Nitrogen Addition Affects Nitrous Oxide Emissions of Rainfed Lucerne Grassland
Abstract
:1. Introduction
2. Materials and Methods
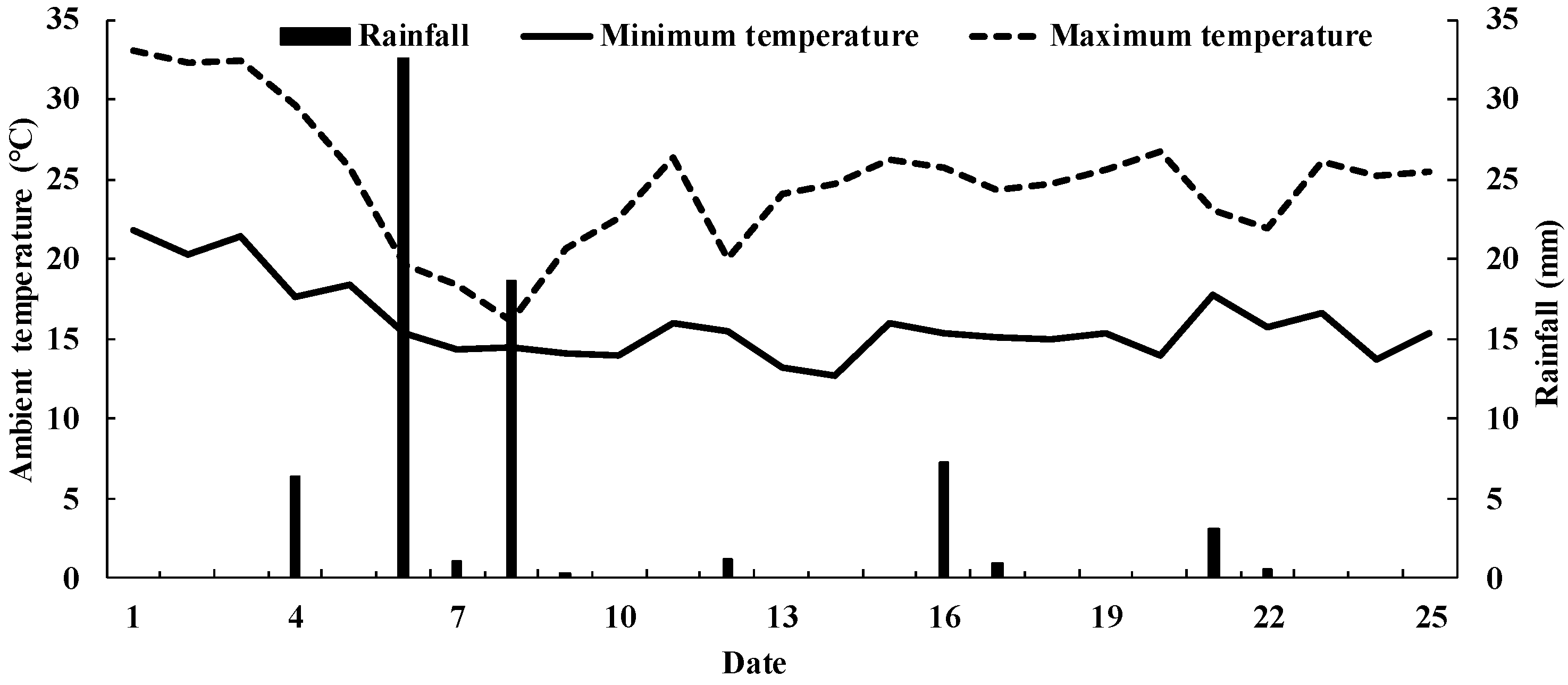
2.1. Site Description
2.2. Experimental Design and N2O Flux Measurements
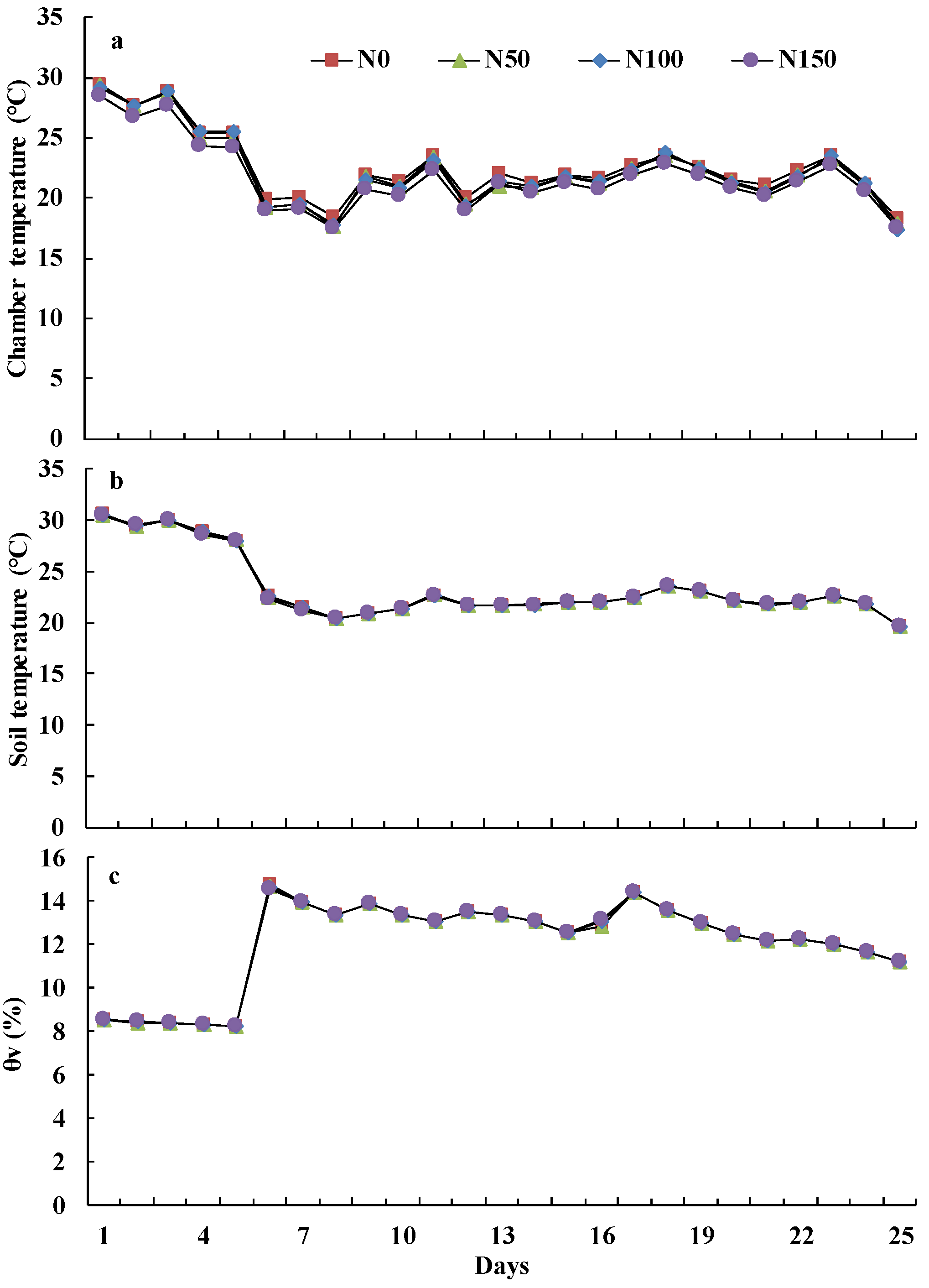
2.3. Soil Measurement and Analysis
2.4. Plant Measurement and Analysis
2.5. Statistical Analysis
3. Results
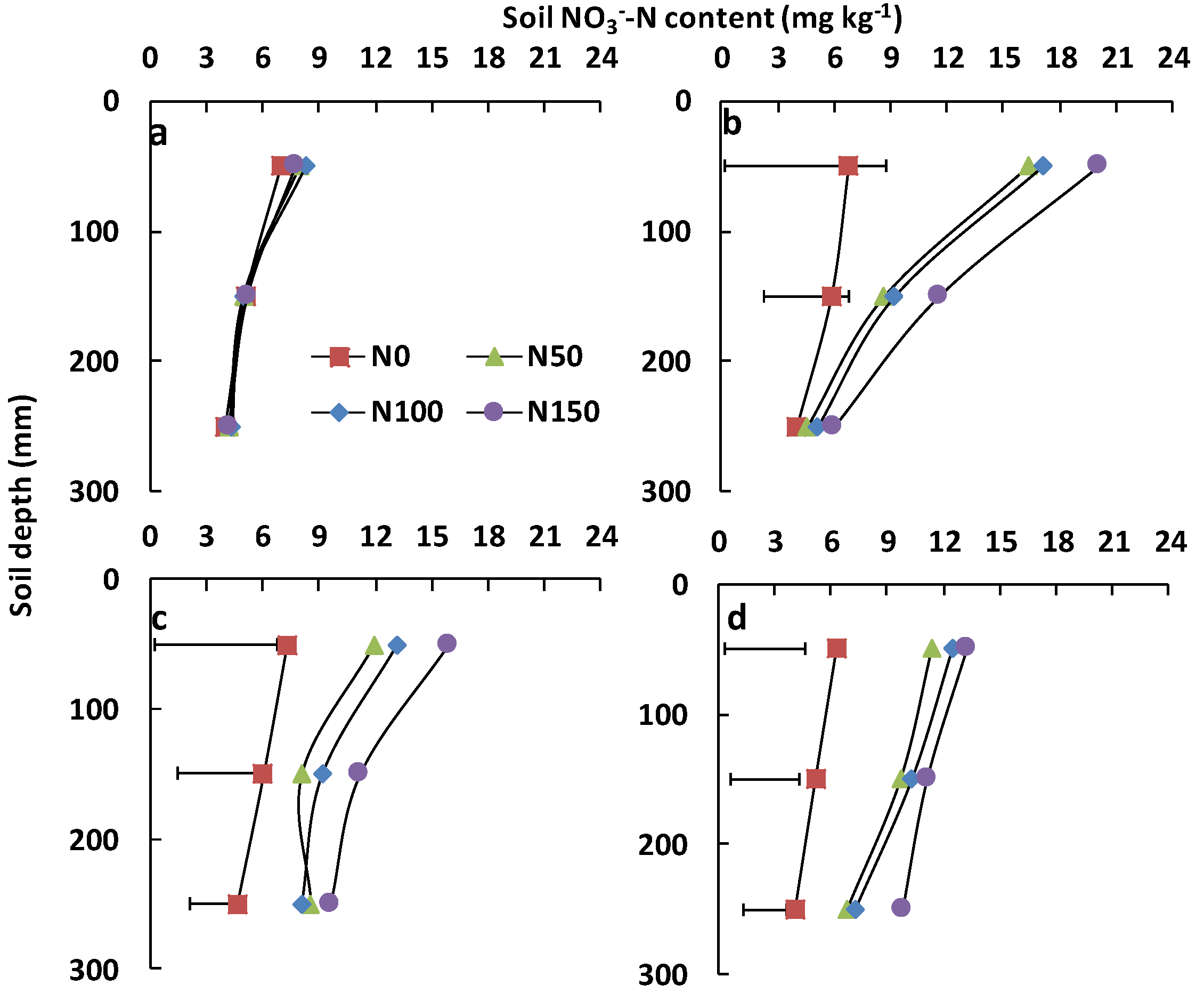
3.1. Soil Physical and Chemical Properties
3.2. Grassland Yield, Height and Leaf Area Index
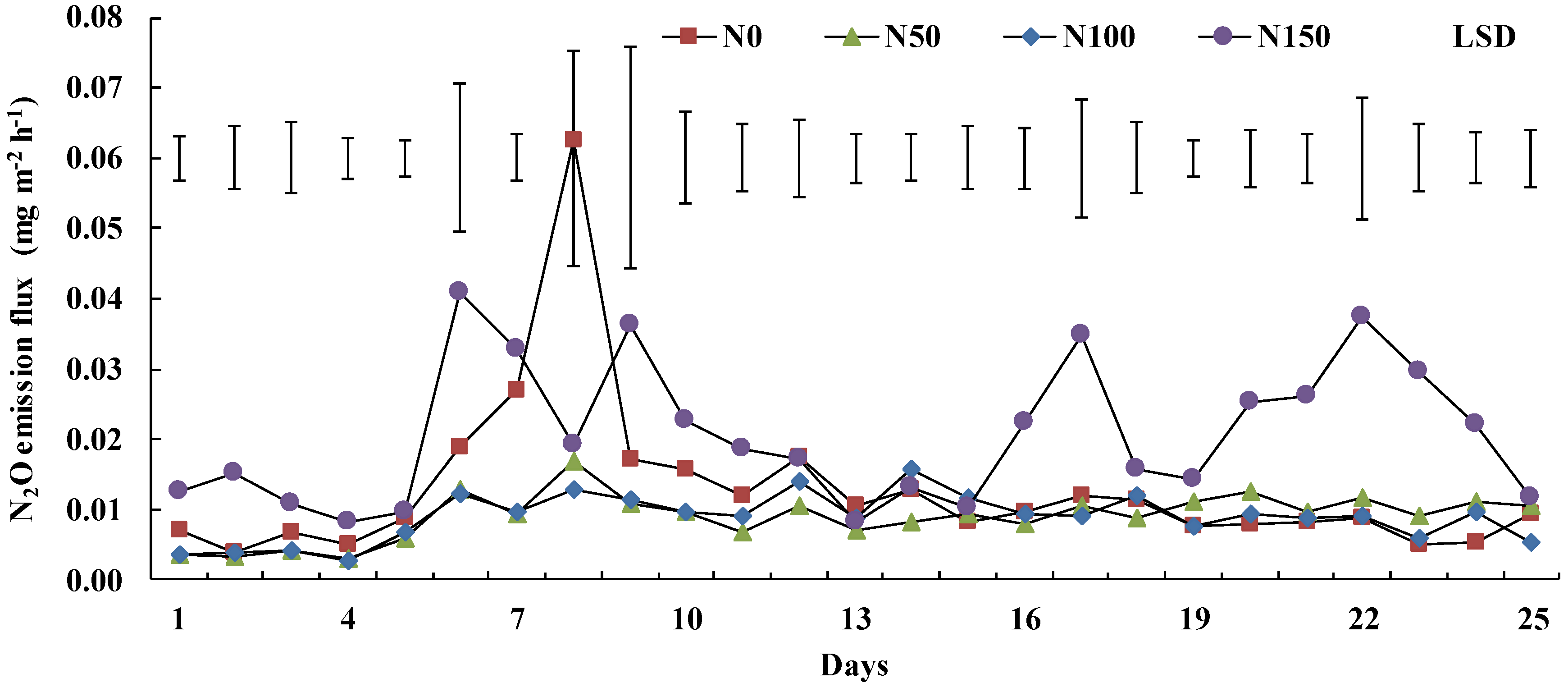
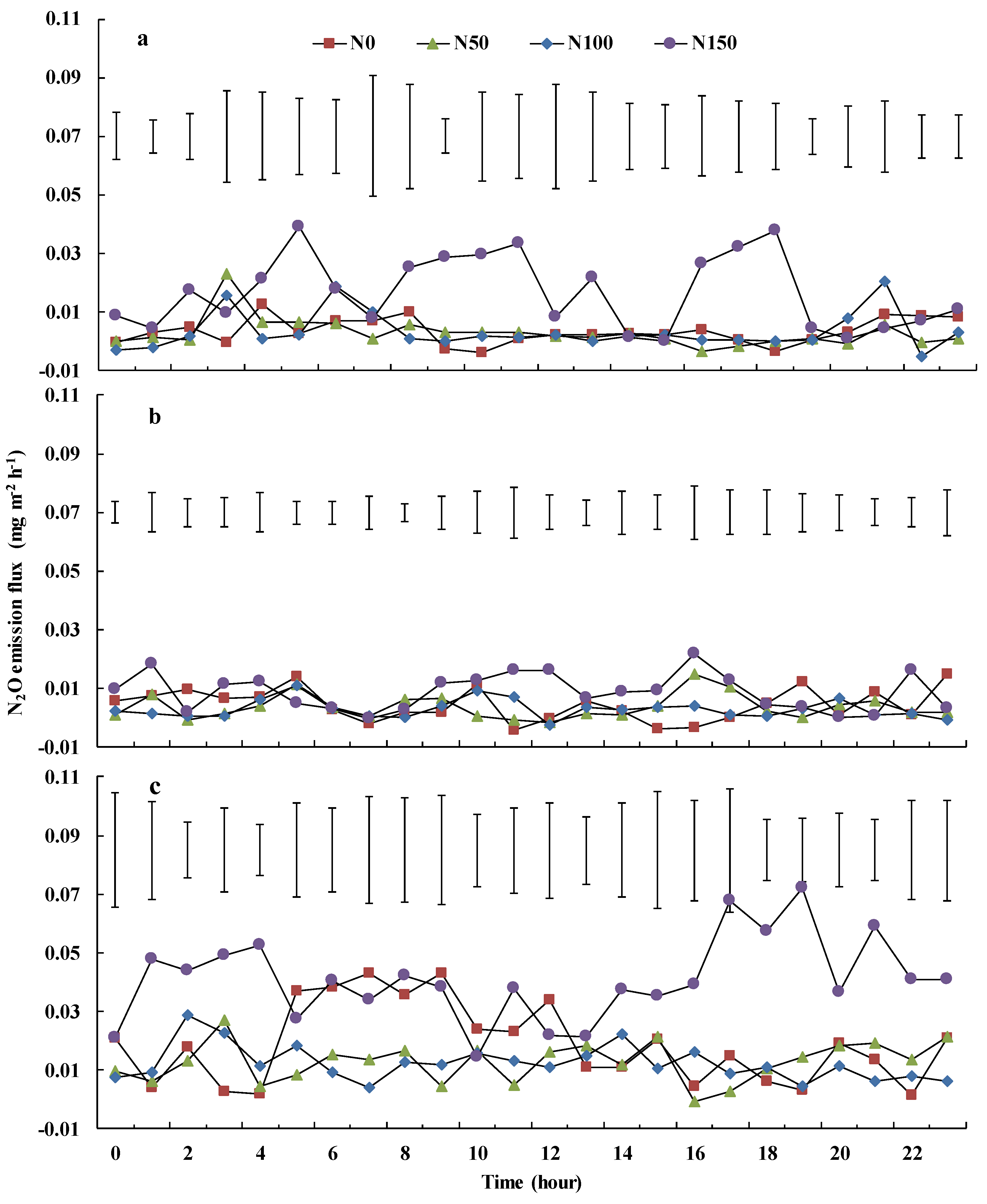
3.3. Soil N2O Emissions and Diurnal Variability
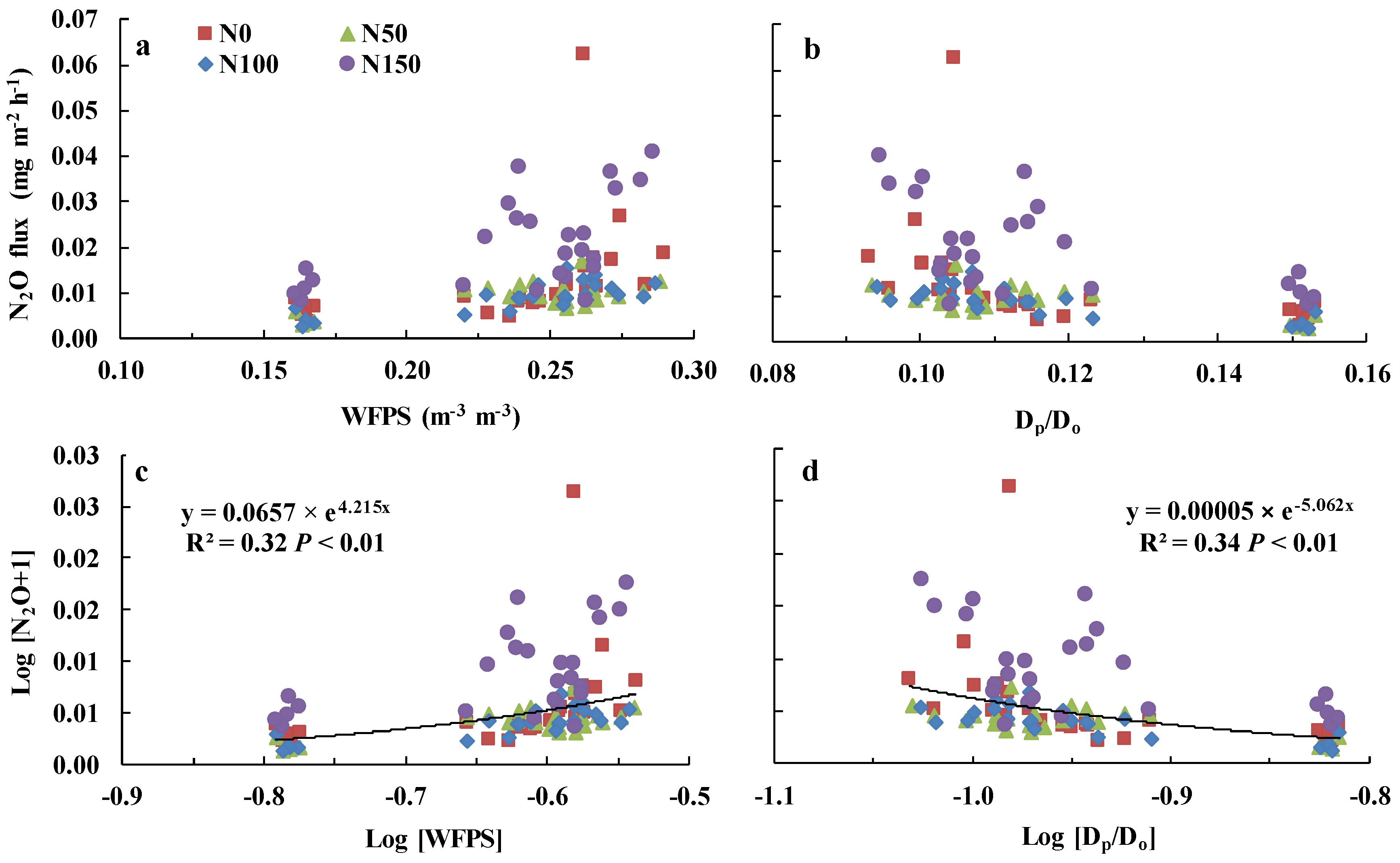
3.4. Relationships between Soil N2O Emissions and Soil Water Content
4. Discussion
4.1. Soil Nitrogen Dynamics
4.2. Soil N2O Emissions and Diurnal Variability
4.3. Relationship of N2O with WFPS and Dp/Do
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change: Working Group II: Impacts, Adaptation and Vulnerability; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Cole, C.V.; Duxbury, J.; Freney, J.; Heinemeyer, O.; Minami, K.; Mosier, A.; Paustian, N.; Rosenberg, N.; Smpson, N.; Sauerbeck, D.; et al. Global estimates of potential mitigation of greenhouse gas emissions by agriculture. Nutr. Cycl. Agroecosyst. 1997, 49, 221–228. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloway, J.N.; Winiwarter, W.; Leip, A.; Leach, A.M.; Bleeker, A.; Erisman, J.W. Nitrogen footprints: Past, present and future. Environ. Res. Lett. 2014, 9, 11. [Google Scholar] [CrossRef]
- Hong, F. Alfalfa Science; China Agriculture Press: Beijing, China, 2009; pp. 97–132. [Google Scholar]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.A. Quantifying the loss mechanisms of nitrogen. J. Soil Water Conserv. 2002, 57, 389–398. [Google Scholar]
- Shen, Y.; Li, L.; Chen, W.; Robertson, M.; Unkovich, M.; Bellotti, W.; Probert, M. Soil water, soil nitrogen and productivity of lucerne-wheat sequences on deep silt loams in a summer dominant rainfall environment. Field Crops Res. 2009, 111, 97–108. [Google Scholar] [CrossRef]
- Bremner, J.M. Sources of nitrous oxide in soils. Nutr. Cycl. Agroecosyst. 1997, 49, 7–16. [Google Scholar] [CrossRef]
- Rochette, P.; Janzen, H.H. Towards a revised coefficient for estimating N2O emissions from legumes. Nutr. Cycl. Agroecosyst. 2005, 73, 171–179. [Google Scholar] [CrossRef]
- Mahama, G.Y.; Prasad, P.V.V.; Roozeboom, K.L.; Nippert, J.B.; Rice, C.W. Reduction of nitrogen fertilizer requirements and nitrous oxide emissions using legume cover crops in a no-tillage sorghum production system. Sustainability 2020, 12, 4403. [Google Scholar] [CrossRef]
- Deng, X.P.; Shan, L.; Zhang, H.; Turner, N.C. Improving agricultural water use efficiency in arid and semiarid areas of China. Agric Water Manag. 2006, 80, 23–40. [Google Scholar] [CrossRef]
- Barton, L.; Gleeson, D.B.; Maccarone, L.D.; Zúñiga, L.P.; Murphy, D.V. Is liming soil a strategy for mitigating nitrous oxide emissions from semi-arid soils? Soil Biol. Biochem. 2013, 62, 28–35. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Gao, D.; Bai, E.; Li, M.; Zhao, C.; Yu, K.; Hagedorn, F. Responses of soil nitrogen and phosphorus cycling to drying and rewetting cycles: A meta-analysis. Soil Biol. Biochem. 2020, 148, 107896. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Rochette, P.; Angers, D.A.; Bélanger, G.; Chantigny, M.H.; Prévost, D.; Lévesque, G. Emissions of N2O from alfalfa and soybean crops in eastern canada. Soil Sci. Soc. Am. J. 2004, 68, 493–506. [Google Scholar] [CrossRef]
- Friedl, J.; Scheer, C.; Rowlings, D.W.; McIntosh, H.V.; Strazzabosco, A.; Warner, D.I.; Grace, P. Denitrification losses from an intensively managed sub-tropical pasture—Impact of soil moisture on the partitioning of N2 and N2O emissions. Soil Biol. Biochem. 2016, 92, 58–66. [Google Scholar] [CrossRef]
- Farquharson, R.; Baldock, J. Concepts in modelling N2O emissions from land use. Plant Soil 2008, 309, 147–167. [Google Scholar] [CrossRef]
- Davidson, E.A.; Trumbore, S.E. Gas diffusivity and production of CO2 in deep soils of the eastern Amazon. Tellus Ser. B 1995, 47, 550–565. [Google Scholar] [CrossRef] [Green Version]
- Millington, R.J. Gas dffusion in porous media. Science 1959, 130, 100–102. [Google Scholar] [CrossRef]
- Chamindu Deepagoda, T.K.K.; Clough, T.J.; Thomas, S.M.; Balaine, N.; Elberling, B. Density effects on soil-water characteristics, soil-gas diffusivity, and emissions of N2O and N2 from a re-packed pasture soil. Soil Sci. Soc. Am. J. 2019, 83, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Balaine, N.; Clough, T.J.; Beare, M.H.; Thomas, S.M.; Meenken, E.D.; Ross, J.G. Changes in relative gas diffusivity explain soil nitrous oxide flux dynamics. Soil Sci. Soc. Am. J. 2013, 77, 1496–1505. [Google Scholar] [CrossRef]
- Owens, J.; Clough, T.J.; Laubach, J.; Hunt, J.E.; Venterea, R.T.; Phillips, R.L. Nitrous oxide fluxes, soil oxygen, and denitrification potential of urine- and non-urine-treated soil under different irrigation frequencies. J. Environ. Qual. 2016, 45, 1169–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaine, N.; Clough, T.J.; Beare, M.H.; Thomas, S.M.; Meenken, E.D. Soil gas diffusivity controls N2O and N2 emissions and their ratio. Soil Sci. Soc. Am. J. 2016, 80, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Rolston, D.E.; Moldrup, P. Gas Diffusivity. In Methods of Soil Analysis, Part 4 Physical Methods; Topp, G.C., Dane, J.H., Eds.; SSSA: Madison, WI, USA, 2002; pp. 1113–1139. [Google Scholar]
- Harrison-Kirk, T.; Thomas, S.M.; Clough, T.J.; Beare, M.H.; van der Weerden, T.J.; Meenken, E.D. Compaction influences N2O and N2 emissions from 15N-labeled synthetic urine in wet soils during successive saturation/drainage cycles. Soil Biol. Biochem. 2015, 88, 178–188. [Google Scholar] [CrossRef]
- Li, Y.; Clough, T.J.; Moinet, G.Y.K.; Whitehead, D. Emissions of nitrous oxide, dinitrogen and carbon dioxide from three soils amended with carbon substrates under varying soil matric potentials. Eur. J. Soil Sci. 2021, 72, 2261–2275. [Google Scholar] [CrossRef]
- Parkin, T.B.; Venterea, R.T.; Hargreaves, S.K. Calculating the detection limits of chamber-based soil greenhouse gas flux measurements. J. Env. Qual. 2012, 41, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Liu, G. Soil Physical and Chemical Analysis and Description of Soil Profiles; Standards Press of China: Beijing, China, 1996; pp. 5–151. [Google Scholar]
- Moldrup, P.; Chamindu Deepagoda, T.K.K.; Hamamoto, S.; Komatsu, T.; Kawamoto, K.; Rolston, D.E.; Wollesen de Jonge, L. Structure-dependent water-induced linear reduction model for predicting gas diffusivity and tortuosity in repacked and intact soil. Vadose Zone J. 2013, 12, 1–11. [Google Scholar] [CrossRef]
- Folina, A.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Katsenios, N.; Efthimiadou, A.; Travlos, I.S.; Roussis, I.; Darawsheh, M.K.; Papastylianou, P.; et al. Evaluation of various nitrogen indices in N-fertilizers with inhibitors in field crops: A review. Agronomy 2021, 11, 418. [Google Scholar] [CrossRef]
- Herrmann, A.; Witter, E. Sources of C and N contributing to the flush in mineralization upon freeze-thaw cycles in soils. Soil Biol. Biochem. 2002, 34, 1495–1505. [Google Scholar] [CrossRef]
- Chapuis-lardy, L.; Wrage, N.; Metay, A.; Chotte, J.L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Change Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- O’Hara, G.W.; Daniel, R.M. Rhizobial denitrification: A review. Soil Biol. Biochem. 1985, 17, 1–9. [Google Scholar] [CrossRef]
- Gates, C.; Muller, W. Nodule and plant development in the soyabean, Glycine max (L.) Merr.: Growth response to nitrogen, phosphorus and sulfur. Aust. J. Bot. 1979, 27, 203–215. [Google Scholar] [CrossRef]
- Teepe, R.; Brumme, R.; Beese, F. Nitrous oxide emissions from soil during freezing and thawing periods. Soil Biol. Biochem. 2001, 33, 1269–1275. [Google Scholar] [CrossRef]
- Burton, D.L.; Beauchamp, E.G. Profile nitrous oxide and carbon dioxide concentrations in a soil subject to freezing. Soil Sci. Soc. Am. J. 1994, 58, 115–122. [Google Scholar] [CrossRef]
- Owens, J. Understanding How Pasture Irrigation Influences Soil Nitrous Oxide Fluxes and Nitrous Oxide Reductase; Lincoln University: Lincoln, New Zealand, 2016. [Google Scholar]
- Rousset, C.; Clough, T.J.; Grace, P.R.; Rowlings, D.W.; Scheer, C. Irrigation scheduling with soil gas diffusivity as a decision tool to mitigate N2O emissions from a urine-affected pasture. Agriculture 2021, 11, 443. [Google Scholar] [CrossRef]
- Li, Y.; Moinet, G.Y.K.; Clough, T.J.; Hunt, J.E.; Whitehead, D. Net ecosystem carbon exchange for Bermuda grass growing in mesocosms as affected by irrigation frequency. Pedosphere 2022, 32, 393–401. [Google Scholar] [CrossRef]
| Layer (mm) | SOC (mg g−1) | TN (mg g−1) | C/N |
|---|---|---|---|
| 0–100 | 10.23 ± 1.61 | 1.12 ± 0.35 | 9.13 ± 1.70 |
| 100–200 | 8.53 ± 0.87 | 0.88 ± 0.21 | 9.74 ± 2.19 |
| 200–300 | 7.54 ± 0.63 | 0.88 ± 0.14 | 8.61 ± 1.14 |
| Treatment | DM Yield (kg ha−1) | Height (mm) | LAI | |
|---|---|---|---|---|
| A | N0 | 4635.93 ± 221.76 | 504.4 ± 26.5 | 2.73 ± 0.20 |
| N50 | 4855.19 ± 537.69 | 551.1 ± 42.3 | 2.95 ± 0.32 | |
| N100 | 4848.89 ± 401.11 | 581.1 ± 36.9 | 3.06 ± 0.27 | |
| N150 | 4985.93 ± 396.86 | 584.4 ± 50.8 | 3.11 ± 0.26 | |
| B | N0 | 3281.56 ± 270.76 | 284.7 ± 20.5 | 1.99 ± 0.53 |
| N50 | 3490.23 ± 467.65 | 318.4 ± 35.5 | 1.88 ± 0.42 | |
| N100 | 3622.83 ± 578.46 | 299.7 ± 25.9 | 2.09 ± 0.37 | |
| N150 | 3594.97 ± 933.39 | 302.3 ± 33.7 | 1.88 ± 0.39 |
| Factor | Sum of Squares | df | Sum of Squares | F | p |
|---|---|---|---|---|---|
| N | 0.008 | 3 | 0.003 | 112.68 | <0.001 |
| R | 0.005 | 9 | 0.001 | 24.41 | <0.001 |
| N × R | 0.010 | 27 | <0.001 | 14.38 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, G.; Shurpali, N.J.; Shen, Y. Nitrogen Addition Affects Nitrous Oxide Emissions of Rainfed Lucerne Grassland. Int. J. Environ. Res. Public Health 2022, 19, 7789. https://doi.org/10.3390/ijerph19137789
Li Y, Wang G, Shurpali NJ, Shen Y. Nitrogen Addition Affects Nitrous Oxide Emissions of Rainfed Lucerne Grassland. International Journal of Environmental Research and Public Health. 2022; 19(13):7789. https://doi.org/10.3390/ijerph19137789
Chicago/Turabian StyleLi, Yuan, Gang Wang, Narasinha J. Shurpali, and Yuying Shen. 2022. "Nitrogen Addition Affects Nitrous Oxide Emissions of Rainfed Lucerne Grassland" International Journal of Environmental Research and Public Health 19, no. 13: 7789. https://doi.org/10.3390/ijerph19137789
APA StyleLi, Y., Wang, G., Shurpali, N. J., & Shen, Y. (2022). Nitrogen Addition Affects Nitrous Oxide Emissions of Rainfed Lucerne Grassland. International Journal of Environmental Research and Public Health, 19(13), 7789. https://doi.org/10.3390/ijerph19137789








