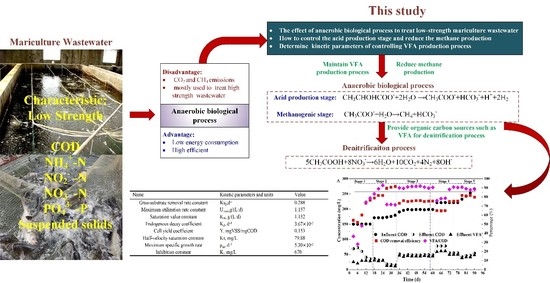
The Control Strategy and Kinetics of VFAs Production in an ASBR Reactor Treating Low-Strength Mariculture Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Configuration and Operation of ASBR
2.2. Seeding Sludge and Synthetic Wastewater
2.3. Analytical Methods
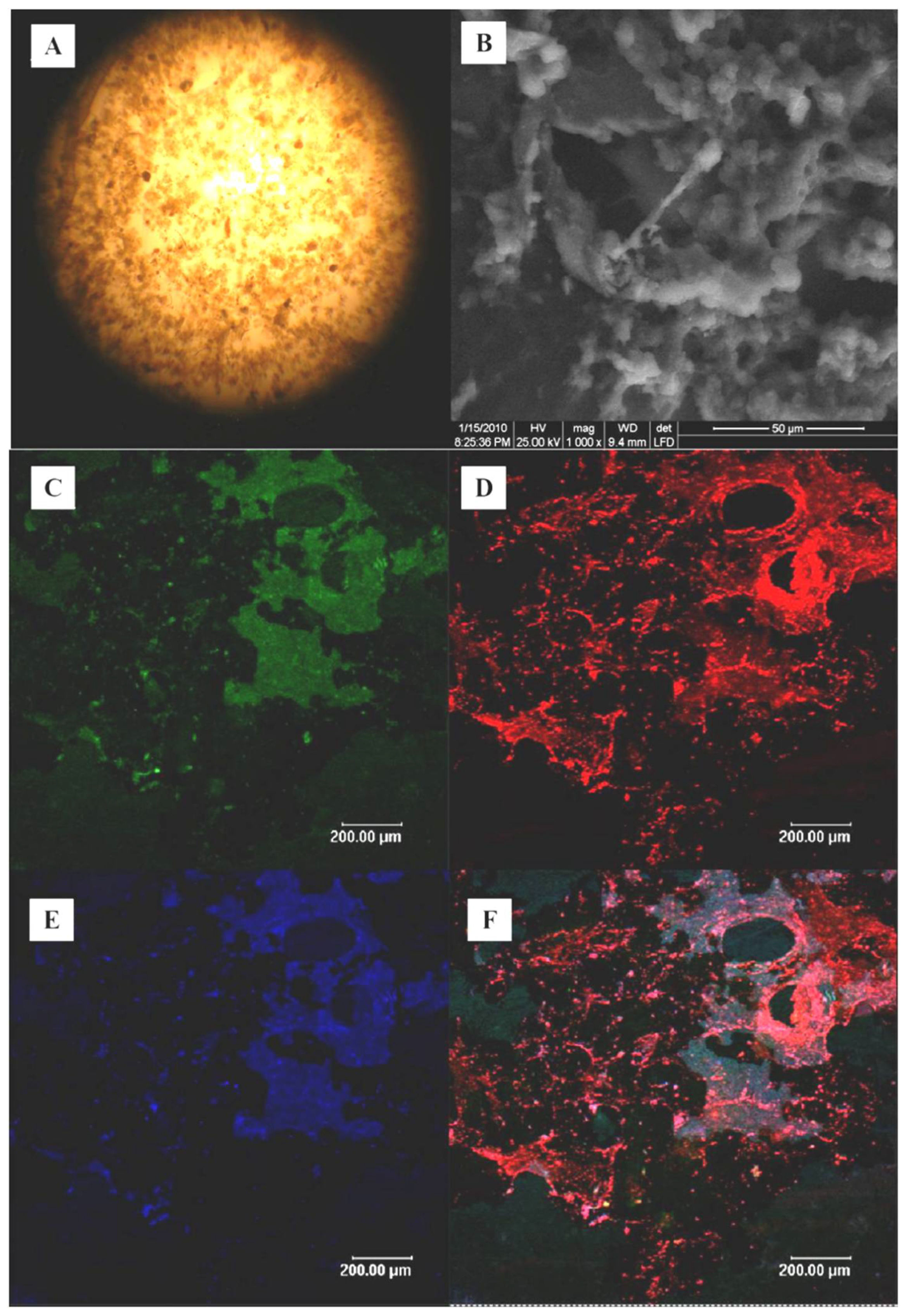
2.4. SEM Analysis and FISH Analysis
2.5. Model Description
3. Results and Discussion
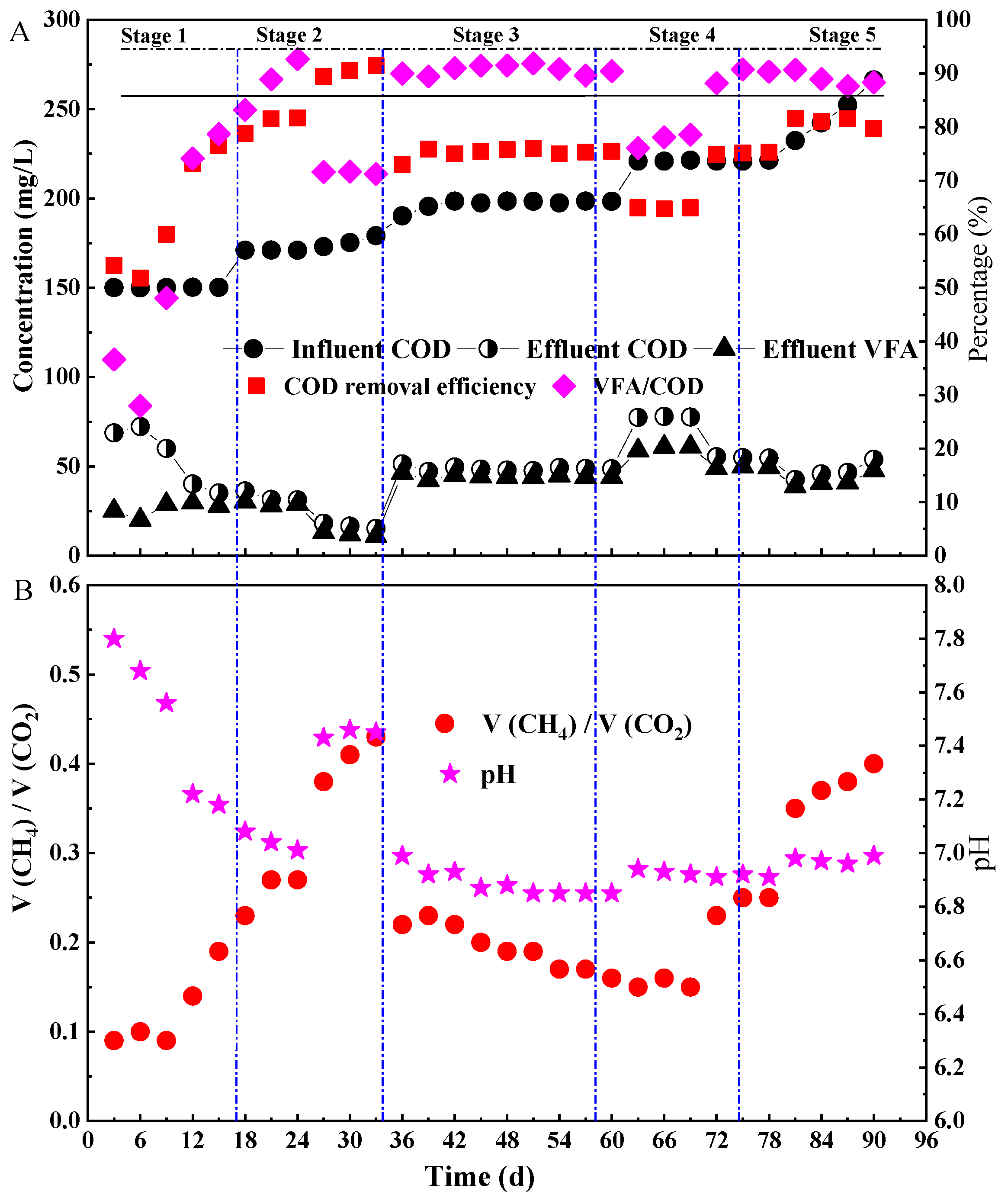
3.1. The Performance of ASBR
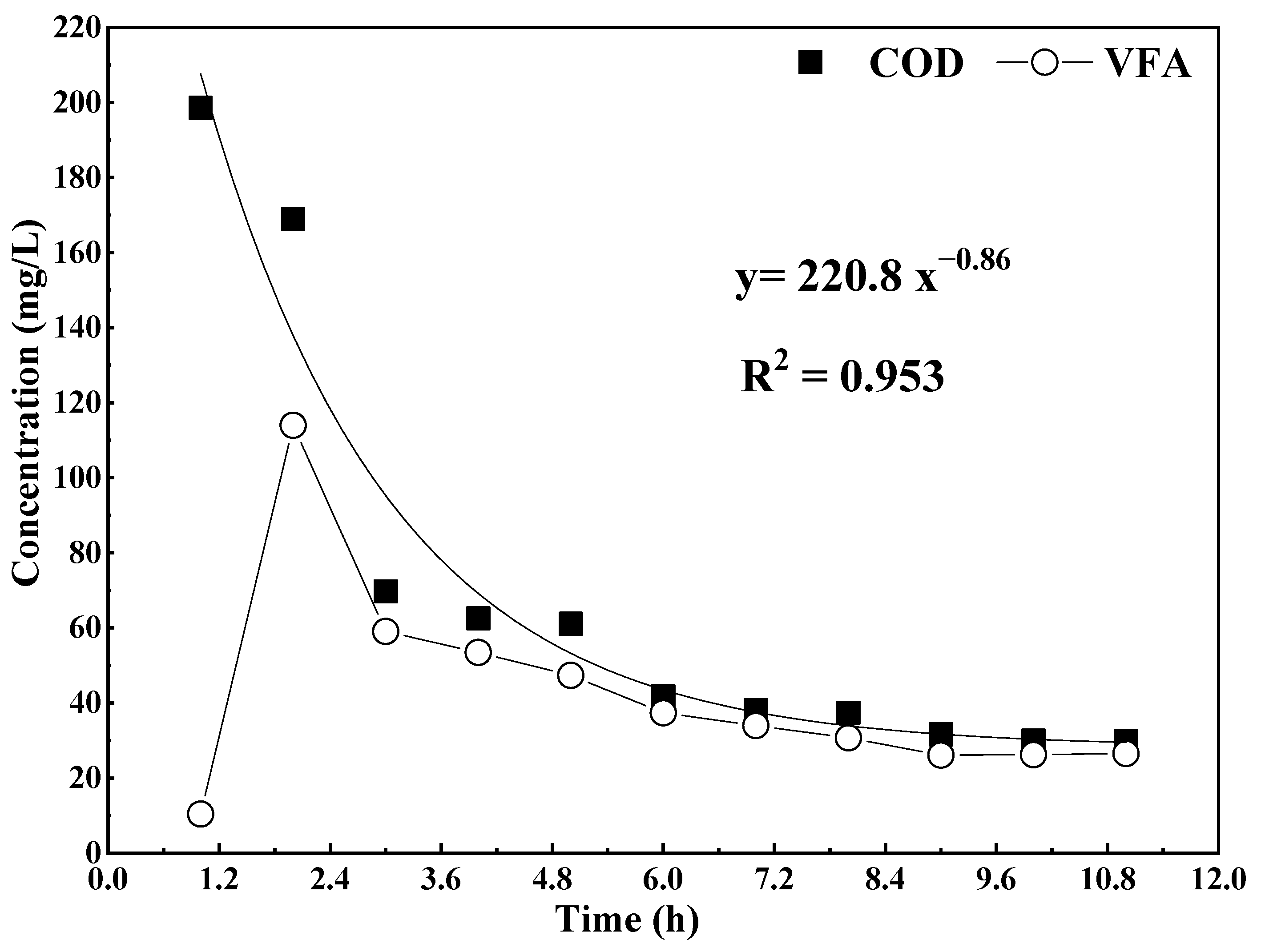
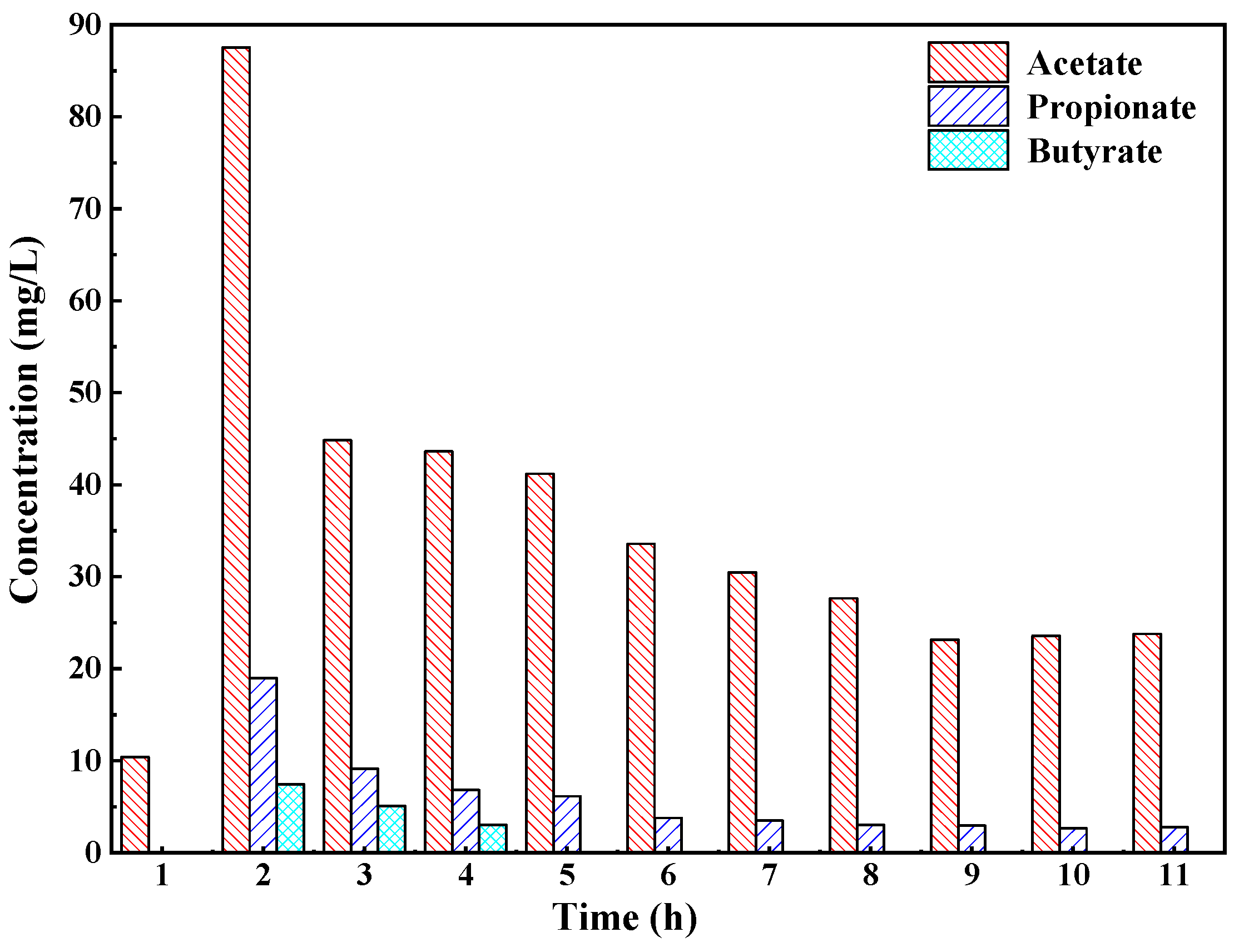
3.2. Cyclic Concentration Profiles of COD and VFA in One ASBR Cycle
3.3. SEM and FISH Analysis
3.4. Kinetics of Anaerobic Fermentation Reaction Treating Low-Strength Mariculture Wastewater
4. Conclusions
- (1)
- The ASBR was successfully started under a low organic load, and the decarbonization effect of the reactor reached more than 75%. The influent COD concentration and HRT were the key factors affecting the VFA accumulation in the ASBR. The VFA accumulation could be realized by increasing the influent COD concentration and shortening the hydraulic retention time.
- (2)
- The reaction order of the anaerobic biological reaction was approximately 2 in this experiment. The VFA accumulation mainly occurred in the first 3 h, and the VFA accumulation reached the maximum in the 2nd hour. The main components of VFA in the reactor were acetate, propionate and butyrate. In the later stage of the reaction, only acetate and propionate existed in the reactor, and their contents accounted for more than 95% of the VFA. The FISH results showed that acidogenic bacteria and methanogens coexisted in the ASBR. The proportions of acidogenic bacteria and methanogens were 39.7% and 46.5%, respectively.
- (3)
- The experimental data of the reactor were simulated using the Grau second-order substrate removal model, Stover–Kincannon model, Monod model and Haldane model, and the kinetic parameters of the anaerobic fermentation reaction treating low organic load wastewater were obtained as the Grau substrate removal rate constant KS2 = 0.288 d−1, maximum rate removal constant Umax = 1.157 g/(L·d), saturation constant KB = 1.132 g/(L·d), endogenous decay coefficient Kd = 3.67 × 10−3 d−1, sludge yield coefficient Y = 0.153 mgVSS/mgCOD and inhibition constant Ki = 670 mg/L.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, L.R.; Clavelle, T.; Klinger, D.H.; Lester, S.E. The ecological and economic potential for offshore mariculture in the Caribbean. Nat. Sustain. 2019, 2, 62–70. [Google Scholar] [CrossRef]
- Ni, M.; Yuan, J.; Hua, J.; Lian, Q.; Guo, A.; Liu, M.; Xin, J.; Wang, H.; Gu, Z. Shrimp–vegetable rotational farming system: An innovation of shrimp aquaculture in the tidal flat ponds of Hangzhou Bay, China. Aquaculture 2020, 518, 734864. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, H.; Junaid, M.; Xu, N.; Zhu, Y.; Tao, H.; Wong, M. Spatiotemporal distribution, source apportionment and risk assessment of typical hormones and phenolic endocrine disrupting chemicals in environmental and biological samples from the mariculture areas in the Pearl River Delta, China. Sci. Total Environ. 2022, 807, 150752. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cuthbertson, A.; Gualtieri, C.; Shao, D. A Review on Mariculture Effluent: Characterization and Management Tools. Water 2020, 12, 2991. [Google Scholar] [CrossRef]
- Shi, R.; Han, T.; Xu, S.; Huang, H.; Qi, Z.; Zhu, Q. Bacterial community responses to the redox profile changes of mariculture sediment. Mar. Pollut. Bull. 2021, 166, 112250. [Google Scholar] [CrossRef]
- Song, W.; Li, Z.; Ding, Y.; Liu, F.; You, H.; Qi, P.; Wang, F.; Li, Y.; Jin, C. Performance of a novel hybrid membrane bioreactor for treating saline wastewater from mariculture: Assessment of pollutants removal and membrane filtration performance. Chem. Eng. J. 2018, 331, 695–703. [Google Scholar] [CrossRef]
- Junior, A.P.B.; Flickinger, D.L.; Henry-Silva, G.G. Sedimentation rates of nutrients and particulate material in pond mariculture of shrimp (Litopenaeus vannamei) carried out with different management strategies. Aquaculture 2021, 534, 736307. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, L.; Jin, C.; Zhao, Y.; Gao, M.; She, Z.; Wang, G. Metagenomics and Network Analysis Elucidating the Coordination between Fermentative Bacteria and Microalgae in a Novel Bacterial-Algal Coupling Reactor (BACR) for Mariculture Wastewater Treatment. Water Res. 2022, 215, 118256. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, L.; Shao, M.; Hu, F.; Wang, G.; Zhao, Y.; Gao, M.; Jin, C.; She, Z. Denitrification performance evaluation and kinetics analysis with mariculture solid wastes (MSW) derived carbon source in marine recirculating aquaculture systems (RAS). Bioresour. Technol. 2020, 313, 123649. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.-h.; Chen, X.-j.; Jia, L.-q.; Guo, X.-n.; Chen, R.-s.; Zhang, M.-s.; Chen, Z.-y.; Wang, H.-d. Carbon peak and carbon neutrality in China: Goals, implementation path and prospects. China Geol. 2021, 4, 720–746. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, L.; Shao, M.; Hu, F.; Wang, G.; Zhao, Y.; Gao, M.; Jin, C.; She, Z. Heterotrophic denitrification strategy for marine recirculating aquaculture wastewater treatment using mariculture solid wastes fermentation liquid as carbon source: Optimization of COD/NO3−-N ratio and hydraulic retention time. Bioresour. Technol. 2020, 304, 122982. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhao, C.; Wang, Q.; Lu, S.; She, Z.; Zhao, Y.; Jin, C.; Guo, L.; Li, K.; Gao, M. Impact of carbon/nitrogen ratio on the performance and microbial community of sequencing batch biofilm reactor treating synthetic mariculture wastewater. J. Environ. Manag. 2021, 298, 113528. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, L.; Gao, P.; Yu, D.; Yao, Z.; Gao, M.; Zhao, Y.; Jin, C.; She, Z. Thermophilic bacteria combined with alkyl polyglucose pretreated mariculture solid wastes using as denitrification carbon source for marine recirculating aquaculture wastewater treatment. Sci. Total Environ. 2021, 792, 148447. [Google Scholar] [CrossRef]
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour. Technol. 2020, 302, 122800. [Google Scholar] [CrossRef]
- Li, C.; Cao, J.; Ren, H.; Li, Y.; Tang, S. Comparison on kinetics and microbial community among denitrification process fed by different kinds of volatile fatty acids. Process Biochem. 2015, 50, 447–455. [Google Scholar] [CrossRef]
- Hou, L.; Griswold, N.; Hu, Z. Impact of decreasing hydraulic retention times on the specific affinity of methanogens and their community structures in an anaerobic membrane bioreactor process treating low strength wastewater. Sci. Total Environ. 2020, 739, 140373. [Google Scholar] [CrossRef]
- Pereira, E.L.; Borges, A.C.; Da Silva, G.J. Effect of the Progressive Increase of Organic Loading Rate in an Anaerobic Sequencing Batch Reactor for Biodiesel Wastewater Treatment. Water 2022, 14, 223. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Amann, R.I.; Krumholz, L.; Stahl, D.A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 1990, 172, 762–770. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Mazéas, L.; Sghir, A.; Leblon, G.; Bouchez, T. Insights into networks of functional microbes catalysing methanization of cellulose under mesophilic conditions. Environ. Microbiol. 2009, 11, 889–904. [Google Scholar] [CrossRef]
- Raskin, L.; Stromley, J.M.; Rittmann, B.E.; Stahl, D.A. Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl. Environ. Microbiol. 1994, 60, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Wang, Z.; Zhou, Y.; Ng, W.J. The role of hydrogenotrophic methanogens in an acidogenic reactor. Chemosphere 2015, 140, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, J.; Sui, Q.; Wei, Y. Biogas-pH automation control strategy for optimizing organic loading rate of anaerobic membrane bioreactor treating high COD wastewater. Bioresour. Technol. 2016, 203, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Harb, R.; Laçin, D.; Subaşı, I.; Erguder, T.H. Denitrifying anaerobic methane oxidation (DAMO) cultures: Factors affecting their enrichment, performance and integration with anammox bacteria. J. Environ. Manag. 2021, 295, 113070. [Google Scholar] [CrossRef] [PubMed]
- Russ, L.; Harhangi, H.R.; Schellekens, J.; Verdellen, B.; Kartal, B.; Op den Camp, H.J.M.; Jetten, M.S.M. Genome analysis and heterologous expression of acetate-activating enzymes in the anammox bacterium Kuenenia stuttgartiensis. Arch. Microbiol. 2012, 194, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-L.; Gao, D.-W.; Tao, Y.; Wang, X.-L. C2/C3 fatty acid stress on anammox consortia dominated by Candidatus Jettenia asiatica. Chem. Eng. J. 2014, 253, 402–407. [Google Scholar] [CrossRef]
- Wandera, S.M.; Westerholm, M.; Qiao, W.; Yin, D.; Jiang, M.; Dong, R. The correlation of methanogenic communities’ dynamics and process performance of anaerobic digestion of thermal hydrolyzed sludge at short hydraulic retention times. Bioresour. Technol. 2019, 272, 180–187. [Google Scholar] [CrossRef]
- Benyahya, Y.; Fail, A.; Alali, A.; Sadik, M. Recovery of Household Waste by Generation of Biogas as Energy and Compost as Bio-Fertilizer—A Review. Processes 2022, 10, 81. [Google Scholar] [CrossRef]
- Işik, M.; Sponza, D.T. Substrate removal kinetics in an upflow anaerobic sludge blanket reactor decolorising simulated textile wastewater. Process Biochem. 2005, 40, 1189–1198. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Hashemi, H.; Eslami, H.; Fallahzadeh, R.A.; Khosravi, R.; Askari, R.; Ghahramani, E. Kinetics of biogas production and chemical oxygen demand removal from compost leachate in an anaerobic migrating blanket reactor. J. Environ. Manag. 2018, 206, 707–714. [Google Scholar] [CrossRef]
- Pavlostathis, S.G.; Giraldogomez, E. Kinetic of anaerobic treatment. Water Sci. Technol. 1991, 24, 35–59. [Google Scholar] [CrossRef]
- Lokshina, L.Y.; Vavilin, V.A.; Kettunen, R.H.; Rintala, J.A.; Holliger, C.; Nozhevnikova, A.N. Evaluation of kinetic coefficients using integrated monod and haldane models for low-temperature acetoclastic methanogenesis. Water Res. 2001, 35, 2913–2922. [Google Scholar] [CrossRef]
- Fukuzaki, S.; Nishio, N.; Nagai, S. Kinetics of the methanogenic fermentation of acetate. Appl. Environ. Microbiol. 1990, 56, 3158–3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
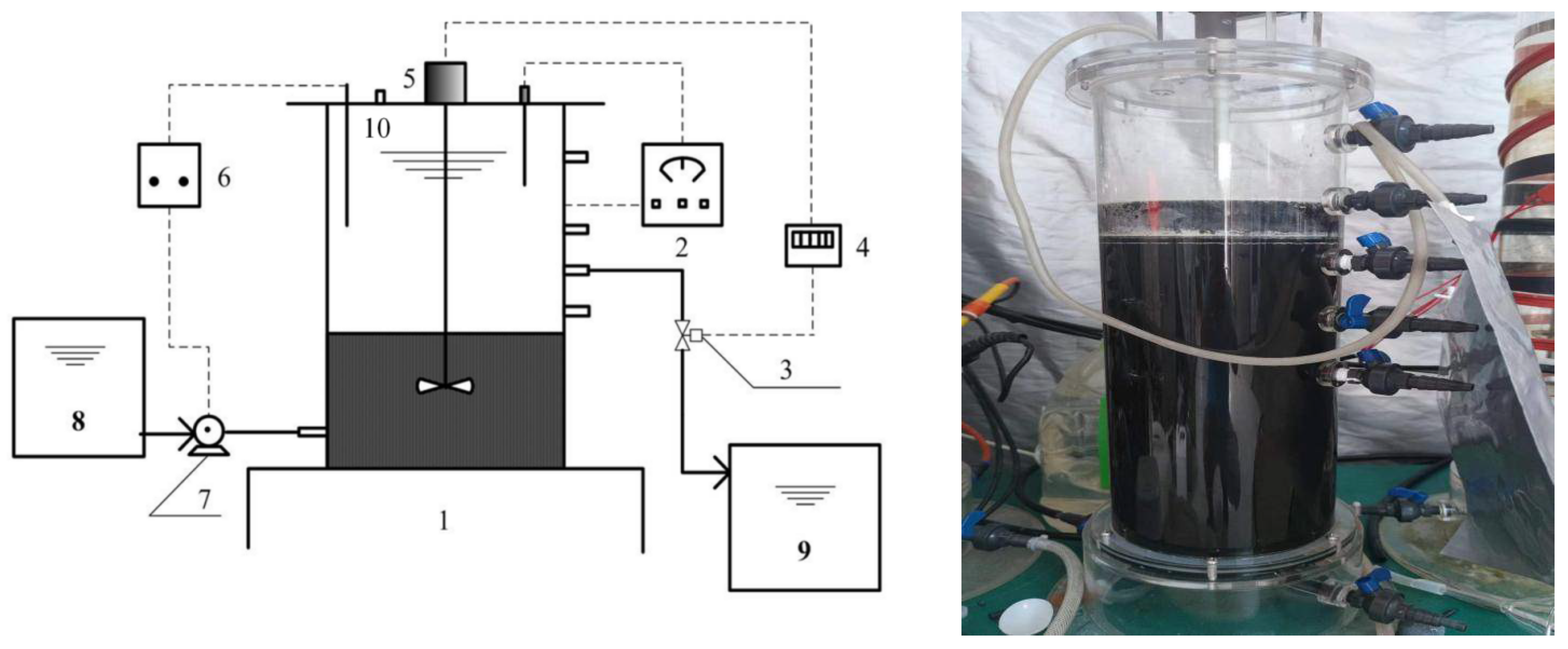
| HRT (d) | Feeding Time (h) | Fermentation Time (h) | Sedimentation Time (h) | Discharge Time (h) |
|---|---|---|---|---|
| 0.387 | 0.5 | 7 | 2 | 0.5 |
| 0.465 | 0.5 | 9 | 2 | 0.5 |
| 0.697 | 0.5 | 13 | 2 | 0.5 |
| 0.852 | 0.5 | 18 | 2 | 0.5 |
| Probe Name | Formamide(%) | Fluorescent Dye | Color | Excitation and Emission Wavelength (nm) |
|---|---|---|---|---|
| EUB338 | 20 | FITC | Green | 488, 528 |
| MS1414 | 20 | Cy3 | Red | 543, 570 |
| BAC307 | 40 | Cy3 | Purple | 630, 670 |
| Model Name | Mathematical Equations | Parameters Meaning |
|---|---|---|
| Grau-2 substrate removal | Si, Se: Influent and effluent substrate concentration (mg/L), respectively X: Biomass concentration in a reactor (mg/L) Ks2: Grau substrate removal rate constant (d−1) | |
| Stover–Kincannon | Umax: The maximum utilization rate constant g/(L·d) KB: The saturation value constant g/(L·d) Q: Flow rate of influent wastewater (L/d) | |
| Monod | X0, Xe: Biomass concentration of influent and effluent wastewater (mg/L), respectively Vb: Volume of sludge bed (L) μ: Specific growth rate (d−1) Kd: Endogenous decay coefficient (d−1) Ks: Half-velocity saturation constant (mg/L) Y: Cell yield coefficient (mg VSS/mg COD) μm: Maximum specific growth rate (d−1) | |
| Haldane | Ki: Inhibition constant (mg/L) |
| Items | Kinetic Parameters and Units | Value | Fitting Results | Correlation Coefficient R2 | Model Name |
|---|---|---|---|---|---|
| Grau substrate removal rate constant | Ks2, d−1 | 0.288 | Y = 1.124X + 0.094 | 0.983 | Grau-2 |
| Maximum utilization rate constant | Umax, g/(L·d) | 1.157 | Y = 0.978X + 8.64 × 10−4 | 0.985 | Stover–Kincannon |
| Saturation value constant | KB, g/(L·d) | 1.132 | Stover–Kincannon | ||
| Endogenous decay coefficient | Kd, d−1 | 3.67 × 10−3 | Y = 6.438X + 0.024 | 0.835 | Monod |
| Cell yield coefficient | Y, mgVSS/mgCOD | 0.153 | Monod | ||
| Half-velocity saturation constant | Ks, mg/L | 79.88 | Monod | ||
| Maximum specific growth rate | μm, d−1 | 5.30 × 10−2 | Monod | ||
| Inhibition constant | Ki, mg/L | 670 | Y = 0.007X2 + 4.69X + 134.7 | 0.852 | Haldane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Zhang, C.; Sun, Q.; Xu, G. The Control Strategy and Kinetics of VFAs Production in an ASBR Reactor Treating Low-Strength Mariculture Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 7858. https://doi.org/10.3390/ijerph19137858
Gao F, Zhang C, Sun Q, Xu G. The Control Strategy and Kinetics of VFAs Production in an ASBR Reactor Treating Low-Strength Mariculture Wastewater. International Journal of Environmental Research and Public Health. 2022; 19(13):7858. https://doi.org/10.3390/ijerph19137858
Chicago/Turabian StyleGao, Fan, Cuiya Zhang, Qinbang Sun, and Guangjing Xu. 2022. "The Control Strategy and Kinetics of VFAs Production in an ASBR Reactor Treating Low-Strength Mariculture Wastewater" International Journal of Environmental Research and Public Health 19, no. 13: 7858. https://doi.org/10.3390/ijerph19137858
APA StyleGao, F., Zhang, C., Sun, Q., & Xu, G. (2022). The Control Strategy and Kinetics of VFAs Production in an ASBR Reactor Treating Low-Strength Mariculture Wastewater. International Journal of Environmental Research and Public Health, 19(13), 7858. https://doi.org/10.3390/ijerph19137858