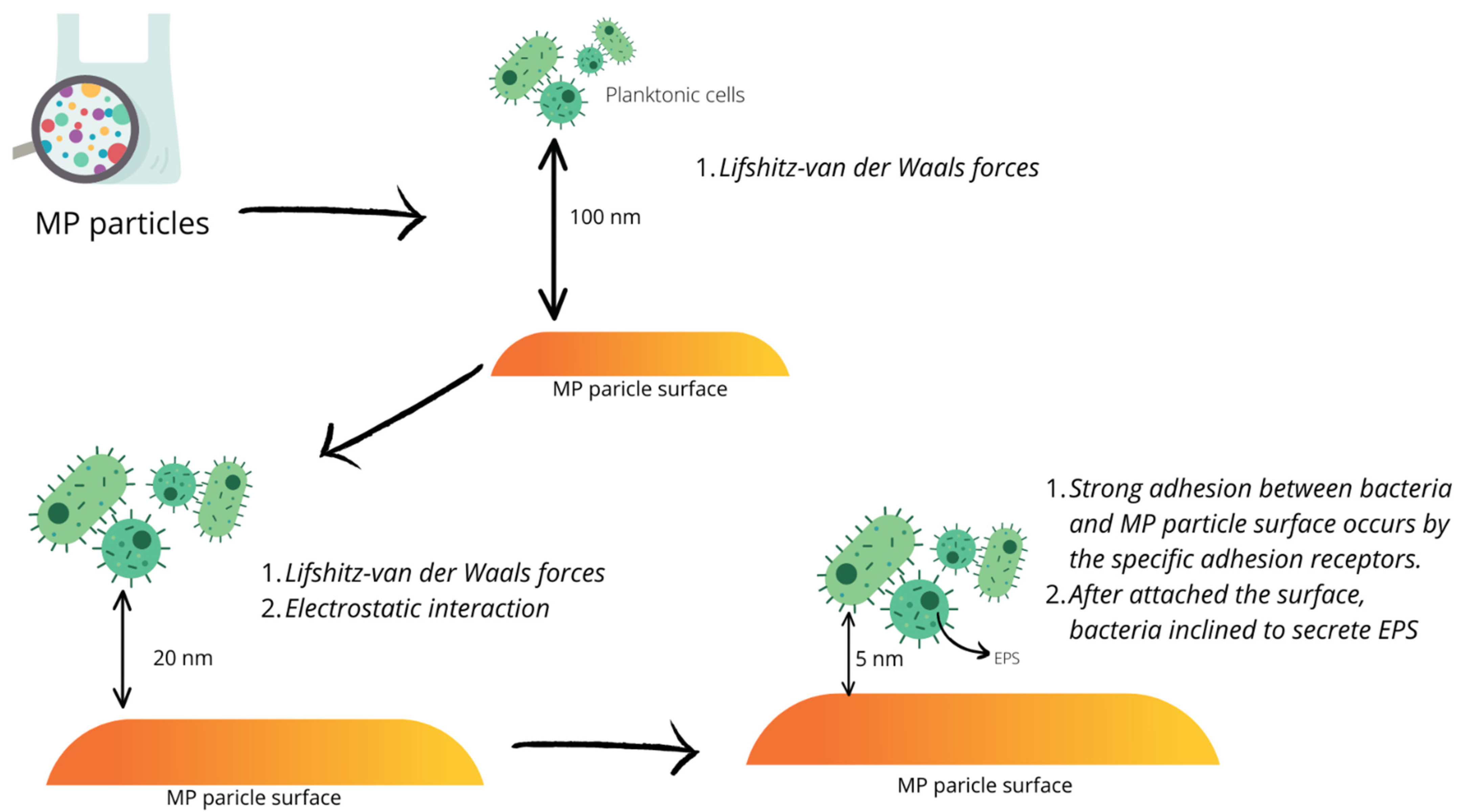
3. Biofilm Formation
4. Microorganisms Detected on Microplastics
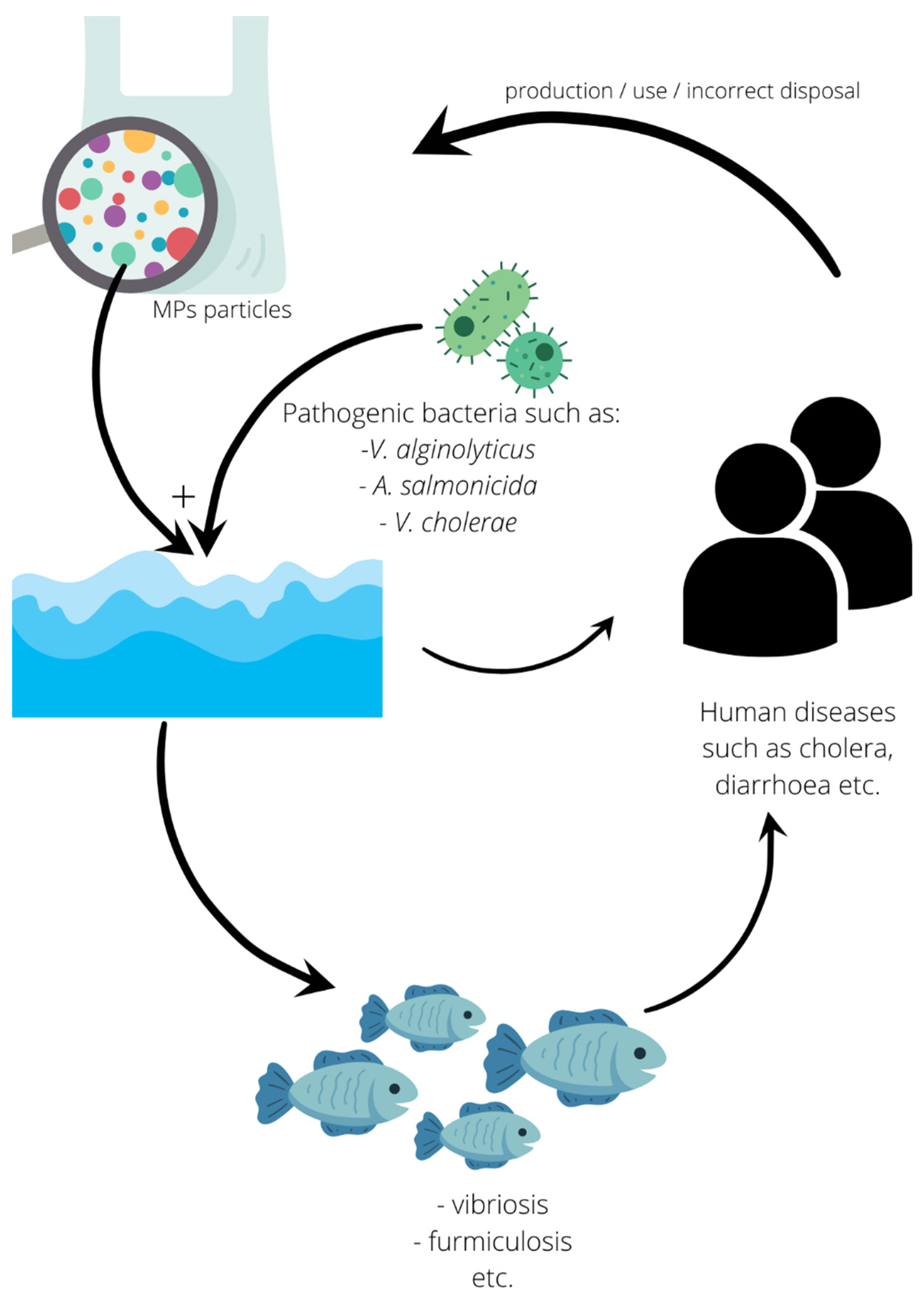
5. Microplastics and Microbial Safety in Aquaculture
5.1. Marine Animals’ Pathogens
5.2. Human Pathogens
5.3. Plant Pathogens
5.4. Other Aspects of Microplastic Influence on Animal Health
5.5. Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, C. Not so many fish in the sea. Curr. Biol. 2017, 27, R439–R443. [Google Scholar] [CrossRef]
- Parata, L.; Sammut, J.; Egan, S. Opportunities for microbiome research to enhance farmed freshwater fish quality and production. Rev. Aquac. 2021, 13, 2027–2037. [Google Scholar] [CrossRef]
- Simonit, S.; Perrings, C. Sustainability and the value of the ‘regulating’ services: Wetlands and water quality in Lake Victoria. Ecol. Econ. 2011, 70, 1189–1199. [Google Scholar] [CrossRef]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and managing microbes in aquaculture—Towards a sustainable industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Cholewińska, P.; Czyż, K.; Nowakowski, P.; Wyrostek, A. The microbiome of the digestive system of ruminants—A review. Anim. Health Res. Rev. 2020, 21, 3–14. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Vadstein, O.; Bergh, Ø.; Gatesoupe, F.-J.; Galindo-Villegas, J.; Mulero, V.; Picchietti, S.; Scapigliati, G.; Makridis, P.; Olsen, Y.; Dierckens, K.; et al. Microbiology and immunology of fish larvae. Rev. Aquac. 2013, 5, S1–S25. [Google Scholar] [CrossRef] [Green Version]
- Van Bonn, W.; LaPointe, A.; Gibbons, S.M.; Frazier, A.; Hampton-Marcell, J.; Gilbert, J. Aquarium microbiome response to ninety-percent system water change: Clues to microbiome management. Zoo Biol. 2015, 34, 360–367. [Google Scholar] [CrossRef]
- Krotman, Y.; Yergaliyev, T.M.; Shani, R.A.; Avrahami, Y.; Szitenberg, A. Dissecting the factors shaping fish skin microbiomes in a heterogeneous inland water system. Microbiome 2020, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.H.; Lin, G.; Fu, G.H.; Wan, Z.Y.; Lee, M.; Wang, L.; Liu, X.J.; Yue, G.H. The intestinal microbiome of fish under starvation. BMC Genom. 2014, 15, 266. [Google Scholar] [CrossRef] [Green Version]
- Arias, C.R.; Koenders, K.; Larsen, A.M. Predominant bacteria associated with red snapper from the Northern Gulf of Mexico. J. Aquat. Anim. Health 2013, 25, 281–289. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; He, D.; Xu, J.; Sun, J.; Li, J.; Pan, X. Environmental behaviors of microplastics in aquatic systems: A systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J. Hazard. Mater. 2022, 423, 126915. [Google Scholar] [CrossRef]
- Plastics Europe. Quarterly Report Q3/2021. European Plastics Manufacturers (EU 27); Plastics Europe AISBL: Brussels, Belgium, 2022. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Galgani, F.; Hanke, G.; Maes, T. Global Distribution, Composition and Abundance of Marine Litter. In Marine Anthropogenic Litter, 1st ed.; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 29–56. [Google Scholar]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- European Chemicals Agency. Restricting the Use of Intentionally Added Microplastic Particles to Consumer or Professional Use Products of Any Kind; ECHA; European Commission: Helsinki, Finland, 2020. [Google Scholar]
- Frère, L.; Maignien, L.; Chalopin, M.; Huvet, A.; Rinnert, E.; Morrison, H.; Kerninon, S.; Cassone, A.-L.; Lambert, C.; Reveillaud, J.; et al. Microplastic bacterial communities in the Bay of Brest: Influence of polymer type and size. Environ. Pollut. 2018, 242, 614–625. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef]
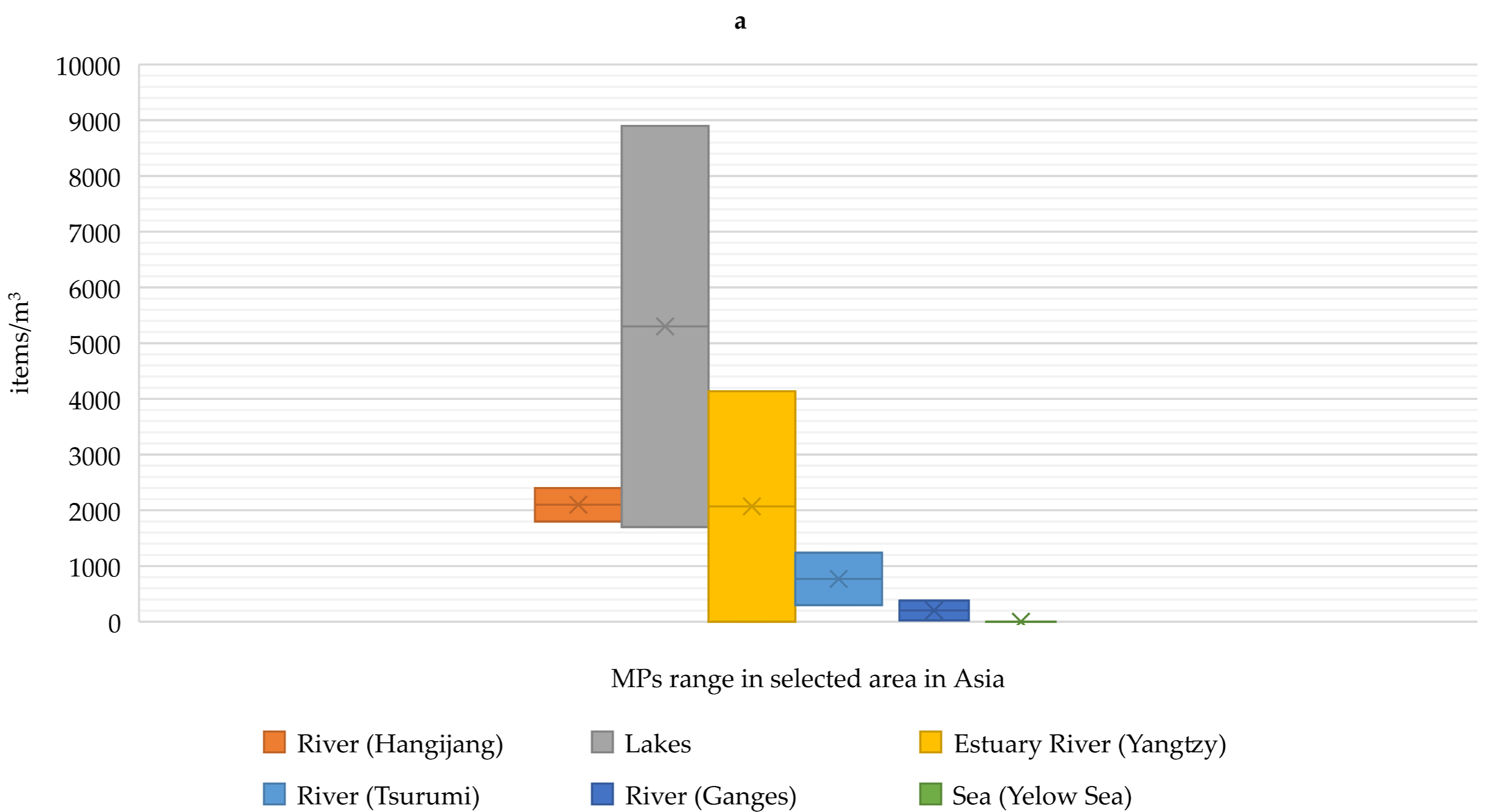
- Ma, J.; Niu, X.; Zhang, D.; Lu, L.; Ye, X.; Deng, W.; Li, Y.; Lin, Z. High levels of microplastic pollution in aquaculture water of fish ponds in the Pearl River Estuary of Guangzhou, China. Sci. Total Environ. 2020, 744, 140679. [Google Scholar] [CrossRef]
- Priscilla, V.; Patria, M.P. Comparison of microplastic abundance in aquaculture ponds of milkfish Chanos chanos (Forsskål, 1775) at Muara Kamal and Marunda, Jakarta Bay. IOP Conf. Ser. Earth Environ. Sci. 2020, 404, 012027. [Google Scholar] [CrossRef]
- Pham, D.N.; Clark, L.; Li, M. Microplastics as hubs enriching antibiotic-resistant bacteria and pathogens in municipal activated sludge. J. Hazard. Mater. Lett. 2021, 2, 100014. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Rowe, I.; Ita-Nagy, D.; Kahhat, R. Microplastics in fisheries and aquaculture: Implications to food sustainability and safety. Curr. Opin. Green Sustain. Chem. 2021, 29, 100464. [Google Scholar] [CrossRef]
- Deng, H.; Wei, R.; Luo, W.; Hu, L.; Li, B.; Di, Y.; Shi, H. Microplastic pollution in water and sediment in a textile industrial area. Environ. Pollut. 2020, 258, 113658. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Su, L.; Craig, N.J.; Du, F.; Wu, C.; Shi, H. Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environ. Pollut. 2019, 246, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze estuary system, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Chen, C.F.; Ju, Y.R.; Lim, Y.C.; Hsu, N.H.; Lu, K.T.; Hsieh, S.L.; Dong, C.D.; Chen, C.W. Microplastics and their affiliated PAHs in the sea surface connected to the southwest coast of Taiwan. Chemosphere 2020, 254, 126818. [Google Scholar] [CrossRef]
- Kameda, Y.; Yamada, N.; Fujita, E. Source- and polymer-specific size distributions of fine microplastics in surface water in an urban river. Environ. Pollut. 2021, 284, 117516. [Google Scholar] [CrossRef]
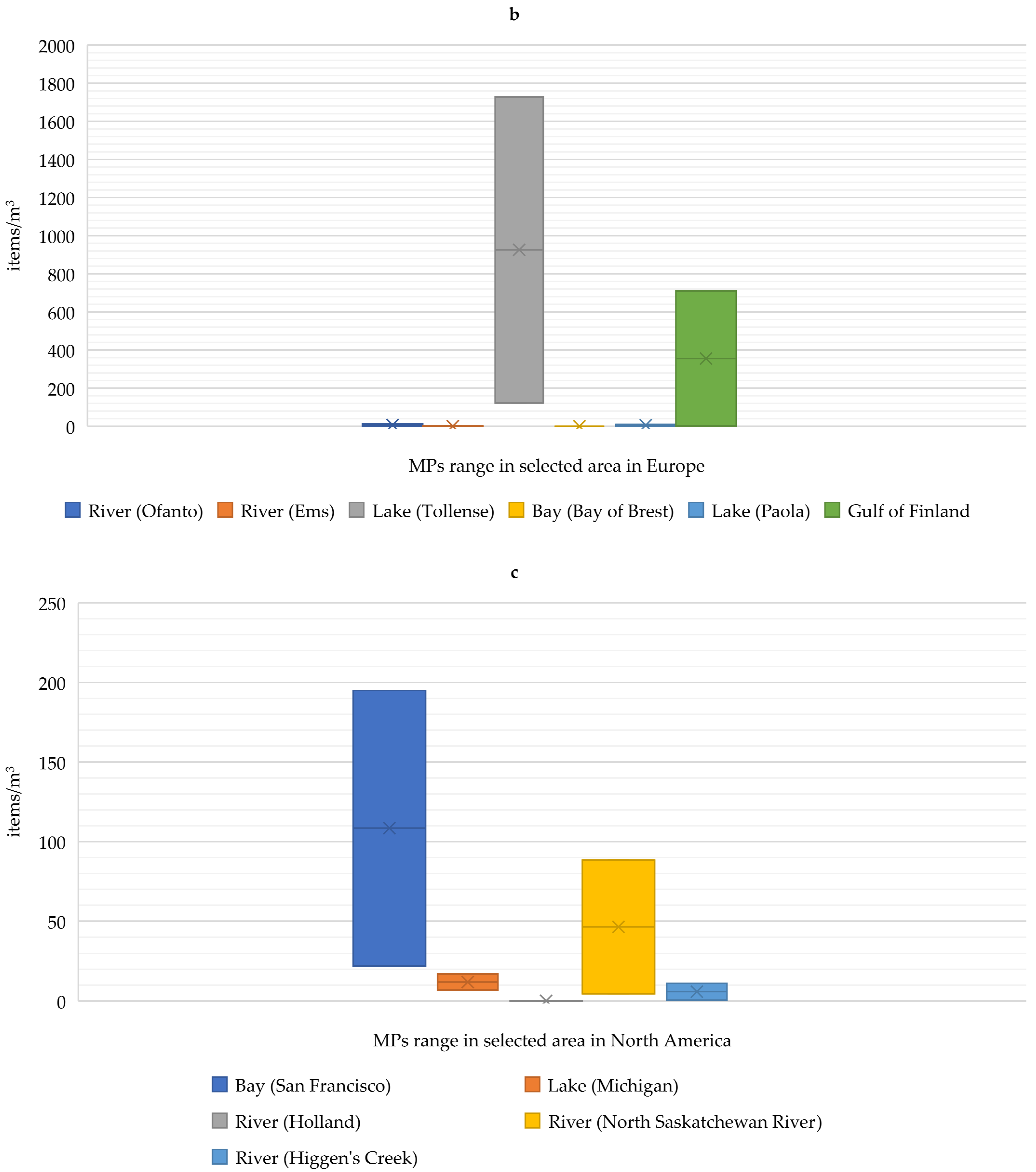
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and their possible sources: The example of Ofanto river in Southeast Italy. Environ. Pollut. 2019, 258, 113284. [Google Scholar] [CrossRef]
- Eibes, P.M.; Gabel, F. Floating microplastic debris in a rural river in Germany: Distribution, types and potential sources and sinks. Sci. Total Environ. 2022, 816, 151641. [Google Scholar] [CrossRef]
- Tamminga, M.; Hengstmann, E.; Deuke, A.K.; Fischer, E.K. Microplastic concentrations, characteristics, and fluxes in water bodies of the Tollense catchment, Germany, with regard to different sampling systems. Environ. Sci. Pollut. Res. 2022, 29, 11345–11358. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.-P.W.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Baroth, A.; Barrett, A.C.; Bhola, S.; Chowdhury, G.W.; Davies, B.F.; Koldewey, H. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 2021, 274, 116348. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liang, J.; Zhu, M.; Zhao, Y.; Zhang, B. Microplastics in seawater and zooplankton from the Yellow Sea. Environ. Pollut. 2018, 242, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Di Pippo, F.; Venezia, C.; Sighicelli, M.; Pietrelli, L.; Di Vito, S.; Nuglio, S.; Rossetti, S. Microplastic-associated biofilms in lentic Italian ecosystems. Water Res. 2020, 187, 116429. [Google Scholar] [CrossRef] [PubMed]
- Uurasjärvi, E.; Pääkkönen, M.; Setälä, O.; Koistinen, A.; Lehtiniemi, M. Microplastics accumulate to thin layers in the stratified Baltic Sea. Environ. Pollut. 2021, 268, 115700. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Felismino, M.E.L.; Helm, P.A.; Rochman, C.M. Microplastic and other anthropogenic microparticles in water and sediments of Lake Simcoe. J. Great Lakes Res. 2021, 47, 180–189. [Google Scholar] [CrossRef]
- Bujaczek, T.; Kolter, S.; Locky, D.; Ross, M.S. Characterization of microplastics and anthropogenic fibers in surface waters of the North Saskatchewan River, Alberta, Canada. Facets 2021, 6, 26–43. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Bråte, I.L.N.; Hurley, R.; Iversen, K.; Beyer, J.; Thomas, K.V.; Steindal, C.C.; Green, N.W.; Olsen, M.; Lusher, A. Mytilus spp. as sentinels for monitoring microplastic pollution in Norwegian coastal waters: A qualitative and quantitative study. Environ. Pollut. 2018, 243, 383–393. [Google Scholar] [CrossRef] [PubMed]
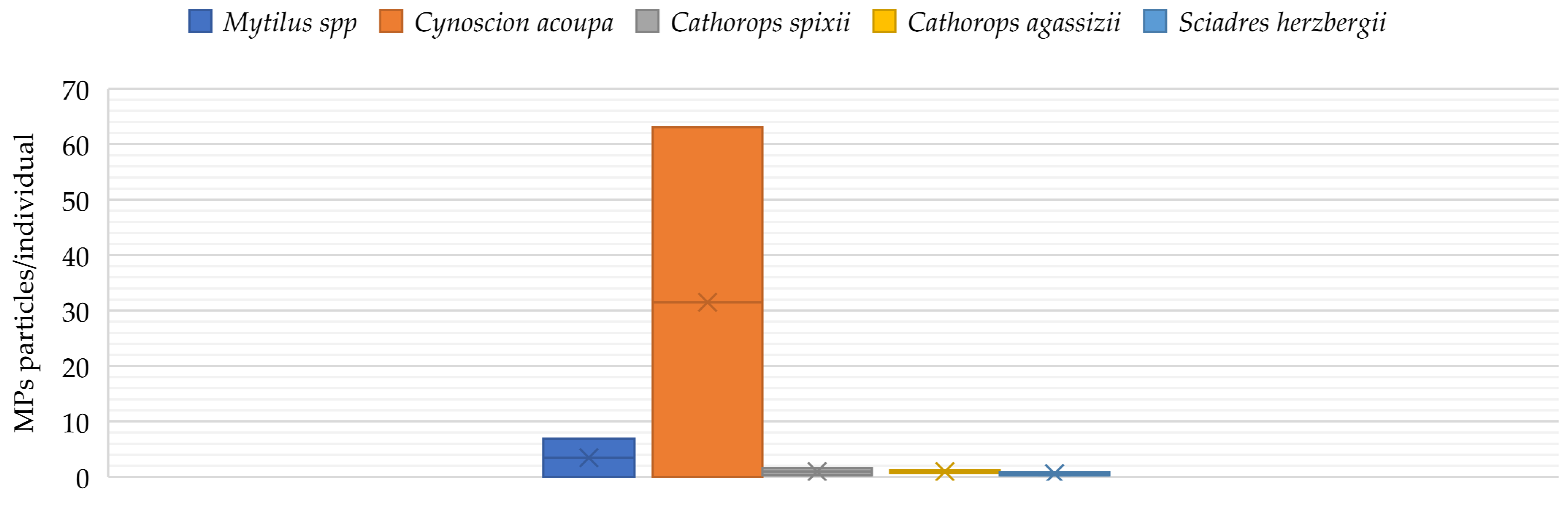
- Ferreira, G.V.B.; Barletta, M.; Lima, A.R.A.; Morley, S.A.; Justino, A.K.S.; Costa, M.F. High intake rates of microplastics in a Western Atlantic predatory fish, and insights of a direct fishery effect. Environ. Pollut. 2018, 236, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef]
- Possatto, F.E.; Barletta, M.; Costa, M.F.; do Sul, J.A.I.; Dantas, D.V. Plastic debris ingestion by marine catfish: An unexpected fisheries impact. Mar. Pollut. Bull. 2011, 62, 1098–1102. [Google Scholar] [CrossRef]
- Nadal, M.A.; Alomar, C.; Deudero, S. High levels of microplastic ingestion by the semipelagic fish bogue Boops boops (L.) around the Balearic Islands. Environ. Pollut. 2016, 214, 517–523. [Google Scholar] [CrossRef]
- Rummel, C.D.; Löder, M.G.J.; Fricke, N.F.; Lang, T.; Griebeler, E.M.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, K.; Hong, S.H.; Park, K.I.; Park, J.W. Impact of polyethylene terephthalate microfiber length on cellular responses in the Mediterranean mussel Mytilus galloprovincialis. Mar. Environ. Res. 2021, 168, 105320. [Google Scholar] [CrossRef]
- Spanjer, A.R.; Liedtke, T.L.; Conn, K.E.; Weiland, L.K.; Black, R.W.; Godfrey, N. Evidence for rapid gut clearance of microplastic polyester fibers fed to Chinook salmon: A tank study. Environ. Pollut. 2020, 265, 115083. [Google Scholar] [CrossRef]
- Kim, L.; Kim, S.A.; Kim, T.H.; Kim, J.; An, Y.J. Synthetic and natural microfibers induce gut damage in the brine shrimp Artemia franciscana. Aquat. Toxicol. 2021, 232, 105748. [Google Scholar] [CrossRef]
- Au, S.Y.; Bruce, T.F.; Bridges, W.C.; Klaine, S.J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wegner, A.; Foekema, E.M.; van den Heuvel-Greve, M.J.; Koelmans, A.A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowski, K.; Cholewińska, P.; Palić, D.; Bednarska, M.; Jarosz, M.; Wiśniewska, I. Estrogen Receptors Mediated Negative Effects of Estrogens and Xenoestrogens in Teleost Fishes—Review. Int. J. Mol. Sci. 2022, 23, 2605. [Google Scholar] [CrossRef] [PubMed]
- Lorite, G.S.; Rodrigues, C.M.; de Souza, A.A.; Kranz, C.; Mizaikoff, B.; Cotta, M.A. The role of conditioning film formation and surface chemical changes on Xylella fastidiosa adhesion and biofilm evolution. J. Colloid Interface Sci. 2011, 359, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.; Chen, T.; Zhou, Q.; Liu, Y.; Wei, J.; Waniek, J.J.; Luo, Y. Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci. Total Environ. 2020, 734, 139237. [Google Scholar] [CrossRef]
- Clutterbuck, A.L.; Woods, E.J.; Knottenbelt, D.C.; Clegg, P.D.; Cochrane, C.A.; Percival, S.L. Biofilms and their relevance to veterinary medicine. Vet. Microbiol. 2007, 121, 1–17. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restrepo-Flórez, J.M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene—A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90. [Google Scholar] [CrossRef]
- Rogers, J.; Dowsett, A.B.; Dennis, P.J.; Lee, J.V.; Keevil, C.W. Influence of plumbing materials on biofilm formation and growth of Legionella pneumophila in potable water systems. Appl. Environ. Microbiol. 1994, 60, 1842–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrish, K.; Fahrenfeld, N.L. Microplastic biofilm in fresh-and wastewater as a function of microparticle type and size class. Environ. Sci. Water Res. Technol. 2019, 5, 495–505. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Agents that inhibit bacterial biofilm formation. Future Med. Chem. 2015, 7, 647–671. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; DeBeer, D.; Caldwell, D.; Korber, D.; James, G. Biofilms the customized microniche. J. Bacteriol. 1994, 176, 2137–2142. [Google Scholar] [CrossRef] [Green Version]
- Zacheus, O.M.; Iivanainen, E.K.; Nissinen, T.K.; Lehtola, M.J.; Martikainen, P.J. Bacterial biofilm formation on polyvinyl chloride, polyethylene and stainless steel exposed to ozonated water. Water Res. 2000, 34, 63–70. [Google Scholar] [CrossRef]
- Dufour, D.; Leung, V.; Lévesque, C.M. Bacterial biofilm: Structure, function, and antimicrobial resistance. Endod. Top. 2010, 22, 2–16. [Google Scholar] [CrossRef]
- Rijnaarts, H.M.; Norde, W.; Bouwer, E.J.; Lyklema, J.; Zehnder, A.J. Bacterial adhesion under static and dynamic conditions. Appl. Environ. Microbiol. 1993, 59, 3255–3265. [Google Scholar] [CrossRef] [Green Version]
- Le Thi, T.T.; Prigent-Combaret, C.; Dorel, C.; Lejeune, P. [15] First stages of biofilm formation: Characterization and quantification of bacterial functions involved in colonization process. Methods Enzymol. 2001, 336, 152–159. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Xue, J. Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ. Sci. Technol. 2021, 55, 12780–12790. [Google Scholar] [CrossRef] [PubMed]
- Garnett, J.A.; Matthews, S. Interactions in bacterial biofilm development: A structural perspective. Curr. Protein Pept. Sci. 2012, 13, 739–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Reisser, J.; Shaw, J.; Hallegraeff, G.; Proietti, M.; Barnes, D.K.; Thums, M.; Wilcox, C.; Hardesty, B.D.; Pattiaratchi, C. Millimeter-sized marine plastics: A new pelagic habitat for microorganisms and invertebrates. PLoS ONE 2014, 9, e100289. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.J.; London, M.G.; Oforji, N.; Ogunsola, A.; Hoellein, T.J. Microplastic selects for convergent microbiomes from distinct riverine sources. Freshw. Sci. 2020, 39, 281–291. [Google Scholar] [CrossRef]
- McGivney, E.; Cederholm, L.; Barth, A.; Hakkarainen, M.; Hamacher-Barth, E.; Ogonowski, M.; Gorokhova, E. Rapid physicochemical changes in microplastic induced by biofilm formation. Front. Bioeng. Biotechnol. 2020, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Zhao, S.; Zhu, L.; Li, D. Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci. Total Environ. 2018, 624, 48–54. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Masó, M.; Garcés, E.; Pagès, F.; Camp, J. Drifting plastic debris as a potential vector for dispersing harmful algal bloom (HAB) species. Sci. Mar. 2003, 67, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Kesy, K.; Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Spatial environmental heterogeneity determines young biofilm assemblages on microplastics in Baltic Sea mesocosms. Front. Microbiol. 2019, 10, 1665. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Fu, Q.; Li, D.; Zhang, Y.; He, J.; Feng, D.; Zhao, Y.; Du, G.; Yu, H.; Ge, C. Microplastic-associated biofilm in an intensive mariculture pond: Temporal dynamics of microbial communities, extracellular polymeric substances and impacts on microplastics properties. J. Clean. Prod. 2021, 319, 128774. [Google Scholar] [CrossRef]
- Wu, X.; Pan, J.; Li, M.; Li, Y.; Bartlam, M.; Wang, Y. Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res. 2019, 165, 114979. [Google Scholar] [CrossRef] [PubMed]
- Viršek, M.K.; Lovšin, M.N.; Koren, Š.; Kržan, A.; Peterlin, M. Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar. Pollut. Bull. 2017, 125, 301–309. [Google Scholar] [CrossRef]
- Van der Meulen, M.D.; De Vriese, L.; Lee, J.; Maes, T.; Van Dalfsen, J.A.; Huvet, A.; Soudant, P.; Robbens, J.; Vethaak, A.D. Socio-Economic Impact of Microplastics in the 2 Seas, Channel and France Manche Region: An Initial Risk Assessment; MICRO Interreg project Iva: Wallonie, France, 2014. [Google Scholar]
- Tan, B.; Li, Y.; Xie, H.; Dai, Z.; Zhou, C.; Qian, Z.-J.; Hong, P.; Liang, Y.; Ren, L.; Sun, S.; et al. Microplastics accumulation in mangroves increasing the resistance of its colonization Vibrio and Shewanella. Chemosphere 2022, 295, 133861. [Google Scholar] [CrossRef]
- Sun, X.; Chen, B.; Xia, B.; Li, Q.; Zhu, L.; Zhao, X.; Gao, Y.; Qu, K. Impact of mariculture-derived microplastics on bacterial biofilm formation and their potential threat to mariculture: A case in situ study on the Sungo Bay, China. Environ. Pollut. 2020, 262, 114336. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, G.; Zhu, Q.; O’Connor, W.; Subashchandrabose, S.; Grainge, I.; Knight, R.; Palanisami, T. Exploring the composition and functions of plastic microbiome using whole-genome sequencing. Environ. Sci. Technol. 2021, 55, 4899–4913. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasunaga, N.; Yamamoto, N. Characteristics of bacterial strains isolated from so-called vibriosis of cultured red sea bream in the winter of 1977. Fish Pathol. 1977, 12, 209–214. [Google Scholar] [CrossRef]
- Gómez-León, J.; Villamil, L.; Lemos, M.L.; Novoa, B.; Figueras, A. Isolation of Vibrio alginolyticus and Vibrio splendidus from aquacultured carpet shell clam (Ruditapes decussatus) larvae associated with mass mortalities. Appl. Environ. Microbiol. 2005, 71, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Dussud, C.; Meistertzheim, A.; Conan, P.; Pujo-Pay, M.; George, M.; Fabre, P.; Coudane, J.; Higgs, P.; Elineau, A.; Pedrotti, M.; et al. Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters. Environ. Pollut. 2018, 236, 807–816. [Google Scholar] [CrossRef]
- Jones, M.K.; Oliver, J.D. Vibrio vulnificus: Disease and pathogenesis. Infect. Immun. 2009, 77, 1723–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.M.; Maldonado, G.C.; Castro, R.O.; de Sá Felizardo, J.; Cardoso, R.P.; Dos Anjos, R.M.; de Araújo, F.V. Dispersal of potentially pathogenic bacteria by plastic debris in Guanabara Bay, RJ, Brazil. Mar. Pollut. Bull. 2019, 141, 561–568. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Liu, T.; Chen, S.; Xiao, H. A metagenomic study of intestinal microbial diversity in relation to feeding habits of surface and cave-dwelling Sinocyclocheilus species. Microb. Ecol. 2020, 79, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Qin, G.; Luo, W.; Lin, Q. A novel pathogenic bacteria (Vibrio fortis) causing enteritis in cultured seahorses, Hippocampus erectus Perry, 1810. J. Fish Dis. 2016, 39, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Wang, Y.G.; Rong, X.J. Isolation and identification of causative pathogen for skin ulcerative syndrome in Apostichopus japonicus. J. Fish China 2006, 30, 118–123. [Google Scholar]
- Hou, D.; Hong, M.; Wang, Y.; Dong, P.; Cheng, H.; Yan, H.; Yao, Z.; Li, D.; Wang, K.; Zhang, D. Assessing the Risks of Potential Bacterial Pathogens Attaching to Different Microplastics during the Summer–Autumn Period in a Mariculture Cage. Microorganisms 2021, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Curren, E.; Leong, S.C.Y. Profiles of bacterial assemblages from microplastics of tropical coastal environments. Sci. Total Environ. 2019, 655, 313–320. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, L.; Jiang, P.; Wang, X.; Liu, K.; Li, C.; Li, D. Seasonal biofilm formation on floating microplastics in coastal waters of intensified marinculture area. Mar. Pollut. Bull. 2021, 171, 112914. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Wu, N.; Zhao, Z.; Xu, W.A.; Ma, Y.; Niu, Z. Colonization characteristics of bacterial communities on plastic debris influenced by environmental factors and polymer types in the Haihe Estuary of Bohai Bay, China. Environ. Sci. Technol. 2019, 53, 10763–10773. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Osborn, A.M.; Duhaime, M.B. Microbes on a bottle: Substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE 2016, 11, e0159289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lu, J.; Wu, J.; Wang, J.; Luo, Y. Potential risks of microplastics combined with superbugs: Enrichment of antibiotic resistant bacteria on the surface of microplastics in mariculture system. Ecotoxicol. Environ. Saf. 2020, 187, 109852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, X.; Dai, M.; Cen, J.; Zhou, L.; Xie, J. Microplastic pollution and its relationship with the bacterial community in coastal sediments near Guangdong Province, South China. Sci. Total Environ. 2021, 760, 144091. [Google Scholar] [CrossRef] [PubMed]
- Oberbeckmann, S.; Labrenz, M. Marine microbial assemblages on microplastics: Diversity, adaptation, and role in degradation. Annu. Rev. Mar. Sci. 2020, 12, 209–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colwell, R.R.; Kaper, J.; Joseph, S.W. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: Occurrence and distribution in Chesapeake Bay. Science 1977, 198, 394–396. [Google Scholar] [PubMed]
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic hitchhikers—Assessing pathogen risks from marine microplastic. Trends Microbiol. 2021, 29, 107–116. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Zhang, Y.; Wu, J.; Luo, Y. Unique bacterial community of the biofilm on microplastics in coastal water. Bull. Environ. Contam. Toxicol. 2021, 107, 597–601. [Google Scholar] [CrossRef]
- Xue, N.; Wang, L.; Li, W.; Wang, S.; Pan, X.; Zhang, D. Increased inheritance of structure and function of bacterial communities and pathogen propagation in plastisphere along a river with increasing antibiotics pollution gradient. Environ. Pollut. 2020, 265, 114641. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Q.; Wang, Y.; Zhang, J.; Xiong, D. Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol. 2018, 78, 279–288. [Google Scholar] [CrossRef]
- Talpur, A.D.; Memon, A.J.; Khan, M.I.; Ikhwanuddin, M.; Daniel, M.D.; Abol-Munafi, A.B. Pathogenicity and antibiotic sensitivity of pathogenic flora associated with the gut of blue swimming crab, Portunus pelagicus (Linnaeus, 1857). J. Anim. Vet. Adv. 2011, 10, 2106–2119. [Google Scholar] [CrossRef]
- Laganà, P.; Caruso, G.; Corsi, I.; Bergami, E.; Venuti, V.; Majolino, D.; La Ferla, R.; Azzaro, M.; Cappello, S. Do plastics serve as a possible vector for the spread of antibiotic resistance? First insights from bacteria associated to a polystyrene piece from King George Island (Antarctica). Int. J. Hyg. Environ. Health 2019, 222, 89–100. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, M.; Liu, Y.; Su, Y.; Xu, T.; Yu, M.; Zhang, X.H. Comparison of cultivable bacterial communities associated with Pacific white shrimp (Litopenaeus vannamei) larvae at different health statuses and growth stages. Aquaculture 2016, 451, 163–169. [Google Scholar] [CrossRef]
- Radisic, V.; Nimje, P.S.; Bienfait, A.M.; Marathe, N.P. Marine plastics from Norwegian west coast carry potentially virulent fish pathogens and opportunistic human pathogens harboring new variants of antibiotic resistance genes. Microorganisms 2020, 8, 1200. [Google Scholar] [CrossRef] [PubMed]
- Carballo, J.; Seoane, R.M.; Nieto, T.P. Adhesion of Aeromonas salmonicida to materials used in aquaculture. Bull.-Eur. Assoc. Fish Pathol. 2000, 20, 77–82. [Google Scholar]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Toranzo, A.E.; Magariños, B. Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: A review. Dis. Aquat. Org. 2006, 71, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, C.; Li, H.; Zhang, P.; Liu, X. The impact of microplastic-microbe interactions on animal health and biogeochemical cycles: A mini-review. Sci. Total Environ. 2021, 773, 145697. [Google Scholar] [CrossRef] [PubMed]
- MacEachern, D.; McCullough, J.; Duchin, J.; Tran, M.; MacDonald, K.; Marfin, A.; Jones, J.; Newton, A.; Tarr, C.; Talkington, D.; et al. Notes from the field: Vibrio mimicus infection from consuming crayfish-Spokane, Washington, June 2010. Morb. Mortal. Wkly. Rep. 2010, 59, 1374. [Google Scholar]
- Naik, R.K.; Naik, M.M.; D’Costa, P.M.; Shaikh, F. Microplastics in ballast water as an emerging source and vector for harmful chemicals, antibiotics, metals, bacterial pathogens and HAB species: A potential risk to the marine environment and human health. Mar. Pollut. Bull. 2019, 149, 110525. [Google Scholar] [CrossRef]
- Strom, M.S.; Paranjpye, R.N. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2000, 2, 177–188. [Google Scholar] [CrossRef]
- Colwell, R.R.; Grimes, D.J. Vibrio diseases of marine fish populations. Helgoländer Meeresunters. 1984, 37, 265–287. [Google Scholar] [CrossRef] [Green Version]
- Pumipuntu, N.; Indrawattana, N. Vibrio parahaemolyticus: A seafood-borne pathogen. J. Trop. Med. Parasitol. 2017, 40, 50–62. [Google Scholar]
- Ramamurthy, T.; Chowdhury, G.; Pazhani, G.P.; Shinoda, S. Vibrio fluvialis: An emerging human pathogen. Front. Microbiol. 2014, 5, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, V.T.; Reveillaud, J.; Zettler, E.; Mincer, T.J.; Murphy, L.; Amaral-Zettler, L.A. Oligotyping reveals community level habitat selection within the genus Vibrio. Front. Microbiol. 2014, 5, 563. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Oliver, D.M.; McCarron, A.; Quilliam, R.S. Colonisation of plastic pellets (nurdles) by E. coli at public bathing beaches. Mar. Pollut. Bull. 2019, 139, 376–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quilliam, R.S.; Jamieson, J.; Oliver, D.M. Seaweeds and plastic debris can influence the survival of faecal indicator organisms in beach environments. Mar. Pollut. Bull. 2014, 84, 201–207. [Google Scholar] [CrossRef]
- Pazos, R.S.; Suárez, J.C.; Gómez, N. Study of the plastisphere: Biofilm development and presence of faecal indicator bacteria on microplastics from the Río de la Plata estuary. Ecosistemas 2020, 29, 2069. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ü.N.; Başaran Kankılıç, G.; Akca, G.; Çırak, T.; Erdoğan, Ş. Microplastics in a dam lake in Turkey: Type, mesh size effect, and bacterial biofilm communities. Environ. Sci. Pollut. Res. Int. 2020, 27, 45688–45698. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kavvadia, P.K.; Mantadakis, E.; Kofteridis, D.P.; Bliziotis, I.A.; Saloustros, E.; Maraki, S.; Samonis, G. Morganella morganii infections in a general tertiary hospital. Infection 2006, 34, 315–321. [Google Scholar] [CrossRef]
- Nemec, A.; Musílek, M.; Maixnerová, M.; De Baere, T.; Van Der Reijden, T.J.K.; Vaneechoutte, M.; Dijkshoorn, L. Acinetobacter beijerinckii sp. Nov. and Acinetobacter gyllenbergii sp. nov., haemolytic organisms isolated from humans. Int. J. Syst. Evol. Microbiol. 2009, 59, 118–124. [Google Scholar] [CrossRef]
- Harrison, J.P.; Schratzberger, M.; Sapp, M.; Osborn, A.M. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef] [Green Version]
- Shariff, M.; Beri, K. Exacerbation of bronchiectasis by Pseudomonas monteilii: A case report. BMC Infect. Dis. 2017, 17, 511. [Google Scholar] [CrossRef] [Green Version]
- Gani, M.; Rao, S.; Miller, M.; Scoular, S. Pseudomonas mendocina bacteremia: A case study and review of literature. Am. J. Case Rep. 2019, 20, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Holt, H.M.; Gahrn-Hansen, B.; Bruun, B. Shewanella algae and Shewanella putrefaciens: Clinical and microbiological characteristics. Clin. Microbiol. Infect. 2005, 11, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgson, D.J.; Bréchon, A.L.; Thompson, R.C. Ingestion and fragmentation of plastic carrier bags by the amphipod Orchestia gammarellus: Effects of plastic type and fouling load. Mar. Pollut. Bull. 2018, 127, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Vroom, R.J.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef]
- Fabra, M.; Williams, L.; Watts, J.E.; Hale, M.S.; Couceiro, F.; Preston, J. The plastic Trojan horse: Biofilms increase microplastic uptake in marine filter feeders impacting microbial transfer and organism health. Sci. Total Environ. 2021, 797, 149217. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Li, M.; Cai, Z.; Gong, H.; Yan, M. Microplastics as an aquatic pollutant affect gut microbiota within aquatic animals. J. Hazard. Mater. 2022, 423, 127094. [Google Scholar] [CrossRef]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.; Fu, Z.; Jin, Y. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef]
- Fackelmann, G.; Sommer, S. Microplastics and the gut microbiome: How chronically exposed species may suffer from gut dysbiosis. Mar. Pollut. Bull. 2019, 143, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef] [PubMed]
- von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; D’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as vehicles of environmental PAHs to marine organisms: Combined chemical and physical hazards to the Mediterranean mussels, Mytilus galloprovincialis. Front. Mar. Sci. 2018, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Li, W.; Chen, X.; He, Y.; Zhang, X.; Gong, H. A preliminary study of the association between colonization of microorganism on microplastics and intestinal microbiota in shrimp under natural conditions. J. Hazard. Mater. 2021, 408, 124882. [Google Scholar] [CrossRef]
- Masó, M.; Fortuño, J.M.; de Juan, S.; Demestre, M. Microfouling communities from pelagic and benthic marine plastic debris sampled across Mediterranean coastal waters. Sci. Mar. 2016, 80, 117–127. [Google Scholar] [CrossRef] [Green Version]
| Group | Selected Taxa | Reference |
|---|---|---|
| Bacteria | Sinobacteraceae, Burkholderiales, Alphaproteobacteria, Sphingomonas, Verrucomicrobia, Gammaproteobacteria, Opitutae, Saprospispiraceae, Rhizobiales, Novosphingobium, Aquabacterium, Acinetobacter, Xanthobacteraceae, Azospirillum, Acinetobacter, Sinobacteraceae | [77,78,80] |
| Diatoms | Amphora, Achanathes, Cymbella, Grammatophora, Haslea, Licophora, Microtabella, Minidiscus, Thalassionema, Thalassiosira, Chaetoceros, Mastogloia, Navicula, Nitzschia, Sellaphora, Strauroneis | [80] |
| Coccoliths | Calcidiscus, Emiliania, Gephyrocapsa, Umbellospharea, Umbilicospharea, Coccolithus, Calciosolenia | [77] |
| Insect eggs | Halobates | [77] |
| Barnacles | Lepas | [77] |
| Dinoflagellates | Alexandrium, Ceratium | [80] |
| Ciliates | Ephelota | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholewińska, P.; Moniuszko, H.; Wojnarowski, K.; Pokorny, P.; Szeligowska, N.; Dobicki, W.; Polechoński, R.; Górniak, W. The Occurrence of Microplastics and the Formation of Biofilms by Pathogenic and Opportunistic Bacteria as Threats in Aquaculture. Int. J. Environ. Res. Public Health 2022, 19, 8137. https://doi.org/10.3390/ijerph19138137
Cholewińska P, Moniuszko H, Wojnarowski K, Pokorny P, Szeligowska N, Dobicki W, Polechoński R, Górniak W. The Occurrence of Microplastics and the Formation of Biofilms by Pathogenic and Opportunistic Bacteria as Threats in Aquaculture. International Journal of Environmental Research and Public Health. 2022; 19(13):8137. https://doi.org/10.3390/ijerph19138137
Chicago/Turabian StyleCholewińska, Paulina, Hanna Moniuszko, Konrad Wojnarowski, Przemysław Pokorny, Natalia Szeligowska, Wojciech Dobicki, Ryszard Polechoński, and Wanda Górniak. 2022. "The Occurrence of Microplastics and the Formation of Biofilms by Pathogenic and Opportunistic Bacteria as Threats in Aquaculture" International Journal of Environmental Research and Public Health 19, no. 13: 8137. https://doi.org/10.3390/ijerph19138137
APA StyleCholewińska, P., Moniuszko, H., Wojnarowski, K., Pokorny, P., Szeligowska, N., Dobicki, W., Polechoński, R., & Górniak, W. (2022). The Occurrence of Microplastics and the Formation of Biofilms by Pathogenic and Opportunistic Bacteria as Threats in Aquaculture. International Journal of Environmental Research and Public Health, 19(13), 8137. https://doi.org/10.3390/ijerph19138137