Epidemiologic Implication of the Association between Herpes Simplex Virus Infection and the Risk of Type 1 Diabetes Mellitus: A Nationwide Case-Control Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
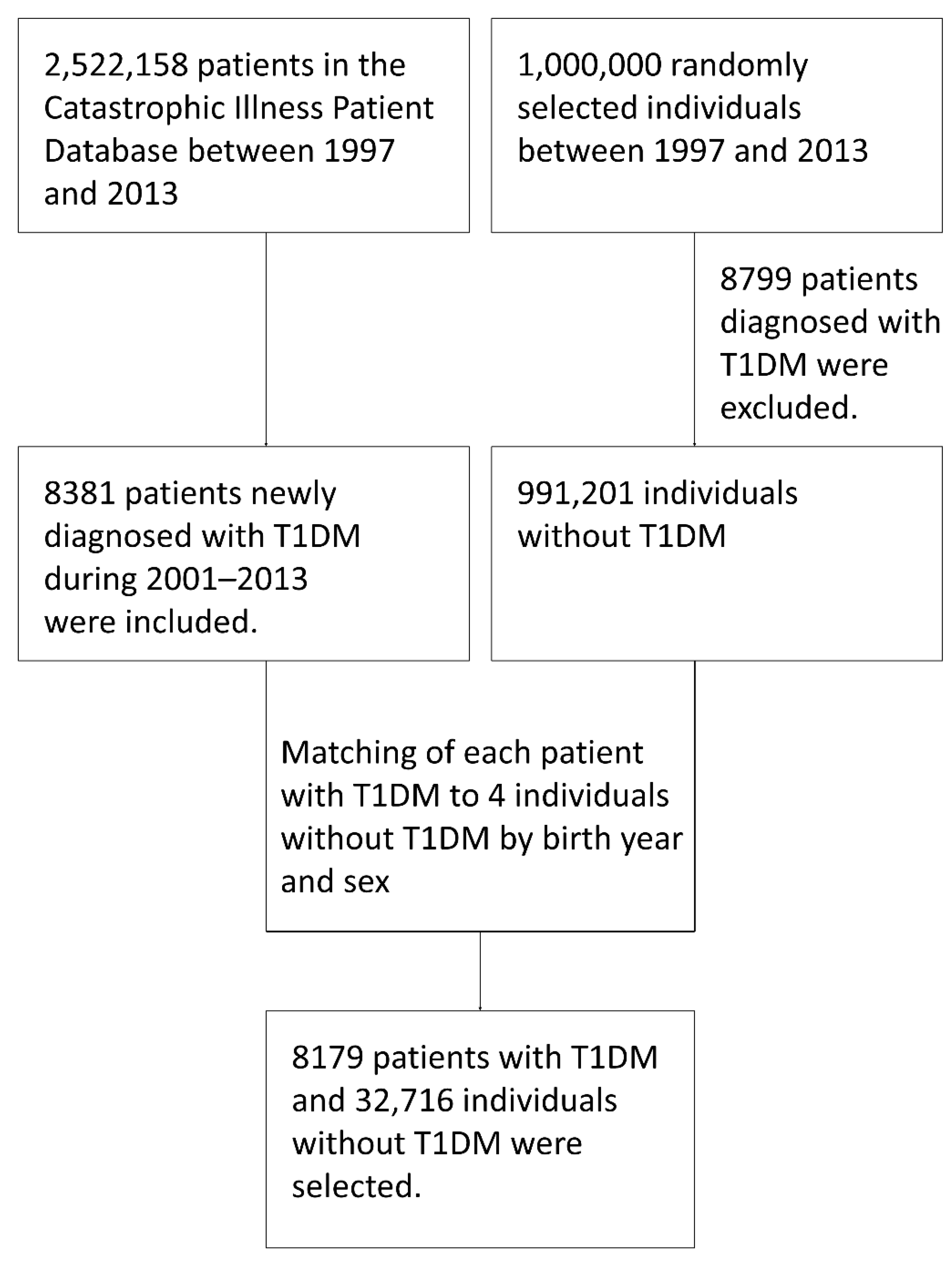
2.1. Subjects and Database Source
2.2. Study Design
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. ICD-9-CM Codes Used to Identify Diagnoses of Type 1 Diabetes Mellitus, History of Human Herpesvirus Infection, and Comorbidities
| Diagnosis Code | Description |
|---|---|
| 250.01 | Diabetes mellitus without mention of complication, Type I [juvenile type], not stated as uncontrolled |
| 250.03 | Diabetes mellitus without mention of complication, Type I [juvenile type], uncontrolled |
| 250.11 | Diabetes with ketoacidosis, Type I [juvenile type], not stated as |
| 250.13 | Diabetes with ketoacidosis, Type I [juvenile type], uncontrolled |
| 250.21 | Diabetes with hyperosmolar coma, Type I [juvenile type], not stated as |
| 250.23 | Diabetes with hyperosmolar coma, Type I [juvenile type], uncontrolled |
| 250.31 | Diabetes with other coma, Type I [juvenile type], not stated as uncontrolled |
| 250.33 | Diabetes with other coma, Type I [juvenile type], uncontrolled |
| 250.41 | Diabetes with renal manifestations, Type I [juvenile type], not stated as |
| 250.43 | Diabetes with renal manifestations, Type I [juvenile type], uncontrolled |
| 250.51 | Diabetes with ophthalmic manifestations, Type I [juvenile type], not stated as uncontrolled |
| 250.53 | Diabetes with ophthalmic manifestations, Type I [juvenile type], uncontrolled |
| 250.61 | Diabetes with neurological manifestations, Type I [juvenile type], not stated as uncontrolled |
| 250.63 | Diabetes with neurological manifestations, Type I [juvenile type], uncontrolled |
| 250.71 | Diabetes with peripheral circulatory disorders, Type I [juvenile type], not stated as uncontrolled |
| 250.73 | Diabetes with peripheral circulatory disorders, Type I [juvenile type], uncontrolled |
| 250.81 | Diabetes with other specified manifestations, Type I [juvenile type], not stated as uncontrolled |
| 250.83 | Diabetes with other specified manifestations, Type I [juvenile type], uncontrolled |
| 250.91 | Diabetes with unspecified complication, Type I [juvenile type], not stated as uncontrolled |
| 250.93 | Diabetes with unspecified complication, Type I [juvenile type], uncontrolled |
| Diagnosis Code | Description |
|---|---|
| Varicella zoster virus, varicella | |
| 052 | Chickenpox |
| 052.0 | Postvaricella encephalitis |
| 052.1 | Varicella (hemorrhagic) pneumonitis |
| 052.7 | Chickenpox with other specified complications |
| 052.8 | Chickenpox with unspecified complication |
| 052.9 | Varicella without mention of complication |
| Varicella zoster virus, zoster | |
| 053 | Herpes zoster |
| 053.0 | Herpes zoster with meningitis |
| 053.1 | Herpes zoster with other nervous system complications |
| 053.10 | Herpes zoster with unspecified nervous system complication |
| 053.11 | Geniculate herpes zoster |
| 053.12 | Postherpetic trigeminal neuralgia |
| 053.13 | Postherpetic polyneuropathy |
| 053.19 | Herpes zoster with other nervous system complications |
| 053.2 | Herpes zoster with ophthalmic complications |
| 053.20 | Herpes zoster dermatitis of eyelid |
| 053.21 | Herpes zoster keratoconjunctivitis |
| 053.22 | Herpes zoster iridocyclitis |
| 053.29 | Herpes zoster with other ophthalmic complication |
| 053.7 | Herpes zoster with other complications |
| 053.71 | Otitis externa due to herpes zoster |
| 053.79 | Herpes zoster with other specified complications |
| 053.8 | Herpes zoster with unspecified complication |
| 053.9 | Herpes zoster without mention of complication |
| Herpes simplex virus | |
| 054 | Herpes simplex |
| 054.0 | Eczema herpeticum |
| 054.1 | Genital herpes |
| 054.10 | Genital herpes, unspecified |
| 054.11 | Herpetic vulvovaginitis |
| 054.12 | Herpetic ulceration of vulva |
| 054.13 | Herpetic infection of penis |
| 054.19 | Other genital herpes |
| 054.2 | Herpetic gingivostomatitis |
| 054.3 | Herpetic meningoencephalitis |
| 054.4 | Herpes simplex with ophthalmic complications |
| 054.40 | Herpes simplex with unspecified ophthalmic complications |
| 054.41 | Herpes simplex dermatitis of eyelid |
| 054.42 | Dendritic keratitis |
| 054.43 | Herpes simplex disciform keratitis |
| 054.44 | Herpes simplex iridocyclitis |
| 054.49 | Herpes simplex with other ophthalmic complications |
| 054.5 | Herpetic septicemia |
| 054.6 | Herpetic whitlow |
| 054.7 | Herpes simplex with other complications |
| 054.71 | Visceral herpes simplex |
| 054.72 | Herpes simplex meningitis |
| 054.73 | Herpes simplex otitis externa |
| 054.79 | Herpes simplex with other specified complications |
| 054.8 | Herpes simplex with unspeciified complication |
| 054.9 | Herpes simplex without mention of complication |
| Epstein–Barr Virus | |
| 075 | Infectious mononucleosis |
| Human cytomegalovirus | |
| 078.5 | Cytomegaloviral disease |
| 484.1 | Pneumonia in cytomegalic inclusion disease |
| Diagnosis Code | Description |
|---|---|
| Atopic diseases | |
| Allergic rhinitis | |
| 477 | Allergic rhinitis |
| 477.0 | Allergic rhinitis due to pollen |
| 477.1 | Allergic rhinitis due to food |
| 477.8 | Allergic rhinitis due to other allergen |
| Asthma | |
| 493 | Asthma |
| 493.0 | Extrinsic asthma |
| 493.00 | Extrinsic asthma without mention of status asthmaticus |
| 493.01 | Extrinsic asthma with status asthmaticus |
| 493.02 | Extrinsic asthma with acute exacerbation |
| 493.1 | Intrinsic asthma |
| 493.10 | Intrinsic asthma without mention of status asthmaticus |
| 493.11 | Intrinsic asthma with status asthmaticus |
| 493.12 | Intrinsic asthma with acute exacerbation |
| Atopic dermatitis | |
| 691 | Atopic dermatitis and related conditions |
| 691.8 | Other atopic dermatitis and related conditions |
| Autoimmune thyroid diseases | |
| Graves’ disease | |
| 242.0 | Toxic diffuse goiter |
| 242.00 | Toxic diffuse goiter without mention of thyrotoxic crisis or storm |
| 242.01 | Toxic diffuse goiter with mention of thyrotoxic crisis or storm |
| Hashimoto’s thyroiditis | |
| 245.2 | Chronic lymphocytic thyroiditis |
| Enterovirus infection | |
| 008.67 | Enteritis due to enterovirus |
| 047 | Meningitis due to enterovirus |
| 047.0 | Meningitis due to Coxsackievirus |
| 047.1 | Meningitis due to echovirus |
| 048 | Other enterovirus diseases of central nervous system |
| 074 | Specific diseases due to Coxsackievirus |
| 074.0 | Herpangina |
| 074.1 | Epidemic pleurodynia |
| 074.2 | Coxsackievirus carditis |
| 074.20 | Coxsackievirus carditis |
| 074.21 | Pericarditis due to Coxsackievirus |
| 074.23 | Myocarditis due to Coxsackievirus |
| 074.3 | Hand-foot-and-mouth disease |
| 074.8 | Other specified diseases due to Coxsackievirus, |
| 079.1 | Echovirus infection in conditions classified elsewhere and of unspecified site, |
| 079.2 | Coxsackievirus infection in conditions classified elsewhere and of unspecified site |
References
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Fard, H.H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- The DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet. Med. 2006, 23, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.C.; Dahlquist, G.G.; Gyurus, E.; Green, A.; Soltesz, G.; Group, E.S. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–2020: A multicentre prospective registration study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Maclaren, N.K. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 1994, 331, 1428–1436. [Google Scholar]
- Todd, J.A.; Bain, S.C. A practical approach to identification of susceptibility genes for IDDM. Diabetes 1992, 41, 1029–1034. [Google Scholar] [CrossRef]
- Maclaren, N.; Riley, W.; Skordis, N.; Atkinson, M.; Spillar, R.; Silverstein, J.; Klein, R.; Vadheim, C.; Rotter, J. Inherited susceptibility to insulin-dependent diabetes is associated with HLA-DR1, while DR5 is protective. Autoimmunity 1988, 1, 197–205. [Google Scholar] [CrossRef]
- Dahlquist, G.G.; Pundziūte-Lyckå, A.; Nyström, L. Birthweight and risk of type 1 diabetes in children and young adults: A population-based register study. Diabetologia 2005, 48, 1114–1117. [Google Scholar] [CrossRef][Green Version]
- Stene, L.C.; Magnus, P.; Lie, R.T.; Søvik, O.; Joner, G. Birth weight and childhood onset type 1 diabetes: Population based cohort study. Br. Med. J. 2001, 322, 889–892. [Google Scholar] [CrossRef]
- Kibirige, M.; Metcalf, B.; Renuka, R.; Wilkin, T.J. Testing the accelerator hypothesis: The relationship between body mass and age at diagnosis of type 1 diabetes. Diabetes Care 2003, 26, 2865–2870. [Google Scholar] [CrossRef]
- Virtanen, S.M.; Saukkonen, T.; Savilahti, E.; Ylönen, K.; Räsänen, L.; Aro, A.; Knip, M.; Tuomilehto, J.; Akerblom, H.K. Diet, cow’s milk protein antibodies and the risk of IDDM in Finnish children. Childhood Diabetes in Finland Study Group. Diabetologia 1994, 37, 381–387. [Google Scholar] [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Norris, J.M.; Yin, X.; Lamb, M.M.; Barriga, K.; Seifert, J.; Hoffman, M.; Orton, H.D.; Barón, A.E.; Clare-Salzler, M.; Chase, H.P.; et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. J. Am. Med. Assoc. 2007, 298, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, K.T.; Boettler, T.; von Herrath, M. Virus infections in type 1 diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007682. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, C.S.; Dib, S.A. Enterovirus and type 1 diabetes: What is the matter? World J. Diabetes 2015, 6, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Gamble, D.R.; Kinsley, M.L.; FitzGerald, M.G.; Bolton, R.; Taylor, K.W. Viral antibodies in diabetes mellitus. Br. Med. J. 1969, 3, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.C.; Rawlinson, W.D.; Craig, M.E. Enterovirus infection and type 1 diabetes mellitus: Systematic review and meta-analysis of observational molecular studies. Br. Med. J. 2011, 342, d35. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Takahashi, Y.; Kishimoto, M.; Nakajima, Y.; Nakanishi, K.; Kajio, H.; Noda, M. A case of fulminant type 1 diabetes associated with significant elevation of mumps titers. Endocr. J. 2008, 55, 561–564. [Google Scholar] [CrossRef]
- Watanabe, N. Conversion to type 1 diabetes after H1N1 influenza infection: A case report. J. Diabetes 2011, 3, 103. [Google Scholar] [CrossRef]
- Johnson, R.W.; Alvarez-Pasquin, M.J.; Bijl, M.; Franco, E.; Gaillat, J.; Clara, J.G.; Labetoulle, M.; Michel, J.P.; Naldi, L.; Sanmarti, L.S.; et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: A multidisciplinary perspective. Ther. Adv. Vaccines 2015, 3, 109–120. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.; Eun, H.M.; McArthur, R.G.; Yoon, J.W. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet 1988, 2, 1–4. [Google Scholar] [CrossRef]
- van der Werf, N.; Hillebrands, J.L.; Klatter, F.A.; Bos, I.; Bruggeman, C.A.; Rozing, J. Cytomegalovirus infection modulates cellular immunity in an experimental model for autoimmune diabetes. Clin. Dev. Immunol. 2003, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wallstrom, G.; Davis, A.; Wang, J.; Park, J.; Throop, A.; Steel, J.; Yu, X.; Wasserfall, C.; Schatz, D.; et al. Immunoproteomic Profiling of Antiviral Antibodies in New-Onset Type 1 Diabetes Using Protein Arrays. Diabetes 2016, 65, 285–296. [Google Scholar] [CrossRef]
- Rachel Lu, J.F.; Chiang, T.L. Evolution of Taiwan’s health care system. Health Econ. Policy Law 2011, 6, 85–107. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Shields, M.D.; Carson, D.J.; Patterson, C.C. A meta-analysis of the association between childhood type 1 diabetes and atopic disease. Diabetes Care 2003, 26, 2568–2574. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Liu, S.; Yu, J. Autoimmune thyroid disease and type 1 diabetes mellitus: Same pathogenesis; new perspective? Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820958329. [Google Scholar] [CrossRef]
- Borysewicz-Sańczyk, H.; Sawicka, B.; Wawrusiewicz-Kurylonek, N.; Głowińska-Olszewska, B.; Kadłubiska, A.; Gościk, J.; Szadkowska, A.; Łosiewicz, A.; Młynarski, W.; Kretowski, A.; et al. Genetic Association Study of IL2RA, IFIH1, and CTLA-4 Polymorphisms with Autoimmune Thyroid Diseases and Type 1 Diabetes. Front. Pediatr. 2020, 8, 481. [Google Scholar] [CrossRef]
- Gamble, D.R.; Taylor, K.W.; Cumming, H. Coxsackie viruses and diabetes mellitus. Br. Med. J. 1973, 4, 260–262. [Google Scholar] [CrossRef]
- Helfand, R.F.; Gary, H.E., Jr.; Freeman, C.Y.; Anderson, L.J.; Pallansch, M.A. Serologic evidence of an association between enteroviruses and the onset of type 1 diabetes mellitus. Pittsburgh Diabetes Research Group. J. Infect. Dis. 1995, 172, 1206–1211. [Google Scholar] [CrossRef]
- Oikarinen, S.; Tauriainen, S.; Hober, D.; Lucas, B.; Vazeou, A.; Sioofy-Khojine, A.; Bozas, E.; Muir, P.; Honkanen, H.; Ilonen, J.; et al. Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes 2014, 63, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, M.; Hyöty, H.; Knip, M.; Ilonen, J.; Reijonen, H.; Vähäsalo, P.; Roivainen, M.; Lonnrot, M.; Leinikki, P.; Hovi, T.; et al. Islet cell antibody seroconversion in children is temporally associated with enterovirus infections. Childhood Diabetes in Finland (DiMe) Study Group. J. Infect. Dis. 1997, 175, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Salminen, K.; Sadeharju, K.; Lönnrot, M.; Vähäsalo, P.; Kupila, A.; Korhonen, S.; Ilonen, J.; Simell, O.; Knip, M.; Hyöty, H. Enterovirus infections are associated with the induction of beta-cell autoimmunity in a prospective birth cohort study. J. Med. Virol. 2003, 69, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Andréoletti, L.; Hober, D.; Hober-Vandenberghe, C.; Belaich, S.; Vantyghem, M.C.; Lefebvre, J.; Wattré, P. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J. Med. Virol. 1997, 52, 121–127. [Google Scholar] [CrossRef]
- Nairn, C.; Galbraith, D.N.; Taylor, K.W.; Clements, G.B. Enterovirus variants in the serum of children at the onset of Type 1 diabetes mellitus. Diabet. Med. 1999, 16, 509–513. [Google Scholar] [CrossRef]
- Yoon, J.W.; Onodera, T.; Notkins, A.L. Virus-induced diabetes mellitus. XV. Beta cell damage and insulin-dependent hyperglycemia in mice infected with coxsackie virus B4. J. Exp. Med. 1978, 148, 1068–1080. [Google Scholar] [CrossRef]
- Hou, J.; Said, C.; Franchi, D.; Dockstader, P.; Chatterjee, N.K. Antibodies to glutamic acid decarboxylase and P2-C peptides in sera from coxsackie virus B4-infected mice and IDDM patients. Diabetes 1994, 43, 1260–1266. [Google Scholar] [CrossRef]
- Fujiya, A.; Ochiai, H.; Mizukoshi, T.; Kiyota, A.; Shibata, T.; Suzuki, A.; Ohashi, N.; Sobajima, H. Fulminant type 1 diabetes mellitus associated with a reactivation of Epstein-Barr virus that developed in the course of chemotherapy of multiple myeloma. J. Diabetes Investig. 2010, 1, 286–289. [Google Scholar] [CrossRef]
- Parkkonen, P.; Hyöty, H.; Ilonen, J.; Reijonen, H.; Ylä-Herttuala, S.; Leinikki, P. Antibody reactivity to an Epstein-Barr virus BERF4-encoded epitope occurring also in Asp-57 region of HLA-DQ8 beta chain. Childhood Diabetes in Finland Study Group. Clin. Exp. Immunol. 1994, 95, 287–293. [Google Scholar]
- Hiemstra, H.S.; Schloot, N.C.; van Veelen, P.A.; Willemen, S.J.; Franken, K.L.; van Rood, J.J.; de Vries, R.R.; Chaudhuri, A.; Behan, P.O.; Drijfhout, J.W.; et al. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA. 2001, 98, 3988–3991. [Google Scholar] [CrossRef]
- Honeyman, M.C.; Stone, N.L.; Harrison, L.C. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: Potential for mimicry with rotavirus and other environmental agents. Mol. Med. 1998, 4, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.C.; Morse, S.A.; Mietzner, T.; Miller, S. Jawetz, Melnick, & Adelberg’s Medical Microbiology, 27th ed.; McGraw Hill: New York, NY, USA, 2016; pp. 457–464. [Google Scholar]
- Cheng, C.L.; Kao, Y.H.Y.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Gau, J.P.; Liu, C.Y.; Yang, M.H.; Chiang, S.C.; Hsu, H.C.; Hong, Y.C.; Hsiao, L.T.; Liu, J.H.; Chiou, T.J.; et al. A nation-wide analysis of venous thromboembolism in 497,180 cancer patients with the development and validation of a risk-stratification scoring system. Thromb. Haemost. 2012, 108, 225–235. [Google Scholar] [PubMed]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Yang, Y.H.K.; Lai, E.C.C. Taiwan’s national health insurance research database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [PubMed]
| T1DM | Non-T1DM | ||||
|---|---|---|---|---|---|
| Variable | n | (%) | n | (%) | p-Value |
| Total | 8179 | 32,716 | |||
| Gender | 1.0 | ||||
| Female | 4309 | (52.7) | 17,236 | (52.7) | |
| Male | 3870 | (47.3) | 15,480 | (47.3) | |
| Age | 1.0 | ||||
| ≤1 | 25 | (0.3) | 100 | (0.3) | |
| 1–3 | 149 | (1.8) | 596 | (1.8) | |
| 3–5 | 217 | (2.6) | 868 | (2.6) | |
| 5–10 | 989 | (12.1) | 3956 | (12.1) | |
| 10–18 | 2002 | (24.5) | 8008 | (24.5) | |
| >18 | 4797 | (58.6) | 19,188 | (58.6) | |
| History of HHV infection | 0.074 | ||||
| VZV, varicella | 237 | (2.90) | 968 | (2.96) | |
| VZV, zoster | 110 | (1.34) | 352 | (1.08) | |
| HSV | 149 | (1.82) | 479 | (1.46) | |
| EBV | 6 | (0.07) | 26 | (0.08) | |
| HCMV | 1 | (0.01) | 3 | (0.01) | |
| No | 7676 | (93.9) | 30,888 | (94.4) | |
| Allergic rhinitis | 0.474 | ||||
| Yes | 1523 | (18.6) | 5980 | (18.3) | |
| No | 6656 | (81.4) | 26,736 | (81.7) | |
| Asthma | 0.005 * | ||||
| Yes | 772 | (9.4) | 2768 | (8.5) | |
| No | 7407 | (90.6) | 29,948 | (91.5) | |
| Atopic dermatitis | <0.001 * | ||||
| Yes | 296 | (3.6) | 939 | (2.9) | |
| No | 7883 | (96.4) | 31,777 | (97.1) | |
| Graves’ disease | <0.001 * | ||||
| Yes | 341 | (4.2) | 111 | (0.3) | |
| No | 7838 | (98.0) | 32,605 | (99.7) | |
| Hashimoto’s thyroiditis | <0.001 * | ||||
| Yes | 134 | (1.6) | 35 | (0.1) | |
| No | 8045 | (98.4) | 32,681 | (99.9) | |
| History of enterovirus infection | 0.008 * | ||||
| Yes | 595 | (7.3) | 2115 | (6.5) | |
| No | 7584 | (92.7) | 30,601 | (93.5) | |
| Crude | Adjusted | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value |
| HHV type | ||||
| VZV, varicella | 0.99 (0.85–1.14) | 0.865 | 0.97 (0.84–1.13) | 0.691 |
| VZV, zoster | 1.26 (1.01–1.57) | 0.037 * | 1.17 (0.94–1.47) | 0.167 |
| HSV | 1.26 (1.04–1.51) | 0.018 * | 1.21 (1.01–1.47) | 0.048 * |
| EBV and HCMV | 0.97 (0.43–2.21) | 0.942 | 0.95 (0.42–2.18) | 0.911 |
| No | Reference | Reference | ||
| Allergic rhinitis | ||||
| Yes | 1.03 (0.96–1.10) | 0.450 | 0.97 (0.91–1.05) | 0.459 |
| No | Reference | Reference | ||
| Asthma | ||||
| Yes | 1.14 (1.05–1.25) | 0.003 * | 1.13 (1.02–1.24) | 0.014 * |
| No | Reference | Reference | ||
| Atopic dermatitis | ||||
| Yes | 1.28 (1.12–1.46) | <0.001 * | 1.23 (1.07–1.41) | 0.004 * |
| No | Reference | Reference | ||
| Graves’ disease | ||||
| Yes | 14.5 (11.5–18.3) | <0.001 * | 13.7 (10.8–17.3) | <0.001 * |
| No | Reference | Reference | ||
| Hashimoto’s thyroiditis | ||||
| Yes | 15.3 (10.6–22.2) | <0.001 * | 13.1 (8.9–19.2) | <0.001 * |
| No | Reference | Reference | ||
| History of enterovirus infection | ||||
| Yes | 1.19 (1.06–1.32) | 0.002 * | 1.16 (1.04–1.30) | 0.008 * |
| No | Reference | Reference | ||
| Crude | Adjusted | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value |
| HHV type | ||||
| VZV, varicella | 1.01 (0.86–1.18) | 0.946 | 0.98 (0.84–1.16) | 0.813 |
| VZV, zoster | 1.19 (0.68–2.09) | 0.539 | 1.18 (0.67–2.08) | 0.579 |
| HSV | 1.39 (1.11–1.75) | 0.004 * | 1.35 (1.08–1.70) | 0.010 * |
| EBV and HCMV | 0.60 (0.21–1.71) | 0.337 | 0.59 (0.21–1.68) | 0.320 |
| No | Reference | Reference | ||
| Allergic rhinitis | ||||
| Yes | 1.08 (0.99–1.18) | 0.094 | 1.04 (0.94–1.14) | 0.576 |
| No | Reference | Reference | ||
| Asthma | ||||
| Yes | 1.09 (0.98–1.21) | 0.127 | 1.03 (0.92–1.16) | 0.466 |
| No | Reference | Reference | ||
| Atopic dermatitis | ||||
| Yes | 1.23 (1.04–1.46) | 0.017 * | 1.20 (1.01–1.42) | 0.042 * |
| No | Reference | Reference | ||
| Graves’ disease | ||||
| Yes | 20.7 (8.6–49.5) | <0.001 * | 18.1 (7.5–43.8) | <0.001 * |
| No | Reference | Reference | ||
| Hashimoto’s thyroiditis | ||||
| Yes | 27.3 (11.6–64.4) | <0.001 * | 24.9 (10.5–58.9) | <0.001 * |
| No | Reference | Reference | ||
| History of enterovirus infection | ||||
| Yes | 1.19 (1.07–1.34) | 0.002 * | 1.17 (1.04–1.31) | 0.007 * |
| No | Reference | Reference | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-C.; Liao, J.-Y. Epidemiologic Implication of the Association between Herpes Simplex Virus Infection and the Risk of Type 1 Diabetes Mellitus: A Nationwide Case-Control Study in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 7832. https://doi.org/10.3390/ijerph19137832
Wang S-C, Liao J-Y. Epidemiologic Implication of the Association between Herpes Simplex Virus Infection and the Risk of Type 1 Diabetes Mellitus: A Nationwide Case-Control Study in Taiwan. International Journal of Environmental Research and Public Health. 2022; 19(13):7832. https://doi.org/10.3390/ijerph19137832
Chicago/Turabian StyleWang, Shao-Chang, and Jung-Yu Liao. 2022. "Epidemiologic Implication of the Association between Herpes Simplex Virus Infection and the Risk of Type 1 Diabetes Mellitus: A Nationwide Case-Control Study in Taiwan" International Journal of Environmental Research and Public Health 19, no. 13: 7832. https://doi.org/10.3390/ijerph19137832
APA StyleWang, S.-C., & Liao, J.-Y. (2022). Epidemiologic Implication of the Association between Herpes Simplex Virus Infection and the Risk of Type 1 Diabetes Mellitus: A Nationwide Case-Control Study in Taiwan. International Journal of Environmental Research and Public Health, 19(13), 7832. https://doi.org/10.3390/ijerph19137832






