Effects of Polybrominated Diphenyl Ethers on Hormonal and Reproductive Health in E-Waste-Exposed Population: A Systematic Review
Abstract
:1. Introduction
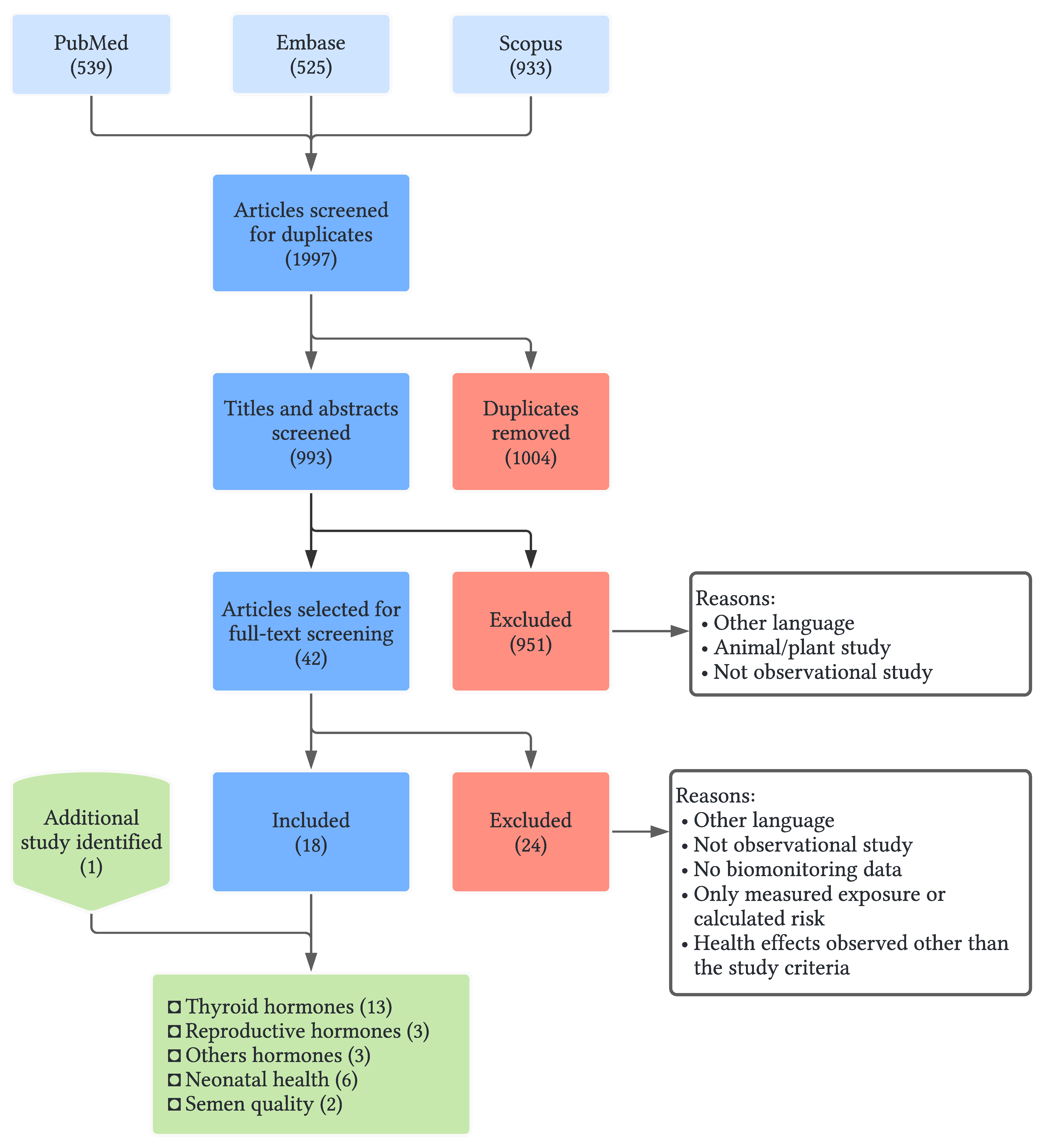
2. Methods
- ◯
- Population: Nearby residents of e-waste recycling sites or workers;
- ◯
- Exposure: Polybrominated diphenyl ethers (PBDEs);
- ◯
- Reference group: Non-exposed population or population with minimal risk of exposure to e-waste;
- ◯
- Outcome: Hormonal and/or reproductive health effects observed due to exposure to PBDEs through e-waste.
2.1. Search Strategy, Study Selection and Data Extraction
2.2. Quality Assessment
3. Results
4. Discussion
4.1. PBDE Exposure Levels
4.2. Hormonal Health
4.3. Reproductive Health
4.4. Limitations and Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeBel, S. Fast Machines, Slow Violence: ICTs, Planned Obsolescence, and E-waste. Globalizations 2016, 13, 300–309. [Google Scholar] [CrossRef]
- Bel, G.; van Brunschot, C.; Easen, N.; Gray, V.; Kuehr, R.; Milios, A.; Mylvakanam, I.; Pennington, J.; The Secretariat of the Basel and Stockholm Conventions. A New Circular Vision for Electronics—Time for a Global Reboot; Platform for Accelerating the Circular Economy (PACE) and UN E-Waste Coalition: Geneva, Switzerland, 2019. [Google Scholar]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows, and the Circular Economy Potential; United Nations University/United Nations Institute for Training and Research: Bonn, Germany; International Telecommunication Union: Geneva, Switzerland; International Solid Waste Association: Rotterdam, The Netherlands, 2020; p. 120. [Google Scholar]
- World Health Organisation. Children and Digital Dumpsites. E-Waste Exposure and Child Health; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Cai, C.; Chen, L.; Huang, H.; Liu, Y.; Yu, S.; Liu, Y.; Tao, S.; Liu, W. Effects of temperature on the emission of particulate matter, polycyclic aromatic hydrocarbons, and polybrominated diphenyl ethers from the thermal treatment of printed wiring boards. J. Hazard. Mater. 2019, 380, 120849. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.; Goldizen, F.C.; Sly, P.D.; Brune, M.-N.; Neira, M.; van den Berg, M.; Norman, R.E. Health consequences of exposure to e-waste: A systematic review. Lancet Glob. Health 2013, 1, e350–e361. [Google Scholar] [CrossRef] [Green Version]
- Kiliaris, P.; Papaspyrides, C.D. Chapter 1—Polymers on Fire. In Polymer Green Flame Retardants; Papaspyrides, C.D., Kiliaris, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–43. [Google Scholar] [CrossRef]
- Yasin, S.; Behary, N.; Perwuelz, A.; Guan, J. Life cycle assessment of flame retardant cotton textiles with optimized end-of-life phase. J. Clean. Prod. 2018, 172, 1080–1088. [Google Scholar] [CrossRef]
- Aschberger, K.; Campia, I.; Pesudo, L.Q.; Radovnikovic, A.; Reina, V. Chemical alternatives assessment of different flame retardants—A case study including multi-walled carbon nanotubes as synergist. Environ. Int. 2017, 101, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhou, H.; Wang, D.; Zha, J.; Xu, Y.; Rao, K.; Ma, M.; Huang, S.; Wang, Z. PBBs, PBDEs, and PCBs in foods collected from e-waste disassembly sites and daily intake by local residents. Sci. Total Environ. 2009, 407, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Meironyte, D.; Noren, K.; Bergman, A. Analysis of polybrominated diphenyl ethers in swedish human milk: A time-related trend study, 1972-1997. J. Toxicol. Environ. Health Part A 1999, 58, 329–341. [Google Scholar] [CrossRef]
- Tue, N.M.; Takahashi, S.; Suzuki, G.; Isobe, T.; Viet, P.H.; Kobara, Y.; Seike, N.; Zhang, G.; Sudaryanto, A.; Tanabe, S. Contamination of indoor dust and air by polychlorinated biphenyls and brominated flame retardants and relevance of non-dietary exposure in Vietnamese informal e-waste recycling sites. Environ. Int. 2013, 51, 160–167. [Google Scholar] [CrossRef]
- Anh, H.Q.; Nam, V.D.; Tri, T.M.; Ha, N.M.; Ngoc, N.T.; Mai, P.T.N.; Anh, D.H.; Minh, N.H.; Tuan, N.A.; Minh, T.B. Polybrominated diphenyl ethers in plastic products, indoor dust, sediment and fish from informal e-waste recycling sites in Vietnam: A comprehensive assessment of contamination, accumulation pattern, emissions, and human exposure. Environ. Geochem. Health 2017, 39, 935–954. [Google Scholar] [CrossRef]
- Die, Q.; Nie, Z.; Huang, Q.; Yang, Y.; Fang, Y.; Yang, J.; He, J. Concentrations and occupational exposure assessment of polybrominated diphenyl ethers in modern Chinese e-waste dismantling workshops. Chemosphere 2019, 214, 379–388. [Google Scholar] [CrossRef]
- Matsukami, H.; Suzuki, G.; Someya, M.; Uchida, N.; Tue, N.M.; Tuyen, L.H.; Viet, P.H.; Takahashi, S.; Tanabe, S.; Takigami, H. Concentrations of polybrominated diphenyl ethers and alternative flame retardants in surface soils and river sediments from an electronic waste-processing area in northern Vietnam, 2012–2014. Chemosphere 2017, 167, 291–299. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme. SC-8/10: Listing of Decabromodiphenyl Ether; United Nations Environment Programme: Stockholm, Sweden, 2017. [Google Scholar]
- Abbasi, G.; Li, L.; Breivik, K. Global Historical Stocks and Emissions of PBDEs. Environ. Sci. Technol. 2019, 53, 6330–6340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-H.; Hu, Y.-J.; Luo, P.; Bao, L.-J.; Qiu, J.-W.; Leung, K.M.Y.; Zeng, E.Y. Occurrence of Halogenated Flame Retardants in Sediment off an Urbanized Coastal Zone: Association with Urbanization and Industrialization. Environ. Sci. Technol. 2014, 48, 8465–8473. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, C.; Han, W.; Song, J.; Li, H.; Zhang, Y.; Jing, X.; Wu, W. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ. Res. 2020, 187, 109531. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Rosseland, C.; Berge, G.; Polder, A. Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 2015, 74, 170–180. [Google Scholar] [CrossRef]
- Tittlemier, S.; Halldorson, T.; Stern, G.; Tomy, T. Vapor pressures, aqueous solubilities, and Henry’s law constants of some brominated flame retardants. Environ. Toxicol. Chem. 2002, 21, 1804–1810. [Google Scholar] [CrossRef]
- Law, R.J.; Covaci, A.; Harrad, S.; Herzke, D.; Abdallah, M.A.E.; Fernie, K.; Toms, L.M.L.; Takigami, H. Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environ. Int. 2014, 65, 147–158. [Google Scholar] [CrossRef]
- Carlsson, P.; Vrana, B.; Sobotka, J.; Borgå, K.; Bohlin Nizzetto, P.; Varpe, Ø. New brominated flame retardants and dechlorane plus in the Arctic: Local sources and bioaccumulation potential in marine benthos. Chemosphere 2018, 211, 1193–1202. [Google Scholar] [CrossRef]
- De Wit, C.A.; Alaee, M.; Muir, D.C.G. Levels and trends of brominated flame retardants in the Arctic. Chemosphere 2006, 64, 209–233. [Google Scholar] [CrossRef]
- Malliari, E.; Kalantzi, O.I. Children’s exposure to brominated flame retardants in indoor environments—A review. Environ. Int. 2017, 108, 146–169. [Google Scholar] [CrossRef]
- Watanabe, I.; Sakai, S.-I. Environmental release and behavior of brominated flame retardants. Environ. Int. 2003, 29, 665–682. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Liu, H.-Y.; Cheng, Z.; Man, Y.-B.; Zhang, K.-S.; Wei, W.; Du, J.; Wong, M.-H.; Wang, H.-S. Polybrominated diphenyl ethers (PBDEs) in human samples of mother–newborn pairs in South China and their placental transfer characteristics. Environ. Int. 2014, 73, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, M.; Vorkamp, K.; Thomsen, M.; Knudsen, L.E. Human internal and external exposure to PBDEs—A review of levels and sources. Int. J. Hyg. Environ. Health 2009, 212, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Song, Q.; Yuan, W.; Ruan, J.; Duan, H.; Li, Y.; Li, J. Human exposure to PBDEs in e-waste areas: A review. Environ. Pollut. 2020, 267, 115634. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, Q.; Li, X.; Li, N.; Chi, P.; Chen, J.; Wang, Z.; Hao, C. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-β: In vitro and in silico investigations. Environ. Health Perspect. 2010, 118, 602–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Kester, M.H.; Andersson, P.L.; Legler, J.; Brouwer, A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 2006, 92, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Cai, Z. In vitro metabolism of hydroxylated polybrominated diphenyl ethers and their inhibitory effects on 17β-estradiol metabolism in rat liver microsomes. Environ. Sci. Pollut. Res. 2012, 19, 3219–3227. [Google Scholar] [CrossRef]
- Levy, M.; Howlett, T. Chapter 19: Endocrine disease. In Kumar & Clark’s Clinical Medicine, 8th ed.; Kumar, P., Clark, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 937–1000. [Google Scholar]
- Rajender, S.; Monica, M.G.; Walter, L.; Agarwal, A. Thyroid, spermatogenesis, and male infertility. Front. Biosci. Elite 2011, 3, 843–855. [Google Scholar] [CrossRef]
- Shields, B.M.; Knight, B.A.; Hill, A.; Hattersley, A.T.; Vaidya, B. Fetal thyroid hormone level at birth is associated with fetal growth. J. Clin. Endocrinol. Metab. 2011, 96, E934–E938. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, T.J.; Sutton, P. The Navigation Guide systematic review methodology: A rigorous and transparent method for translating environmental health science into better health outcomes. Environ. Health Perspect. 2014, 122, 1007–1014. [Google Scholar] [CrossRef]
- Lam, J.; Lanphear, B.P.; Bellinger, D.; Axelrad, D.A.; McPartland, J.; Sutton, P.; Davidson, L.; Daniels, N.; Sen, S.; Woodruff, T.J. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ. Health Perspect. 2017, 125, 086001. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, A.; Nomiyama, K.; Minh Tue, N.; Trang, P.T.K.; Hung Viet, P.; Takahashi, S.; Tanabe, S. Residue profiles of organohalogen compounds in human serum from e-waste recycling sites in North Vietnam: Association with thyroid hormone levels. Environ. Res. 2015, 137, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Gravel, S.; Lavoué, J.; Bakhiyi, B.; Lavoie, J.; Roberge, B.; Patry, L.; Bouchard, M.F.; Verner, M.A.; Zayed, J.; Labrèche, F. Multi-exposures to suspected endocrine disruptors in electronic waste recycling workers: Associations with thyroid and reproductive hormones. Int. J. Hyg. Environ. Health 2020, 225, 113445. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.C.; Liu, T.; Yang, Y.; Yu, S.; Gao, Y.; Huang, W.; Xiao, J.; Ma, W.; Rutherford, S.; Zhang, Y. Changes in thyroid hormone related proteins and gene expression induced by polychlorinated biphenyls and halogen flame retardants exposure of children in a Chinese e-waste recycling area. Sci. Total Environ. 2020, 742, 140597. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.C.; Yu, S.; Wu, D.; Huang, J.; Liu, T.; Xiao, J.; Huang, W.; Gao, Y.; Li, X.; Zeng, W.; et al. Disruption of thyroid hormone regulated proteins and gene expression by polychlorinated biphenyls, polybrominated diphenyl ethers and new flame retardants in residents of an e-waste region. Environ. Pollut. 2019, 254, 112925. [Google Scholar] [CrossRef] [PubMed]
- Julander, A.; Karlsson, M.; Hagström, K.; Ohlson, C.G.; Engwall, M.; Bryngelsson, I.L.; Westberg, H.; van Bavel, B. Polybrominated diphenyl ethers—Plasma levels and thyroid status of workers at an electronic recycling facility. Int. Arch. Occup. Environ. Health 2005, 78, 584–592. [Google Scholar] [CrossRef]
- Lv, Q.X.; Wang, W.; Li, X.H.; Yu, L.; Zhang, Y.; Tian, Y. Polychlorinated biphenyls and polybrominated biphenyl ethers in adipose tissue and matched serum from an E-waste recycling area (Wenling, China). Environ. Pollut. 2015, 199, 219–226. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Liu, Q.; Wang, F.; Nie, J.; Qian, Y. Examining the relationship between brominated flame retardants (BFR) exposure and changes of thyroid hormone levels around e-waste dismantling sites. Int. J. Hyg. Environ. Health 2010, 213, 369–380. [Google Scholar] [CrossRef]
- Xu, P.; Lou, X.; Ding, G.; Shen, H.; Wu, L.; Chen, Z.; Han, J.; Han, G.; Wang, X. Association of PCB, PBDE and PCDD/F body burdens with hormone levels for children in an e-waste dismantling area of Zhejiang Province, China. Sci. Total Environ. 2014, 499, 55–61. [Google Scholar] [CrossRef]
- Xu, P.; Lou, X.; Ding, G.; Shen, H.; Wu, L.; Chen, Z.; Han, J.; Wang, X. Effects of PCBs and PBDEs on thyroid hormone, lymphocyte proliferation, hematology and kidney injury markers in residents of an e-waste dismantling area in Zhejiang, China. Sci. Total Environ. 2015, 536, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, J.; Zeng, X.; Lu, F.; Chen, A.; Huo, X. Elevated serum polybrominated diphenyl ethers and alteration of thyroid hormones in children from Guiyu, China. PLoS ONE 2014, 9, e113699. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Lin, B.G.; Chen, X.C.; Qiao, J.; Li, L.Z.; Liang, Y.; Zhang, G.Z.; Jia, Y.; Zhou, X.Q.; Chen, C.R.; et al. Polybrominated diphenyl ethers in human serum, semen and indoor dust: Effects on hormones balance and semen quality. Sci. Total Environ. 2019, 671, 1017–1025. [Google Scholar] [CrossRef]
- Zheng, J.; He, C.T.; Chen, S.J.; Yan, X.; Guo, M.N.; Wang, M.H.; Yu, Y.J.; Yang, Z.Y.; Mai, B.X. Disruption of thyroid hormone (TH) levels and TH-regulated gene expression by polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and hydroxylated PCBs in e-waste recycling workers. Environ. Int. 2017, 102, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.Y.; Li, X.H.; Zhang, Y.; Yang, Y.L.; Wang, W.Y.; Tian, Y. Partitioning of polybrominated biphenyl ethers from mother to fetus and potential health-related implications. Chemosphere 2017, 170, 207–215. [Google Scholar] [CrossRef]
- Guo, L.C.; Pan, S.; Yu, S.; Liu, T.; Xiao, J.; Zhu, B.; Qu, Y.; Huang, W.; Li, M.; Li, X.; et al. Human Sex Hormone Disrupting Effects of New Flame Retardants and Their Interactions with Polychlorinated Biphenyls, Polybrominated Diphenyl Ethers, a Case Study in South China. Environ. Sci. Technol. 2018, 52, 13935–13941. [Google Scholar] [CrossRef]
- Yu, Y.J.; Lin, B.G.; Liang, W.B.; Li, L.Z.; Hong, Y.D.; Chen, X.C.; Xu, X.Y.; Xiang, M.D.; Huang, S. Associations between PBDEs exposure from house dust and human semen quality at an e-waste areas in South China-A pilot study. Chemosphere 2018, 198, 266–273. [Google Scholar] [CrossRef]
- Li, M.; Huo, X.; Pan, Y.; Cai, H.; Dai, Y.; Xu, X. Proteomic evaluation of human umbilical cord tissue exposed to polybrominated diphenyl ethers in an e-waste recycling area. Environ. Int. 2018, 111, 362–371. [Google Scholar] [CrossRef]
- Wu, K.; Xu, X.; Liu, J.; Guo, Y.; Li, Y.; Huo, X. Polybrominated diphenyl ethers in umbilical cord blood and relevant factors in neonates from Guiyu, China. Environ. Sci. Technol. 2010, 44, 813–819. [Google Scholar] [CrossRef]
- Xu, L.; Huo, X.; Zhang, Y.; Li, W.; Zhang, J.; Xu, X. Polybrominated diphenyl ethers in human placenta associated with neonatal physiological development at a typical e-waste recycling area in China. Environ. Pollut. 2015, 196, 414–422. [Google Scholar] [CrossRef]
- Xu, X.; Yekeen, T.A.; Xiao, Q.; Wang, Y.; Lu, F.; Huo, X. Placental IGF-1 and IGFBP-3 expression correlate with umbilical cord blood PAH and PBDE levels from prenatal exposure to electronic waste. Environ. Pollut. 2013, 182, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Hamazaki, K.; Tsuchida, A.; Inadera, H.; The Japan, E.; Children’s Study Jecs, G. House Dust Avoidance during Pregnancy and Subsequent Infant Development: The Japan Environment and Children’s Study. Int. J. Environ. Res. Public Health 2021, 18, 4277. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Cheng, J.; Tang, Z.; Yin, H.; Zhang, M. Global distribution and trends of polybrominated diphenyl ethers in human blood and breast milk: A quantitative meta-analysis of studies published in the period 2000–2019. J. Environ. Manag. 2021, 280, 111696. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.; Berrington de González, A.; Luqmani, Y.; Suresh, A. Family history of benign thyroid disease and cancer and risk of thyroid cancer. Eur. J. Cancer 2004, 40, 754–760. [Google Scholar] [CrossRef]
- Lazarus, J.H. The importance of iodine in public health. Environ. Geochem. Health 2015, 37, 605–618. [Google Scholar] [CrossRef]
- Lee, D.H.; Jacobs, D.R., Jr. Methodological issues in human studies of endocrine disrupting chemicals. Rev. Endocr. Metab. Disord. 2015, 16, 289–297. [Google Scholar] [CrossRef]
- Kuriyama, S.N.; Wanner, A.; Fidalgo-Neto, A.A.; Talsness, C.E.; Koerner, W.; Chahoud, I. Developmental exposure to low-dose PBDE-99: Tissue distribution and thyroid hormone levels. Toxicology 2007, 242, 80–90. [Google Scholar] [CrossRef]
- He, Q.; Chen, X.; Liu, J.; Li, C.; Xing, H.; Shi, Y.; Tang, Q. Combining Network Pharmacology with Molecular Docking for Mechanistic Research on Thyroid Dysfunction Caused by Polybrominated Diphenyl Ethers and Their Metabolites. BioMed Res. Int. 2021, 2021. [Google Scholar] [CrossRef]
- Huang, X.; Huang, M.; Zuo, Y.; Yi, Z.; Liu, H. Binding characteristics of hydroxylated polybrominated diphenyl ether with thyroid protein and its potential toxicity. J. Mol. Struct. 2021, 1236, 130285. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Beg, M.A. Structural binding perspectives of common plasticizers and a flame retardant, BDE-153, against thyroxine-binding globulin: Potential for endocrine disruption. J. Appl. Toxicol. 2021, 42, 841–851. [Google Scholar] [CrossRef]
- Marsan, E.S.; Bayse, C.A. Halogen-Bonding Interactions of Polybrominated Diphenyl Ethers and Thyroid Hormone Derivatives: A Potential Mechanism for the Inhibition of Iodothyronine Deiodinase. Chemistry 2017, 23, 6625–6633. [Google Scholar] [CrossRef] [PubMed]
- Turyk Mary, E.; Persky Victoria, W.; Imm, P.; Knobeloch, L.; Chatterton, R.; Anderson Henry, A. Hormone Disruption by PBDEs in Adult Male Sport Fish Consumers. Environ. Health Perspect. 2008, 116, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Eagle, S.; Anthopolos, R.; Wolkin, A.; Miranda Marie, L. Associations between Polybrominated Diphenyl Ether (PBDE) Flame Retardants, Phenolic Metabolites, and Thyroid Hormones during Pregnancy. Environ. Health Perspect. 2011, 119, 1454–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.P.; Li, C.H.; Guo, L.H.; Ren, X.M.; Zhang, J.Q. Binding and activity of polybrominated diphenyl ether sulfates to thyroid hormone transport proteins and nuclear receptors. Environ. Sci. Processes Impacts 2019, 21, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.M.; Guo, L.H. Molecular toxicology of polybrominated diphenyl ethers: Nuclear hormone receptor mediated pathways. Environ. Sci. Processes Impacts 2013, 15, 702–708. [Google Scholar] [CrossRef]
- Hallgren, S.; Darnerud, P.O. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology 2002, 177, 227–243. [Google Scholar] [CrossRef]
- Yang, J.; Chan, K.M. Evaluation of the toxic effects of brominated compounds (BDE-47, 99, 209, TBBPA) and bisphenol a (BPA) using a zebrafish liver cell line, ZFL. Aquat. Toxicol. 2015, 159, 138–147. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Jiang, Y.; Lei, Y.; Lu, L.; Zhou, J.; Huang, H.; Fang, D.; Tao, G. Effect on metabolic enzymes and thyroid receptors induced by BDE-47 by activation the pregnane X receptor in HepG2, a human hepatoma cell line. Toxicol. Vitr. 2014, 28, 1377–1385. [Google Scholar] [CrossRef]
- Szabo, D.T.; Richardson, V.M.; Ross, D.G.; Diliberto, J.J.; Kodavanti, P.R.S.; Birnbaum, L.S. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression Involved in thyroid hormone metabolism in male rat pups. Toxicol. Sci. 2009, 107, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Lundstedt-Enkel, K.; Karlsson, D.; Darnerud, P.O. Interaction study with rats given two flame retardants: Polybrominated diphenyl ethers (Bromkal 70-5 DE) and chlorinated paraffins (Cereclor 70L). J. Chemom. 2010, 24, 710–718. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wang, H.; Li, J.; Shan, Z.; Teng, W.; Teng, X. The Correlation between Polybrominated Diphenyl Ethers (PBDEs) and Thyroid Hormones in the General Population: A Meta-Analysis. PLoS ONE 2015, 10, e0126989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, P.I.; Stapleton, H.M.; Mukherjee, B.; Hauser, R.; Meeker, J.D. Associations between brominated flame retardants in house dust and hormone levels in men. Sci. Total Environ. 2013, 445, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Makey, C.M.; McClean, M.D.; Braverman, L.E.; Pearce, E.N.; Sjödin, A.; Weinberg, J.; Webster, T.F. Polybrominated diphenyl ether exposure and reproductive hormones in North American men. Reprod. Toxicol. 2016, 62, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Eskenazi, B.; Rauch, S.A.; Tenerelli, R.; Huen, K.; Holland, N.T.; Lustig, R.H.; Kogut, K.; Bradman, A.; Sjödin, A.; Harley, K.G. In utero and childhood DDT, DDE, PBDE and PCBs exposure and sex hormones in adolescent boys: The CHAMACOS study. Int. J. Hyg. Environ. Health 2017, 220, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Hond, E.; Tournaye, H.; De Sutter, P.; Ombelet, W.; Baeyens, W.; Covaci, A.; Cox, B.; Nawrot, T.S.; Van Larebeke, N.; D’Hooghe, T. Human exposure to endocrine disrupting chemicals and fertility: A case–control study in male subfertility patients. Environ. Int. 2015, 84, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.-Y.; Zheng, Z.; Ren, X.-M.; Andersson, P.L.; Guo, L.-H. Structure-Dependent Activity of Polybrominated Diphenyl Ethers and Their Hydroxylated Metabolites on Estrogen Related Receptor γ: In Vitro and in Silico Study. Environ. Sci. Technol. 2018, 52, 8894–8902. [Google Scholar] [CrossRef]
- Meerts, I.A.; Letcher, R.J.; Hoving, S.; Marsh, G.; Bergman, A.; Lemmen, J.G.; van der Burg, B.; Brouwer, A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 2001, 109, 399–407. [Google Scholar] [CrossRef]
- Sheikh, I.A. Endocrine-disrupting potential of polybrominated diphenyl ethers (PBDEs) on androgen receptor signaling: A structural insight. Struct. Chem. 2021, 32, 887–897. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, W.; Xia, P.; Zhang, X.; Yu, H. Qualitative and quantitative simulation of androgen receptor antagonists: A case study of polybrominated diphenyl ethers. Sci. Total Environ. 2017, 603, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Lu, M.; Lin, S.; Cai, Z. Glucuronidation of hydroxylated polybrominated diphenyl ethers and their modulation of estrogen UDP-glucuronosyltransferases. Chemosphere 2012, 86, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Forhead, A.J.; Fowden, A.L. Thyroid hormones in fetal growth and prepartum maturation. J. Endocrinol. 2014, 221, R87–R103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.T.; Deng, X.K.; Zhao, Y.Y.; Li, J.L.; Song, Q.; Zhang, Y.H.; Yang, Q.; Chen, S.Q. Concentrations of Polybrominated Diphenyl Ethers in Maternal Blood, Placental Size, and Risk for Fetal Growth Restriction: A Nested Case-control Study. Biomed. Environ. Sci. 2020, 33, 821–828. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Q.; Cao, Z.; Su, X.; Hua, J.; Zhang, Y.; He, X. Umbilical cord blood PBDEs concentrations in relation to placental size at birth. Chemosphere 2018, 201, 20–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Q.; Ge, W.; Jin, Y.; Chen, S.; Zhao, Y.; Xiao, X.; Zhang, Y. Associations between in utero exposure to polybrominated diphenyl ethers, pathophysiological state of fetal growth and placental DNA methylation changes. Environ. Int. 2019, 133, 105255. [Google Scholar] [CrossRef]
- Eick, S.M.; Hom Thepaksorn, E.K.; Izano, M.A.; Cushing, L.J.; Wang, Y.; Smith, S.C.; Gao, S.; Park, J.-S.; Padula, A.M.; DeMicco, E.; et al. Associations between prenatal maternal exposure to per- and polyfluoroalkyl substances (PFAS) and polybrominated diphenyl ethers (PBDEs) and birth outcomes among pregnant women in San Francisco. Environ. Health 2020, 19, 100. [Google Scholar] [CrossRef]
- Pearce, J.L.; Neelon, B.; Bloom, M.S.; Buckley, J.P.; Ananth, C.V.; Perera, F.; Vena, J.; Hunt, K. Exploring associations between prenatal exposure to multiple endocrine disruptors and birth weight with exposure continuum mapping. Environ. Res. 2021, 200, 111386. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Xie, Y.; Tian, Y.-K.; Liu, H.; He, C.-D.; An, S.-L.; Chen, W.; Zhou, Y.-Z.; Zhong, X.-N. Associations Between Polybrominated Diphenyl Ethers Concentrations in Human Placenta and Small for Gestational Age in Southwest China. Front. Public Health 2022, 10, 116. [Google Scholar] [CrossRef]
- Harley, K.; Chevrier, J.; Aguilar Schall, R.; Sjodin, A.; Bradman, A.; Eskenazi, B. Association of Prenatal Exposure to Polybrominated Diphenyl Ethers and Infant Birth Weight. Am. J. Epidemiol. 2011, 174, 885–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wang, C.; Cui, C.; Ding, G.; Zhou, Y.; Jin, J.; Gao, Y.; Tian, Y. Prenatal exposure to polybrominated diphenyl ethers and birth outcomes. Environ. Pollut. 2015, 206, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Espinosa, M.-J.; Costa, O.; Vizcaino, E.; Murcia, M.; Fernandez-Somoano, A.; Iñiguez, C.; Llop, S.; Grimalt, J.O.; Ballester, F.; Tardon, A. Prenatal Exposure to Polybrominated Flame Retardants and Fetal Growth in the INMA Cohort (Spain). Environ. Sci. Technol. 2015, 49, 10108–10116. [Google Scholar] [CrossRef] [PubMed]
- Robledo Candace, A.; Yeung, E.; Mendola, P.; Sundaram, R.; Maisog, J.; Sweeney Anne, M.; Barr Dana, B.; Louis Germaine, M.B. Preconception Maternal and Paternal Exposure to Persistent Organic Pollutants and Birth Size: The LIFE Study. Environ. Health Perspect. 2015, 123, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Ouidir, M.; Buck Louis, G.M.; Kanner, J.; Grantz, K.L.; Zhang, C.; Sundaram, R.; Rahman, M.L.; Lee, S.; Kannan, K.; Tekola-Ayele, F.; et al. Association of Maternal Exposure to Persistent Organic Pollutants in Early Pregnancy With Fetal Growth. JAMA Pediatrics 2020, 174, 149–161. [Google Scholar] [CrossRef]
- Morreale De Escobar, G.; Obregon, M.J.; Escobar Del Rey, F. Clinical perspective: Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J. Clin. Endocrinol. Metab. 2000, 85, 3975–3987. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Deng, J.; Shi, X.; Liu, C.; Yu, K.; Zhou, B. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic–pituitary–thyroid axis of zebrafish larvae. Aquat. Toxicol. 2010, 97, 226–233. [Google Scholar] [CrossRef]
- Li, J.; Ma, W.; Zhao, Y.; Jin, Y.; Xiao, X.; Ge, W.; Shi, H.; Zhang, Y. Lactational exposure of polybrominated diphenyl ethers and its association with infant developmental measurements. J. Hazard. Mater. 2020, 388, 122031. [Google Scholar] [CrossRef]
- Miranda, M.L.; Anthopolos, R.; Wolkin, A.; Stapleton, H.M. Associations of birth outcomes with maternal polybrominated diphenyl ethers and thyroid hormones during pregnancy. Environ. Int. 2015, 85, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Guo, F.; Aamir, M.; Liu, Y.; Tang, M.; Liu, W. Multicenter biomonitoring of polybrominated diphenyl ethers (PBDEs) in colostrum from China: Body burden profile and risk assessment. Environ. Res. 2019, 179, 108828. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Zhang, Y.; Zhou, Y.; Shi, R.; Cui, C.; Gao, Y.; Tian, Y. Polybrominated diphenyl ethers in cord blood and perinatal outcomes from Laizhou Wan Birth Cohort, China. Environ. Sci. Pollut. Res. 2018, 25, 20802–20808. [Google Scholar] [CrossRef]
- Chan, S.Y.; Andrews, M.H.; Lingas, R.; McCabe, C.J.; Franklyn, J.A.; Kilby, M.D.; Matthews, S.G. Maternal nutrient deprivation induces sex-specific changes in thyroid hormone receptor and deiodinase expression in the fetal guinea pig brain. J. Physiol. 2005, 566, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, N.; Barnett, A.G.; Sly, P.D.; Callan, A.C.; Stasinska, A.; Heyworth, J.S.; Hinwood, A.L.; Knibbs, L.D. Prenatal exposure to mixtures of persistent environmental chemicals and fetal growth outcomes in Western Australia. Int. J. Hyg. Environ. Health 2022, 240, 113899. [Google Scholar] [CrossRef]
- Gross, R.S.; Ghassabian, A.; Vandyousefi, S.; Messito, M.J.; Gao, C.; Kannan, K.; Trasande, L. Persistent organic pollutants exposure in newborn dried blood spots and infant weight status: A case-control study of low-income Hispanic mother-infant pairs. Environ. Pollut. 2020, 267, 115427. [Google Scholar] [CrossRef]
- Koren, G.; Carnevale, A.; Ling, J.; Ozsarfati, J.; Kapur, B.; Bagli, D. Fetal exposure to polybrominated diphenyl ethers and the risk of hypospadias: Focus on the congeners involved. J. Pediatric Urol. 2019, 15, 405.e1. [Google Scholar] [CrossRef] [PubMed]
- Poon, S.; Koren, G.; Carnevale, A.; Aleksa, K.; Ling, J.; Ozsarfati, J.; Kapur, B.M.; Bagli, D. Association of In Utero Exposure to Polybrominated Diphenyl Ethers with the Risk of Hypospadias. JAMA Pediatrics 2018, 172, 851–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, O.; Huang, J.Y.; Aleksa, K.; Hales, B.F.; Goodyer, C.G.; Robaire, B.; Chevrier, J.; Chan, P. Exposure to polybrominated diphenyl ethers and phthalates in healthy men living in the greater Montreal area: A study of hormonal balance and semen quality. Environ. Int. 2018, 116, 165–175. [Google Scholar] [CrossRef]
- Akutsu, K.; Takatori, S.; Nozawa, S.; Yoshiike, M.; Nakazawa, H.; Hayakawa, K.; Makino, T.; Iwamoto, T. Polybrominated diphenyl ethers in human serum and sperm quality. Bull. Environ. Contam. Toxicol. 2008, 80, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Song, X.; Zhang, Y.; Zeng, F.; Niu, X.; Wu, J. Investigation of polybrominated diphenyl ethers level in blood and male semen quality in Pearl River Delta region. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2015, 35, 2274–2281. [Google Scholar] [CrossRef]
- Mumford, S.L.; Kim, S.; Chen, Z.; Gore-Langton, R.E.; Boyd Barr, D.; Buck Louis, G.M. Persistent organic pollutants and semen quality: The LIFE Study. Chemosphere 2015, 135, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Toft, G.; Lenters, V.; Vermeulen, R.; Heederik, D.; Thomsen, C.; Becher, G.; Giwercman, A.; Bizzaro, D.; Manicardi, G.C.; Spanò, M.; et al. Exposure to polybrominated diphenyl ethers and male reproductive function in Greenland, Poland and Ukraine. Reprod. Toxicol. 2014, 43, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abdelouahab, N.; AinMelk, Y.; Takser, L. Polybrominated diphenyl ethers and sperm quality. Reprod. Toxicol. 2011, 31, 546–550. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Vita, R. Thyroid dysfunction and semen quality. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418775241. [Google Scholar] [CrossRef]
- Sly, J.L.; Carpenter, D.O. Special vulnerability of children to environmental exposures. Rev. Environ. Health 2012, 27, 151–157. [Google Scholar] [CrossRef] [PubMed]

| Study Authors | Measurements and Samples Collected | Study Design | Participant Characteristics and Location | Comorbidities |
|---|---|---|---|---|
| Eguchi et al. [39] | Thyroid hormones and exposure levels through serum samples | Case–control | 111-E: 77 workers at e-waste recycling site (Bui Dau, Vietnam), and C: 34 residents of unexposed rural area (Duong Quang, Vietnam) | None stated (participants with thyroid disease or taking thyroid-related medications were excluded) |
| Gravel et al. [40] | Thyroid and reproductive hormones and exposure levels through plasma samples | Cross-sectional | 100-E1: 85 workers at e-waste recycling facility (Québec, Canada), and E2: 15 workers from recycling facilities other than e-waste (Québec, Canada) | None stated (participants with thyroid disease or taking thyroid-related medications were excluded) |
| Guo et al. [41] | Thyroid hormones and exposure levels through serum samples | Case–control | 114-E: 57 sixth grade children in e-waste recycling town with >30 years of history (Q City, South China), and C: 57 individuals living 50 km from exposed town with no e-waste exposure (Q City, South China) | Respiratory illness symptoms |
| Guo et al. [42] | Thyroid hormones and exposure levels through blood samples | Case–control | 112-E: 54 residents of e-waste recycling town with > 30 years of history (Q City, South China), and C: 58 individuals living 50 km from exposed town with no e-waste exposure (Q City, South China) | Neurological and respiratory illness symptoms |
| Julander et al. [43] | Thyroid hormones and exposure levels through serum samples | Longitudinal cohort study | 14-E: 14 e-waste recycling workers (3 were excluded due to <4 sampling instances) (Örebro, Sweden) | None—one participant with thyroid disease excluded |
| Lv et al. [44] | Thyroid hormones and exposure levels through maternal serum, and neonatal health through physical examinations | Case–control | 74-E: 64 pregnant women residing in e-waste exposed area for >5 years (Wenling, Taizhou, China), and C: 10 pregnant women residing for <2 years in non-e-waste exposed area (Wenling, Taizhou, China) | None stated |
| Wang et al. [45] | Thyroid hormones and exposure levels through plasma samples | Case–control | 442-E1: 236 individuals occupationally exposed to e-waste (Taizhou, Zhejiang Province, China), E2: 89 individuals non-occupationally exposed (Taizhou, Zhejiang Province, China), and C: 117 individuals with no exposure to e-waste (Taizhou, Zhejiang Province, China) | None stated (participants with thyroid disease or taking thyroid-related medications were excluded) |
| Xu et al. [46] | Thyroid, ACTH, cortisol, and growth hormones and exposure levels through serum samples | Case–control | 45-E: 21 residents of e-waste-exposed town with ≈20 years of history (Luqiao, Taizhou, China), and C: 24 individuals living 100 km from exposed site with no e-waste exposure (Tiantai, Taizhou, China) | None stated |
| Xu et al. [47] | Thyroid hormones (only FT3, FT4, and TSH) and exposure levels through serum samples | Case–control | 55-E: 40 residents of e-waste-exposed region (Luqiao, Taizhou, China), and C: 15 individuals living 200 km from exposed town with no e-waste exposure (Yunhe, Taizhou, China) | None stated |
| Xu et al. [48] | Thyroid (only FT3, FT4, and TSH) and IGF-1 hormones and exposure levels through serum samples | Cross-sectional | 167-E: 162 children in kindergarten in an e-waste-exposed town (Guiyu, Shantou, China) | None stated (participants with thyroid disease or taking thyroid-related medications were excluded) |
| Yu et al. [49] | Thyroid and reproductive hormones and exposure levels through serum samples, and semen quality through semen samples | Cross-sectional | 76-E1: 38 men from e-waste area and living near e-waste dismantling plant (Qingyuan city, South China), and E2: 38 men living near an e-waste dismantling plant (Qingyuan city, South China) | None stated |
| Zheng et al. [50] | Thyroid hormones and exposure levels through serum samples | Cross-sectional | 79-E: 79 e-waste recycling workers (Undescribed town, South China) | None stated (participants with thyroid disease or taking thyroid-related medications were excluded) |
| Zheng et al. [51] | Thyroid hormones and exposure levels through maternal serum samples, and neonatal health through physical examinations | Case–control | 72-E: 48 residents of exposed area for >20 years (Wenling, Taizhou, China), and C: 24 residents of exposed area for < 3 years (Wenling, Taizhou, China) | None stated (participants with thyroid disease or taking thyroid-related medications were excluded) |
| Guo et al. [52] | Reproductive hormones and exposure levels through serum samples | Case–control | 112-E: 54 residents of e-waste recycling town with >30 years of history (Q City, South China), and C: 58 individuals living 50 km from exposed town with no e-waste exposure (Q City, South China) | Neurological and respiratory illness symptoms |
| Yu et al. [53] | Semen quality and exposure levels through semen samples | Case–control | 57-E: 32 residents of e-waste-exposed area with a history of decades (Longtang, Qingyuan, South China), and C: 25 semen samples from non-exposed population through semen bank (Qingyuan city, South China) | None stated |
| Li et al. [54] | Neonatal health through physical examinations, and exposure levels through umbilical cord tissue samples | Case–control | 300-E: 150 women residing in e-waste-exposed area (Guiyu, Shantou, China), and C: 150 women residing in non-exposed area (Haojiang, Shantou, China) | None stated |
| Wu et al. [55] | Neonatal health through physical examinations, and exposure levels through umbilical cord serum samples | Case–control | 167-E: 108 women residing in e-waste-exposed area (Guiyu, Shantou, China), and C: 59 women residing in non-exposed area (Chaonan, Shantou, China) | Upper respiratory tract infection and other diseases including anaemia, acute nephritis, skin disease, Pancreas Bile Syndrome, placental abruption, severe pregnancy-induced hypertension, preeclampsia, prolonged pregnancy, cord around neck, hepatitis A, and pregnancy-induced hypertension syndrome |
| Xu et al. [56] | Neonatal health through physical examinations, and exposure levels through placenta samples | Case–control | 155-E: 69 women residing in e-waste-exposed area (Guiyu, Shantou, China), and C: 86 women residing in non-exposed area (Haojiang, Shantou, China) | None stated |
| Xu et al. [57] | IGF-1 hormone and exposure levels through umbilical cord serum samples, and neonatal health through physical examinations | Case–control | 154-E: 101 women residing in e-waste-exposed area (Guiyu, Shantou, China), and C: 53 women residing in non-exposed area (Chaonan, Shantou, China) | None stated |
| Study Authors | Confounder Adjustments | Significant Associations (p < 0.5) |
|---|---|---|
| Eguchi et al. [39] | Gender, age, BMI, perchlorate, iodide, thiocyanate, cholesterol, triglyceride, γ-GTP, living site, consumption of meat and eggs, and consumption of marine fish | None |
| Gravel et al. [40] | Age, BMI, blood cadmium and lead, and smoking status | TT4: ↑ BDE 209 (males); and FT3: ↓ BDE 153 and 209 |
| Guo et al. [41] | Gender, BMI, and cough | None |
| Guo et al. [42] | Gender, BMI, dyspnoea, chest tightness, and smoking | TT3: ↑ BDE 47 and 99; TT4: ↓ BDE 153, 183 and ∑PBDEs; FT3: ↑ BDE 47; FT4: ↓BDE 153 and 183; and TSH: ↓ BDE 47 and 100 |
| Julander et al. [43] | BMI | TT3: ↑ BDE 183; FT4: ↑BDE 28 and 100; TSH: ↑ BDE 99 and 154 |
| Lv et al. [44] | Maternal age, pre-pregnancy BMI, gestational weeks, and maternal parity | None |
| Wang et al. [45] | Gender, age, plasma total lipids, alcohol consumption, and smoking status | None |
| Xu et al. [46] | None stated | ACTH: ↑ BDE 47, 99, 100, 153 and 154 |
| Xu et al. [47] | Gender and age | FT4: ↓ BDE 47 |
| Xu et al. [48] | Gender, age, and BMI | FT3: ↓ BDE 100 and ∑PBDEs; FT4: ↓ BDE 100, 153, 154 and 183; TSH: ↑ BDE 28, 47, 99, 100, 154, 209 and ∑PBDEs |
| Yu et al. [49] | Age, BMI, abstinence time, smoking status, and alcohol consumption | Serum-TT3: ↑ BDE 183; TT4: ↓ BDE 183; FT3: ↑ BDE 47; E2: ↓BDE 47 Semen-FT3: ↓; TSH: ↓ BDE: 154; total T: ↑ BDE 183; E2: ↓ BDE 154; semen volume: ↓ BDE 47 and 153; total sperm count: ↓ BDE 153 |
| Zheng et al. [50] | Gender, age, BMI, and occupational exposure duration of e-waste recycling. ∑PBDEs were also adjusted for smoking. | TT3: ↑ BDE 47 |
| Zheng et al. [51] | Maternal age, pre-pregnancy BMI, gestational weeks, and maternal parity | TT4: ↓ BDE 99 and 153 |
| Guo et al. [52] | Females—dyspnoea, chest tightness, and palpitation Males—sore throat and loose cough | Males—Total T: ↑BDE 47, 100, 153, 183 and ∑PBDEs; Pr: ↑ BDE 47; LH: ↑ BDE 99, 100; E2: ↑ BDE 47, 209 and ∑PBDEs Females—FSH: ↓ BDE 153, 154, 183 and ∑PBDEs; LH: ↓ BDE 183; E2: ↑ BDE 153 and 183 |
| Yu et al. [53] | Age, BMI, abstinence time, smoking status, and alcohol consumption | Sperm concentration: ↓ BDE 47; total sperm count: ↓ BDE 47 |
| Li et al. [54] | None stated for Spearman’s correlation analysis (used for neonatal health outcome and PBDE concentration relationship analysis) | Head circumference: ↓ ∑PBDEs; birth length: ↑ ∑PBDEs; BMI: ↓ ∑PBDEs; Apgar score: ↓ ∑PBDEs |
| Wu et al. [55] | None stated | Gestational age: ↑ BDE 28; adverse birth outcomes: ↑ BDE 28, 47, 99, 153, 183, ∑PBDEs |
| Xu et al. [56] | Education, parity, smoking status, and alcohol consumption | Head circumference: ↓ BDE 47 and ∑PBDEs; birth length: ↑ BDE 47; BMI: ↓ BDE 47, 99, 100, 183, 209 and ∑PBDEs; Apgar score: ↓ BDE 28, 47, 153, 183 and ∑PBDEs |
| Xu et al. [57] | None stated | IGF-1: ↑ BDE 154 and 209; gestational age: ↑ BDE 100 and 154 |
| Domain | Eguchi et al. [39] | Gravel et al. [40] | Guo et al. [41] | Guo et al. [42] | Julander et al. [43] | Lv et al. [44] | Wang et al. [45] | Xu et al. [46] | Xu et al. [47] | Xu et al. [48] | Yu et al. [49] | Zheng et al. [50] | Zheng et al. [51] | Guo et al. [52] | Yu et al. [53] | Li et al. [54] | Wu et al. [55] | Xu et al. [56] | Xu et al. [57] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study group representation | |||||||||||||||||||
| Knowledge of group assignments | |||||||||||||||||||
| Exposure assessment methods | |||||||||||||||||||
| Outcome assessment methods | |||||||||||||||||||
Confounding | |||||||||||||||||||
| Incomplete outcome data | |||||||||||||||||||
| Selective outcome reporting | |||||||||||||||||||
| Financial conflict of interest | |||||||||||||||||||
Other |
| Criteria | Summary of Criteria | Thyroid Hormones | Reproductive Hormones | Other Hormones | Neonatal Health | Semen Quality |
|---|---|---|---|---|---|---|
| Downgrade Criteria | ||||||
| Risk of bias | Study limitations—a substantial risk of bias across the body of evidence | 0 | 0 | −2 | −1 | −1 |
| Indirectness | Evidence was not directly comparable to the question of interest (i.e., population, exposure, comparator, or outcome) | 0 | 0 | 0 | 0 | 0 |
| Inconsistency | Widely different estimates of effect in similar populations (heterogeneity or variability in results) | −1 | 0 | 0 | 0 | 0 |
| Imprecision | Studies had few participants and few events (wide Cis as judged by reviewers) | −1 | −1 | 0 | 0 | −1 |
| Publication bias | Studies missing from the body of evidence, resulting in an over or underestimation of the true effects from exposure | 0 | 0 | 0 | 0 | 0 |
| Upgrade Criteria | ||||||
| Large magnitude of effect | Upgraded if modelling suggested confounding alone unlikely to explain associations with large effect estimate as judged by reviewers | 0 | 0 | 0 | 0 | 0 |
| Dose–response | Upgraded if consistent relationship between dose and response in one or multiple studies and/or dose–response across studies | 0 | 0 | 0 | 0 | 0 |
| Confounding minimizes effect | Upgraded if consideration of all plausible residual confounders or biases would underestimate the effect or suggest a spurious effect when results show no effect | 0 | 0 | 0 | 0 | 0 |
| Overall quality of evidence | Moderate | Moderate | Low | Moderate | Low | |
| Overall strength of evidence | Limited evidence of toxicity | Limited evidence of toxicity | Inadequate evidence of toxicity | Limited evidence of toxicity | Limited evidence of toxicity | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.; Cortes-Ramirez, J.; Toms, L.-M.; Sooriyagoda, T.; Karatela, S. Effects of Polybrominated Diphenyl Ethers on Hormonal and Reproductive Health in E-Waste-Exposed Population: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 7820. https://doi.org/10.3390/ijerph19137820
Singh V, Cortes-Ramirez J, Toms L-M, Sooriyagoda T, Karatela S. Effects of Polybrominated Diphenyl Ethers on Hormonal and Reproductive Health in E-Waste-Exposed Population: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(13):7820. https://doi.org/10.3390/ijerph19137820
Chicago/Turabian StyleSingh, Vishal, Javier Cortes-Ramirez, Leisa-Maree Toms, Thilakshika Sooriyagoda, and Shamshad Karatela. 2022. "Effects of Polybrominated Diphenyl Ethers on Hormonal and Reproductive Health in E-Waste-Exposed Population: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 13: 7820. https://doi.org/10.3390/ijerph19137820
APA StyleSingh, V., Cortes-Ramirez, J., Toms, L.-M., Sooriyagoda, T., & Karatela, S. (2022). Effects of Polybrominated Diphenyl Ethers on Hormonal and Reproductive Health in E-Waste-Exposed Population: A Systematic Review. International Journal of Environmental Research and Public Health, 19(13), 7820. https://doi.org/10.3390/ijerph19137820








