The Impact of COVID-19 Infection on Cognitive Function and the Implication for Rehabilitation: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- Population: Healthy adults (without pre-existing conditions) with COVID-19 diagnosed using PCR. Studies with patients suffering from neuropsychiatric disorders before the infection were therefore not included in this analysis.
- Exposure: COVID-19 infection.
- Outcome: Any outcomes related to cognitive disorders, loss of cognitive functions, and/or cognitive fatigue.
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Search Results
3.2. Characteristics of the Participants
3.3. Systematic Review
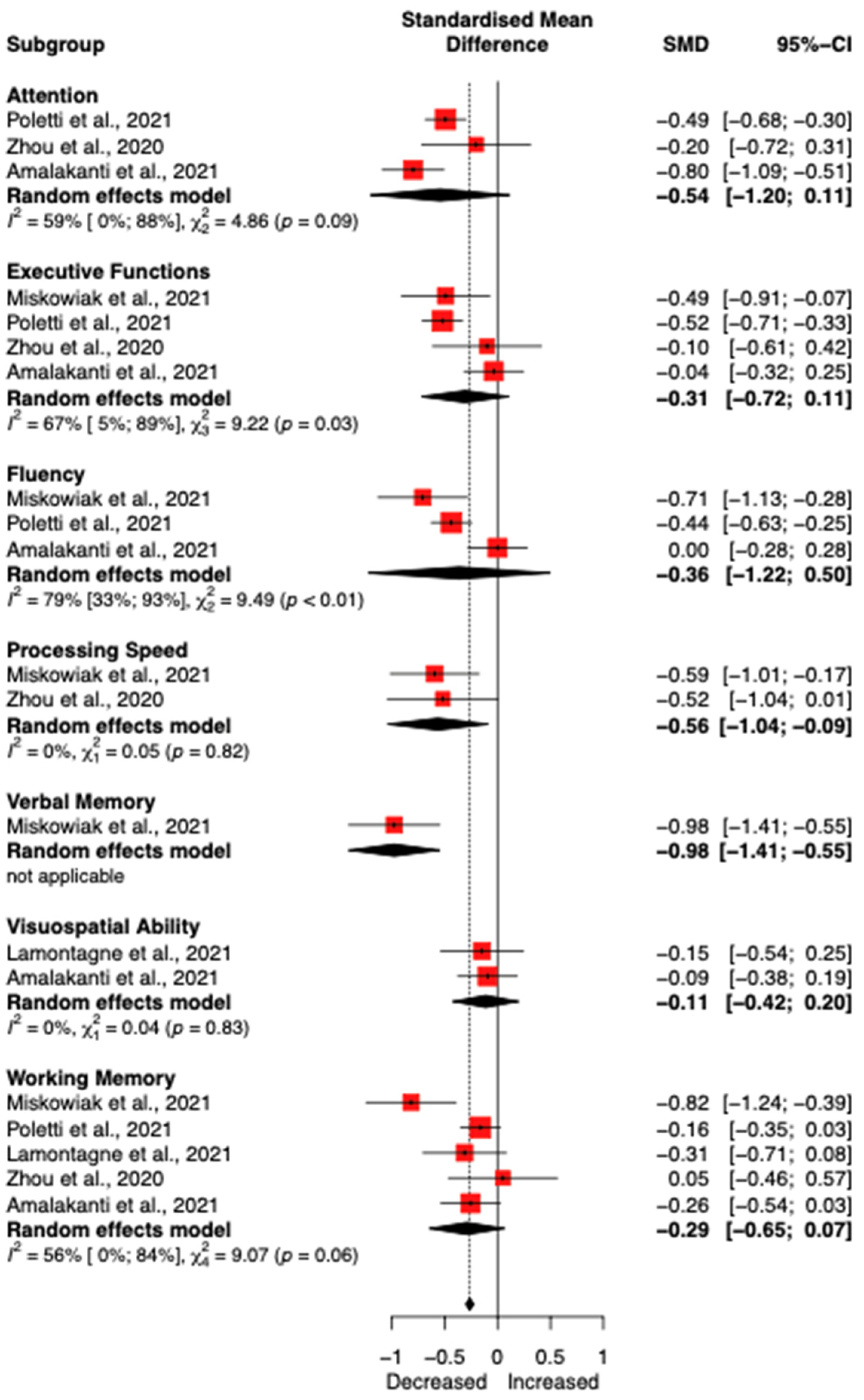
3.4. Meta-Analysis
4. Discussion
4.1. Main Findings
4.2. Limitations of the Systematic Review
4.3. Rehabilitation Strategies
4.4. Implications for the Rehabilitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, E.; Du, H.; Gardner, L. An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Gabutti, G.; d’Anchera, E.; Sandri, F.; Savio, M.; Stefanati, A. Coronavirus: Update Related to the Current Outbreak of COVID-19. Infect. Dis. Ther. 2020, 9, 241–253. [Google Scholar] [CrossRef] [PubMed]
- 2019 Novel Coronavirus COVID-19 (2019-NCoV) Data Repository by Johns Hopkins CSSE. Available online: https://github.com/CSSEGISandData/COVID-19 (accessed on 17 May 2022).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Burn, E.; Tebé, C.; Fernandez-Bertolin, S.; Aragon, M.; Recalde, M.; Roel, E.; Prats-Uribe, A.; Prieto-Alhambra, D.; Duarte-Salles, T. The Natural History of Symptomatic COVID-19 during the First Wave in Catalonia. Nat. Commun. 2021, 12, 777. [Google Scholar] [CrossRef]
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: From an Acute to Chronic Disease? Potential Long-Term Health Consequences. Crit. Rev. Clin. Lab. Sci. 2021, 58, 297–310. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Callard, F.; Perego, E. How and Why Patients Made Long Covid. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef]
- Vogel, A.; Salem, L.C.; Andersen, B.B.; Waldemar, G. Differences in Quantitative Methods for Measuring Subjective Cognitive Decline—Results from a Prospective Memory Clinic Study. Int. Psychogeriatr. 2016, 28, 1513–1520. [Google Scholar] [CrossRef]
- Ofen, N.; Shing, Y.L. From Perception to Memory: Changes in Memory Systems across the Lifespan. Neurosci. Biobehav. Rev. 2013, 37, 2258–2267. [Google Scholar] [CrossRef]
- Bonnechère, B. Evaluation of Processing Speed of Different Cognitive Functions Across the Life Span Using Cognitive Mobile Games. Games Health J. 2022, 11, 132–140. [Google Scholar] [CrossRef]
- Staffolani, S.; Iencinella, V.; Cimatti, M.; Tavio, M. Long COVID-19 Syndrome as a Fourth Phase of SARS-CoV-2 Infection. Infez. Med. 2022, 30, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A Comparison Study of Specificity and Sensitivity in Three Search Tools for Qualitative Systematic Reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsrizi, P.; Gladstone, B.P.; Carrara, E.; Luise, D.; Cona, A.; Bovo, C.; Tacconelli, E. Variation of Effect Estimates in the Analysis of Mortality and Length of Hospital Stay in Patients with Infections Caused by Bacteria-Producing Extended-Spectrum Beta-Lactamases: A Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e030266. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; ISBN 978-1-119-53662-8. [Google Scholar]
- Carter, E.C.; Schönbrodt, F.D.; Gervais, W.M.; Hilgard, J. Correcting for Bias in Psychology: A Comparison of Meta-Analytic Methods. Adv. Methods Pract. Psychol. Sci. 2019, 2, 115–144. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [Green Version]
- Pustejovsky, J.E.; Rodgers, M.A. Testing for Funnel Plot Asymmetry of Standardized Mean Differences. Res. Synth. Methods 2019, 10, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent Neurocognitive Deficits after Recovery from Mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, S.; Chen, J.; Wei, N.; Wang, D.; Lyu, H.; Shi, C.; Hu, S. The Landscape of Cognitive Function in Recovered COVID-19 Patients. J. Psychiatr. Res. 2020, 129, 98–102. [Google Scholar] [CrossRef]
- Alemanno, F.; Houdayer, E.; Parma, A.; Spina, A.; Del Forno, A.; Scatolini, A.; Angelone, S.; Brugliera, L.; Tettamanti, A.; Beretta, L.; et al. COVID-19 Cognitive Deficits after Respiratory Assistance in the Subacute Phase: A COVID-Rehabilitation Unit Experience. PLoS ONE 2021, 16, e0246590. [Google Scholar] [CrossRef]
- Amalakanti, S.; Arepalli, K.V.R.; Jillella, J.P. Cognitive Assessment in Asymptomatic COVID-19 Subjects. Virusdisease 2021, 32, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of Cognitive Function in Patients After COVID-19 Infection. JAMA Netw. Open 2021, 4, e2130645. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Wu, S.; Mera, R.M.; Costa, A.F.; Recalde, B.Y.; Issa, N.P. Cognitive Decline among Individuals with History of Mild Symptomatic SARS-CoV-2 Infection: A Longitudinal Prospective Study Nested to a Population Cohort. Eur. J. Neurol. 2021, 28, 3245–3253. [Google Scholar] [CrossRef]
- Dressing, A.; Bormann, T.; Blazhenets, G.; Schroeter, N.; Walter, L.I.; Thurow, J.; August, D.; Hilger, H.; Stete, K.; Gerstacker, K.; et al. Neuropsychological Profiles and Cerebral Glucose Metabolism in Neurocognitive Long COVID-Syndrome. J. Nucl. Med. 2021, 63, jnumed.121.262677. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive Deficits in People Who Have Recovered from COVID-19. EClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef]
- Hosp, J.A.; Dressing, A.; Blazhenets, G.; Bormann, T.; Rau, A.; Schwabenland, M.; Thurow, J.; Wagner, D.; Waller, C.; Niesen, W.D.; et al. Cognitive Impairment and Altered Cerebral Glucose Metabolism in the Subacute Stage of COVID-19. Brain 2021, 144, 1263–1276. [Google Scholar] [CrossRef]
- Lamontagne, S.J.; Winters, M.F.; Pizzagalli, D.A.; Olmstead, M.C. Post-Acute Sequelae of COVID-19: Evidence of Mood & Cognitive Impairment. Brain Behav. Immun. Health 2021, 17, 100347. [Google Scholar] [CrossRef]
- Mattioli, F.; Stampatori, C.; Righetti, F.; Sala, E.; Tomasi, C.; De Palma, G. Neurological and Cognitive Sequelae of Covid-19: A Four Month Follow-Up. J. Neurol. 2021, 268, 4422–4428. [Google Scholar] [CrossRef]
- Méndez, R.; Balanzá-Martínez, V.; Luperdi, S.C.; Estrada, I.; Latorre, A.; González-Jiménez, P.; Feced, L.; Bouzas, L.; Yépez, K.; Ferrando, A.; et al. Short-Term Neuropsychiatric Outcomes and Quality of Life in COVID-19 Survivors. J. Intern. Med. 2021, 290, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Johnsen, S.; Sattler, S.M.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C.M. Cognitive Impairments Four Months after COVID-19 Hospital Discharge: Pattern, Severity and Association with Illness Variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Norrefalk, J.-R.; Borg, K.; Bileviciute-Ljungar, I. Self-Scored Impairments in Functioning and Disability in Post-COVID Syndrome Following Mild COVID-19 Infection. J. Rehabil. Med. 2021, 53, 2833. [Google Scholar] [CrossRef]
- Patel, R.; Savrides, I.; Cahalan, C.; Doulatani, G.; O’Dell, M.W.; Toglia, J.; Jaywant, A. Cognitive Impairment and Functional Change in COVID-19 Patients Undergoing Inpatient Rehabilitation. Int. J. Rehabil. Res. 2021, 44, 285–288. [Google Scholar] [CrossRef]
- Poletti, S.; Palladini, M.; Mazza, M.G.; De Lorenzo, R. COVID-19 BioB Outpatient Clinic Study Group; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Long-Term Consequences of COVID-19 on Cognitive Functioning up to 6 Months after Discharge: Role of Depression and Impact on Quality of Life. Eur. Arch. Psychiatry Clin. Neurosci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.-F.; Minguet, P.; Colson, C.; Kellens, I.; Chaabane, S.; Delanaye, P.; Cavalier, E.; Chase, J.G.; Lambermont, B.; Misset, B. Post-Intensive Care Syndrome after a Critical COVID-19: Cohort Study from a Belgian Follow-up Clinic. Ann. Intensive Care 2021, 11, 118. [Google Scholar] [CrossRef]
- Solaro, C.; Gamberini, G.; Masuccio, F.G. Cognitive Impairment in Young COVID-19 Patients: The Tip of the Iceberg? Neurol. Sci. 2021, 42, 4865–4866. [Google Scholar] [CrossRef]
- van den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; van Hees, H.W.H.; van Helvoort, H.; van den Boogaard, M.; van der Hoeven, H.; et al. Comprehensive Health Assessment 3 Months After Recovery from Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, e1089–e1098. [Google Scholar] [CrossRef]
- Vyas, A.; Raja Panwar, V.; Mathur, V.; Patel, P.; Mathur, S.; Sharma, A.; Babu Panwar, R.; Gupta, R. Mild Cognitive Impairment in COVID-19 Survivors: Measuring the Brain Fog. Int. J. Ment. Health 2021, 51, 142–151. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, C.; Sun, Y.; Huang, W.; Ye, K. Cognitive Disorders Associated with Hospitalization of COVID-19: Results from an Observational Cohort Study. Brain Behav. Immun. 2021, 91, 383–392. [Google Scholar] [CrossRef]
- Aiello, E.N.; Fiabane, E.; Manera, M.R.; Radici, A.; Grossi, F.; Ottonello, M.; Pain, D.; Pistarini, C. Screening for Cognitive Sequelae of SARS-CoV-2 Infection: A Comparison between the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). Neurol. Sci. 2022, 43, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Bonizzato, S.; Ghiggia, A.; Ferraro, F.; Galante, E. Cognitive, Behavioral, and Psychological Manifestations of COVID-19 in Post-Acute Rehabilitation Setting: Preliminary Data of an Observational Study. Neurol. Sci. 2022, 43, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Rumbea, D.A.; Recalde, B.Y.; Mera, R.M. Cognitive Sequelae of Long COVID May Not Be Permanent: A Prospective Study. Eur. J. Neurol. 2022, 29, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Chen, Y.; Wang, Q.-H.; Wang, L.-R.; Jiang, L.; Yang, Y.; Chen, X.; Li, Y.; Cen, Y.; Xu, C.; et al. One-Year Trajectory of Cognitive Changes in Older Survivors of COVID-19 in Wuhan, China: A Longitudinal Cohort Study. JAMA Neurol. 2022, 79, 509–517. [Google Scholar] [CrossRef]
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-Acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life, and Participation. Am. J. Phys. Med. Rehabil. 2022, 101, 48–52. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Lou, J.J.; Movassaghi, M.; Gordy, D.; Olson, M.G.; Zhang, T.; Khurana, M.S.; Chen, Z.; Perez-Rosendahl, M.; Thammachantha, S.; Singer, E.J.; et al. Neuropathology of COVID-19 (Neuro-COVID): Clinicopathological Update. Free Neuropathol. 2021, 2, 2. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after Breakthrough SARS-CoV-2 Infection. Nat. Med. 2022. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of Long Covid Symptoms after COVID-19 Vaccination: Community Based Cohort Study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk Factors and Disease Profile of Post-Vaccination SARS-CoV-2 Infection in UK Users of the COVID Symptom Study App: A Prospective, Community-Based, Nested, Case-Control Study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- CDC VAERS COVID Vaccine Adverse Event Reports. Available online: https://openvaers.com/covid-data (accessed on 20 June 2022).
- Asadi-Pooya, A.A.; Nemati, H.; Shahisavandi, M.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; et al. Long COVID in Children and Adolescents. World J. Pediatr. 2021, 17, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Ulyte, A.; Puhan, M.A.; Kriemler, S. Long-Term Symptoms After SARS-CoV-2 Infection in Children and Adolescents. JAMA 2021, 326, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Allin, B.; Nugawela, M.D.; Rojas, N.; Dalrymple, E.; Pinto Pereira, S.; Soni, M.; Knight, M.; Cheung, E.Y.; Heyman, I.; et al. Long COVID (Post-COVID-19 Condition) in Children: A Modified Delphi Process. Arch. Dis. Child. 2022, 107, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of Low-Grade Inflammation in Depression: A Systematic Review and Meta-Analysis of CRP Levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lana, S.; Marquié, M.; Ruiz, A.; Boada, M. Cognitive and Neuropsychiatric Manifestations of COVID-19 and Effects on Elderly Individuals with Dementia. Front. Aging Neurosci. 2020, 12, 588872. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social Stress Induces Neurovascular Pathology Promoting Depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27.e1. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- De Roeck, A.; Van Broeckhoven, C.; Sleegers, K. The Role of ABCA7 in Alzheimer’s Disease: Evidence from Genomics, Transcriptomics and Methylomics. Acta Neuropathol. 2019, 138, 201–220. [Google Scholar] [CrossRef] [Green Version]
- Zeng, N.; Zhao, Y.-M.; Yan, W.; Li, C.; Lu, Q.-D.; Liu, L.; Ni, S.-Y.; Mei, H.; Yuan, K.; Shi, L.; et al. A Systematic Review and Meta-Analysis of Long Term Physical and Mental Sequelae of COVID-19 Pandemic: Call for Research Priority and Action. Mol. Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Bronheim, R.S.; Cotter, E.; Skolasky, R.L. Cognitive Impairment Is Associated with Greater Preoperative Symptoms, Worse Health-Related Quality of Life, and Reduced Likelihood of Recovery after Cervical and Lumbar Spine Surgery. N. Am. Spine Soc. J. 2022, 10, 100128. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Long-Term Consequences of COVID-19 at 6 Months and Above: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6865. [Google Scholar] [CrossRef] [PubMed]
- Pistarini, C.; Fiabane, E.; Houdayer, E.; Vassallo, C.; Manera, M.R.; Alemanno, F. Cognitive and Emotional Disturbances Due to COVID-19: An Exploratory Study in the Rehabilitation Setting. Front. Neurol. 2021, 12, 643646. [Google Scholar] [CrossRef]
- Fugazzaro, S.; Contri, A.; Esseroukh, O.; Kaleci, S.; Croci, S.; Massari, M.; Facciolongo, N.C.; Besutti, G.; Iori, M.; Salvarani, C.; et al. Rehabilitation Interventions for Post-Acute COVID-19 Syndrome: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5185. [Google Scholar] [CrossRef] [PubMed]
- Vance, H.; Maslach, A.; Stoneman, E.; Harmes, K.; Ransom, A.; Seagly, K.; Furst, W. Addressing Post-COVID Symptoms: A Guide for Primary Care Physicians. J. Am. Board Fam. Med. 2021, 34, 1229–1242. [Google Scholar] [CrossRef]
- Dixit, S.; Borghi-Silva, A.; Bairapareddy, K.C. Revisiting Pulmonary Rehabilitation during COVID-19 Pandemic: A Narrative Review. Rev. Cardiovasc. Med. 2021, 22, 315–327. [Google Scholar] [CrossRef]
- Halle, M.; Bloch, W.; Niess, A.M.; Predel, H.-G.; Reinsberger, C.; Scharhag, J.; Steinacker, J.; Wolfarth, B.; Scherr, J.; Niebauer, J. Exercise and Sports after COVID-19—Guidance from a Clinical Perspective. Transl. Sports Med. 2021, 4, 310–318. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz Martínez, B.J.; Bernal-Morel, E.; Courel-Ibáñez, J. Post-COVID-19 Syndrome and the Potential Benefits of Exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef]
- Shaw, T.; McGregor, D.; Brunner, M.; Keep, M.; Janssen, A.; Barnet, S. What Is EHealth (6)? Development of a Conceptual Model for EHealth: Qualitative Study with Key Informants. J. Med. Internet Res. 2017, 19, e324. [Google Scholar] [CrossRef]
- Cottrell, M.A.; Galea, O.A.; O’Leary, S.P.; Hill, A.J.; Russell, T.G. Real-Time Telerehabilitation for the Treatment of Musculoskeletal Conditions Is Effective and Comparable to Standard Practice: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2017, 31, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Howard, I.M.; Kaufman, M.S. Telehealth Applications for Outpatients with Neuromuscular or Musculoskeletal Disorders. Muscle Nerve 2018, 58, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gerez, J.J.; Saavedra-Hernandez, M.; Anarte-Lazo, E.; Bernal-Utrera, C.; Perez-Ale, M.; Rodriguez-Blanco, C. Short-Term Effects of a Respiratory Telerehabilitation Program in Confined COVID-19 Patients in the Acute Phase: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 7511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, W.; Zhan, C.; Liu, S.; Yin, Z.; Wang, J.; Chong, Y.; Zheng, C.; Fang, X.; Cheng, W.; et al. A Telerehabilitation Programme in Post-Discharge COVID-19 Patients (TERECO): A Randomised Controlled Trial. Thorax 2021, 77, 697–706. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, C.; Bernal-Utrera, C.; Anarte-Lazo, E.; Saavedra-Hernandez, M.; De-La-Barrera-Aranda, E.; Serrera-Figallo, M.A.; Gonzalez-Martin, M.; Gonzalez-Gerez, J.J. Breathing Exercises versus Strength Exercises through Telerehabilitation in Coronavirus Disease 2019 Patients in the Acute Phase: A Randomized Controlled Trial. Clin. Rehabil. 2022, 36, 486–497. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Sujkowski, A.; Hong, L.; Wessells, R.J.; Todi, S.V. The Protective Role of Exercise against Age-Related Neurodegeneration. Ageing Res. Rev. 2022, 74, 101543. [Google Scholar] [CrossRef]
- Jia, R.-X.; Liang, J.-H.; Xu, Y.; Wang, Y.-Q. Effects of Physical Activity and Exercise on the Cognitive Function of Patients with Alzheimer Disease: A Meta-Analysis. BMC Geriatr. 2019, 19, 181. [Google Scholar] [CrossRef]
- Panza, G.A.; Taylor, B.A.; MacDonald, H.V.; Johnson, B.T.; Zaleski, A.L.; Livingston, J.; Thompson, P.D.; Pescatello, L.S. Can Exercise Improve Cognitive Symptoms of Alzheimer’s Disease? J. Am. Geriatr. Soc. 2018, 66, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Brasure, M.; Desai, P.; Davila, H.; Nelson, V.A.; Calvert, C.; Jutkowitz, E.; Butler, M.; Fink, H.A.; Ratner, E.; Hemmy, L.S.; et al. Physical Activity Interventions in Preventing Cognitive Decline and Alzheimer-Type Dementia: A Systematic Review. Ann. Intern. Med. 2018, 168, 30–38. [Google Scholar] [CrossRef]
- Meng, Q.; Yin, H.; Wang, S.; Shang, B.; Meng, X.; Yan, M.; Li, G.; Chu, J.; Chen, L. The Effect of Combined Cognitive Intervention and Physical Exercise on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Meta-Analysis of Randomized Controlled Trials. Aging Clin. Exp. Res. 2022, 34, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Wahezi, S.E.; Kohan, L.R.; Spektor, B.; Brancolini, S.; Emerick, T.; Fronterhouse, J.M.; Luedi, M.M.; Colon, M.A.; Kitei, P.M.; Anitescu, M.; et al. Telemedicine and Current Clinical Practice Trends in the COVID-19 Pandemic. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, O.; Gillet, A.; Tack, J.; Reychler, G.; Guatteri, M.; Ballarin, A.; Thomas, J.; Espinoza, R.; Bonnier, F.; Norrenberg, M.; et al. Development of a Medium Care Unit Using an Inexperienced Respiratory Staff: Lessons Learned during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 7349. [Google Scholar] [CrossRef]
- Lugo-Agudelo, L.H.; Cruz Sarmiento, K.M.; Spir Brunal, M.A.; Velásquez Correa, J.C.; Posada Borrero, A.M.; Fernanda Mesa Franco, L.; Di Dio Castagna Ianini, R.; Ramírez Pérez Lis, P.A.; Vélez, C.M.; Patiño Lugo, D.F.; et al. Adaptations for Rehabilitation Services during the COVID-19 Pandemic Proposed by Scientific Organizations and Rehabilitation Professionals. J. Rehabil. Med. 2021, 53, jrm00228. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Marshall, C.J.; Dobson, F.; Kasza, J.; Lonsdale, C.; Hinman, R.S. Does a Web-Based Exercise Programming System Improve Home Exercise Adherence for People With Musculoskeletal Conditions?: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2019, 98, 850–858. [Google Scholar] [CrossRef]
- Lambert, T.E.; Harvey, L.A.; Avdalis, C.; Chen, L.W.; Jeyalingam, S.; Pratt, C.A.; Tatum, H.J.; Bowden, J.L.; Lucas, B.R. An App with Remote Support Achieves Better Adherence to Home Exercise Programs than Paper Handouts in People with Musculoskeletal Conditions: A Randomised Trial. J. Physiother. 2017, 63, 161–167. [Google Scholar] [CrossRef]
- Lawford, B.J.; Delany, C.; Bennell, K.L.; Hinman, R.S. “I Was Really Sceptical...But It Worked Really Well”: A Qualitative Study of Patient Perceptions of Telephone-Delivered Exercise Therapy by Physiotherapists for People with Knee Osteoarthritis. Osteoarthr. Cartil. 2018, 26, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Moffet, H.; Tousignant, M.; Nadeau, S.; Mérette, C.; Boissy, P.; Corriveau, H.; Marquis, F.; Cabana, F.; Belzile, É.L.; Ranger, P.; et al. Patient Satisfaction with In-Home Telerehabilitation After Total Knee Arthroplasty: Results from a Randomized Controlled Trial. Telemed. J. e-Health 2017, 23, 80–87. [Google Scholar] [CrossRef]
- Bonnechère, B.; Langley, C.; Sahakian, B.J. The Use of Commercial Computerised Cognitive Games in Older Adults: A Meta-Analysis. Sci. Rep. 2020, 10, 15276. [Google Scholar] [CrossRef]
- Zhang, H.; Huntley, J.; Bhome, R.; Holmes, B.; Cahill, J.; Gould, R.L.; Wang, H.; Yu, X.; Howard, R. Effect of Computerised Cognitive Training on Cognitive Outcomes in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e027062. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Zhao, B.; Liu, Z.; Weng, Y.; Zhou, L. Effectiveness of Computer-Based Training on Post-Stroke Cognitive Rehabilitation: A Systematic Review and Meta-Analysis. Neuropsychol. Rehabil. 2020, 32, 481–497. [Google Scholar] [CrossRef]
- Orgeta, V.; McDonald, K.R.; Poliakoff, E.; Hindle, J.V.; Clare, L.; Leroi, I. Cognitive Training Interventions for Dementia and Mild Cognitive Impairment in Parkinson’s Disease. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lampit, A.; Heine, J.; Finke, C.; Barnett, M.H.; Valenzuela, M.; Wolf, A.; Leung, I.H.K.; Hill, N.T.M. Computerized Cognitive Training in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Neurorehabilit. Neural Repair 2019, 33, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Nandakumar, G. Promoting Healthy Lifestyles Using Information Technology during the COVID-19 Pandemic. Rev. Cardiovasc. Med. 2021, 22, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.; Gordon, W.J.; Stern, A.D. Use of Connected Digital Products in Clinical Research Following the COVID-19 Pandemic: A Comprehensive Analysis of Clinical Trials. BMJ Open 2021, 11, e047341. [Google Scholar] [CrossRef] [PubMed]
- Carl, J.R.; Jones, D.J.; Lindhiem, O.J.; Doss, B.D.; Weingardt, K.R.; Timmons, A.C.; Comer, J.S. Regulating Digital Therapeutics for Mental Health: Opportunities, Challenges, and the Essential Role of Psychologists. Br. J. Clin. Psychol. 2021, 61, 130–135. [Google Scholar] [CrossRef]
- Scott Kruse, C.; Karem, P.; Shifflett, K.; Vegi, L.; Ravi, K.; Brooks, M. Evaluating Barriers to Adopting Telemedicine Worldwide: A Systematic Review. J. Telemed. Telecare 2018, 24, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Rangachari, P.; Mushiana, S.S.; Herbert, K. A Narrative Review of Factors Historically Influencing Telehealth Use across Six Medical Specialties in the United States. Int. J. Environ. Res. Public Health 2021, 18, 4995. [Google Scholar] [CrossRef]
- Almathami, H.K.Y.; Win, K.T.; Vlahu-Gjorgievska, E. Barriers and Facilitators That Influence Telemedicine-Based, Real-Time, Online Consultation at Patients’ Homes: Systematic Literature Review. J. Med. Internet Res. 2020, 22, e16407. [Google Scholar] [CrossRef]
- Engelsma, T.; Jaspers, M.W.M.; Peute, L.W. Considerate MHealth Design for Older Adults with Alzheimer’s Disease and Related Dementias (ADRD): A Scoping Review on Usability Barriers and Design Suggestions. Int. J. Med. Inf. 2021, 152, 104494. [Google Scholar] [CrossRef]
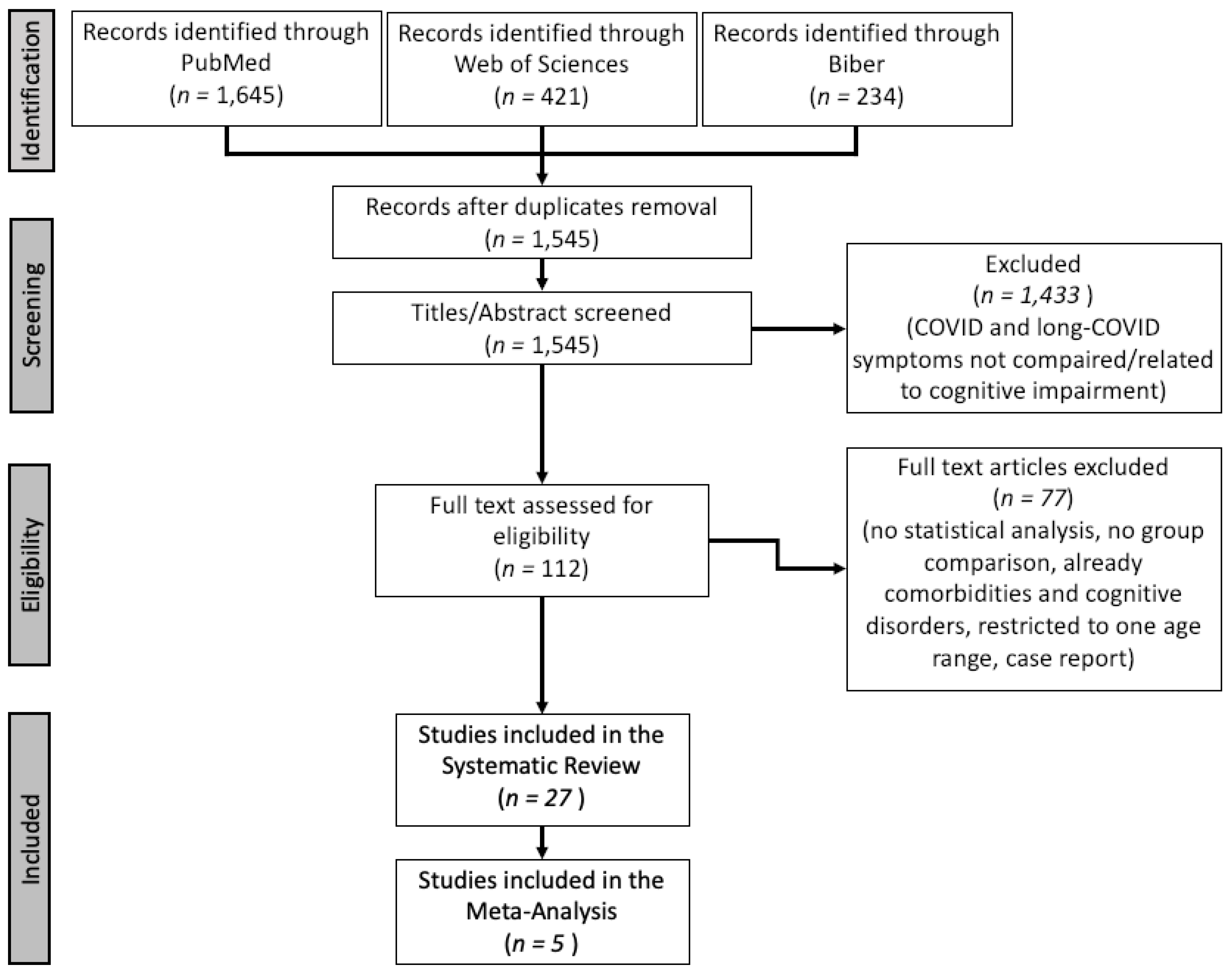
| Study | Country | Recruitment Period | Evaluation Period | Patients | Control | ||||
|---|---|---|---|---|---|---|---|---|---|
| N [% female] | Age | Education | N [% female] | Age | Education | ||||
| Woo et al., 2020 [21] | Germany | July 2020 | 3 months of follow-up | 18 [55%] | 42.2 (14.3) | >12 | 10 [40%] | 38.4 (14.4) | >12 |
| Zhou et al., 2020 [22] | China | Uns. | Uns. | 29 [38%] | 47.0 (10.5) | 12.6 (2.8) | 29 [59%] | 42.5 (6.9) | 12.4 (3.1) |
| Alemanno et al., 2021 [23] | Italy | March to June 2020 | Follow-up: one month after home-discharge | 87 [29%] | 67.2 (12.9) | Uns. | / | / | / |
| Amalakanti et al., 2021 [24] | India | June and July 2020 | Uns. | 93 [52%] | 36.2 (11.7) | Uns. | 102 [55%] | 35.6 (9.8) | Uns. |
| Becker et al., 2021 [25] | USA | April 2020 to May 2021 | Uns. | 740 [63%] | 49.0 (14.2) | 103 less than 12 years | / | / | / |
| Davis et al., 2021 [26] | 56 different countries | September to November 2020 | Follow-up: up to 7 months | 3762 [79%] | 18–80 years old | Uns. | / | / | / |
| Del Brutto et al., 2021 [27] | Ecuador | March to May 2020 | Follow-up: up to 6 months | 50 [63%] | 62.7 (11.9) | Uns. | 28 [63%] | 62.7 (11.9) | Uns. |
| Dressing et al., 2021 [28] | Germany | June 2020 to January 2021 | 202.3 ± 57.5 days after first positive COVID-19-PCR | 31 [64%] | 54.0 (2.1) | Uns. | / | / | / |
| Hampshire et al., 2021 [29] | UK (75,910) and other (5427) | January 2020 to December 2020 | Uns. | 81,337 [55%] | 46.7 (15.7) | * | / | / | / |
| Hosp et al., 2021 [30] | Germany | April to May 2020 | Uns. | 29 [38%] | 65.2 (14.4) | 13.2 (3.0) | / | / | / |
| Lamontagne et al., 2021 [31] | USA & Canada | January 2020 to March 2021 | Uns. | 50 [29%] | 30.8 (9.9) | 16.1 (2.9) | 50 [35%] | 29.1 (9.9) | 15.5 (2.9) |
| Mattioli et al., 2021 [32] | Italy | February 2020 | Follow-up: 4 months | 120 [75%] | 47.8 [26–65] | 16 [8–18] | 30 [73%] | 45.7 [23–62] | 18 [8–18] |
| Méndez et al., 2021 [33] | Spain | March to April 2020 | 1 year after hospital discharge | 171 [42%] | 58.0 [50–68] | 11 [8–16] | / | / | / |
| Miskowiak et al., 2021 [34] | Denmark | March to June 2020 | 3–4 months and 12 months after discharge | 29 [41%] | 56.2 (10.6) | 14.3 (3.9) | 100 [59%] | 56.0 (6.9) | 14.3 (3.0) |
| Norrefalk et al., 2021 [35] | Sweden | Uns. | Follow-up: 6 months | 100 [82%] | 44.5 (10.6) | <9 years (1), 10–12 years (31), >12 years (61), other (7) | / | / | / |
| Patel et al., 2021 [36] | USA | March to August 2020 | Uns. | 77 [36%] | 61.0 (16.6) | Uns. | / | / | / |
| Poletti et al., 2021 [37] | Italy | May 2020 to February 2021 | Follow-up: 1–3 and 6 months | 312 [62%] | 52.6 (8.8) | Uns. | 165 [44%] | 50.5 (9.2) | Uns. |
| Rousseau et al., 2021 [38] | Belgium | March to July 2020 | Follow-up: 3 months | 32 [28%] | 62 [49–68] | Uns. | / | / | / |
| Solaro et al., 2021 [39] | Italy | November 2020 to March 2021 | Uns. | 32 [41%] | 53.7 (4.8) | Uns. | / | / | / |
| Van den Borst et al., 2021 [40] | Netherlands | April to July 2020 | Follow-up: 3 months | 124 [40%] | 59.0 (14.0) | Low (30), Middle (34), High (60) | / | / | / |
| Vyas et al., 2021 [41] | India | April to August 2020 | Uns. | 300 [48%] | 15–70 years old | Uns. | / | / | / |
| Zhou et al., 2021 [42] | China | Uns. | Uns. | 1091 [47%] | 57.1 (9.2) | Uns. | 2793 [52%] | 57.7 (8.6) | Uns. |
| Aiello et al., 2022 [43] | Italy | May 2020 to May 2021 | Uns. | 45 [89%] | 63.3 (11.4) | 11.0 (3.9) | / | / | / |
| Bonizzato et al., 2022 [44] | Italy | Uns. | Follow-up: at discharge and after 3 months | 12 [42%] | 71.3 (10.1) | 7.2 (3.3) | / | / | / |
| Del Brutto et al., 2022 [45] | Ecuador | May to June 2020 | Uns. | 50 [63%] | 62.7 (11.9) | Uns. | 28 [63%] | 62.6 (11.8) | Uns. |
| Liu et al., 2022 [46] | China | February to April 2020 | Uns. | 1438 [52%] | 69 [66–74] | 12 [9–12] | 438 [49%] | 67 [66–74] | 12 [9–12] |
| Tabacof et al., 2022 [47] | USA | March 2020 to March 2021 | Uns. | 156 [69%] | 44 [13–79] | Uns. | / | / | / |
| Study | Assessment Methods | Main Results | Quality * |
|---|---|---|---|
| Woo et al., 2020 [21] | Modified Telephone Interview for Cognitive Status (TICS-M) | Sustained sub-clinical cognitive impairments might be a common complication after recovery from COVID-19 in young adults. | Fair |
| Zhou et al., 2020 [22] | Trail Making Test (TMT), Sign Coding Test (SCT), Continuous Performance Test (CPT), and Digital Span Test (DST) | The study indicated a potential cognitive dysfunction in patients with COVID-19. Sustained attention is linked with the inflammatory level as indicated by CRP. | Fair |
| Alemanno et al., 2021 [23] | MoCA and MMSE | 80% (out of 87 patients) showed neuropsychological impairments and 40% showed mild-to-moderate depression. They partly recovered at one-month follow-up and 43% had post-traumatic stress disorder signs. Those with severe functional deficits showed important cognitive and emotional deficits which might have been influenced by the choice of ventilatory therapy but seem to be age-related. | Good |
| Amalakanti et al., 2021 [24] | MoCA | Even otherwise asymptomatic COVID-19, patients have cognitive impairments, suggesting the need for a detailed psychometric assessment, especially in the elderly population. | Good |
| Becker et al., 2021 [25] | Number Span forward (attention) and backward (working memory), TMT-A and B (processing speed and executive functioning, respectively), phonemic and category fluency (language), and the Hopkins Verbal Learning Test-revised (memory encoding, recall, and recognition) | Relatively high frequency of cognitive impairment several months after COVID-19 recovery. Deficits in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients. | Good |
| Davis et al., 2021 [26] | Two surveys with platform Qualtrics (257 questions) + MRI if memory and/or cognitive dysfunction symptoms | 88.0% of the participants experienced cognitive dysfunction and/or memory loss. By 7 months, lots of the respondents have not yet recovered and have not returned to previous levels of work, and still experience significant symptom burden. | Good |
| Del Brutto et al., 2021 [27] | MoCA | Cognitive decline was highlighted in patients with mild COVID-19 infection | Good |
| Dressing et al., 2021 [28] | Neuropsychological and psychiatric evaluations and Cerebral 18F-FDG PET imaging on 14/31 patients, Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised (BVMT-R), DST, TMT-A and B, Color-Word Interference Test (FWIT), Symbol-Digit Modalities Test (SDMT), semantic and letter fluency test | Minor deficits in cognitive testing six months after infection, suggesting that neuronal causes could possibly be related to the high prevalence of tiredness. | Good |
| Hampshire et al., 2021 [29] | Great British intelligence Test | Recovered COVID-19 patients exhibited significant cognitive deficits vs. controls. Impairments were higher for people who had been hospitalized, but also for non-hospitalized cases who had biological confirmation of COVID-19 infection. | Good |
| Hosp et al., 2021 [30] | The German version of the MoCA and MRI, FDG-PET-SCAN, CSF analysis | MoCA performance was impaired in 18/26 patients. 18FDG PET revealed pathological results in 10/15 patients with predominant frontoparietal hypometabolism. | Good |
| Lamontagne et al., 2021 [31] | Self-reported measures of stress, depression, and anhedonia, as well as the Attention Network Test and cognitive abilities (Attentional Control Scale) | Selective impairment in attention was observed in the COVID-19 group, marked by deficits in executive functioning while alerting and orienting abilities remained intact. Effects were most pronounced among individuals diagnosed 1–4 months before assessment. The COVID-19 recovered group scored significantly higher on perceived stress. | Good |
| Mattioli et al., 2021 [32] | Controlled Oral Word Association by categories, California Verbal Learning Test, TEA attention test, visual reaction times, auditory reaction times, number of errors and of omissions for attention Tower of London test, and MMSE. | No neurological deficits or cognitive impairment in mild-moderate COVID-19 patients 4 months after the diagnosis, but severe emotional disorders were confirmed. | Good |
| Méndez et al., 2021 [33] | Phone questionnaire | Declined cognitive function, psychiatric morbidity and low QoL are observable in moderate to severe COVID-19 survivors, 1 year after hospital discharge. | Good |
| Miskowiak et al., 2021 [34] | Cognitive failure questionnaires and performance-based cognition test battery (Screen for Cognitive Impairment in Psychiatry Danish version and TMT-B) | 59–65% of the 29 patients experience cognitive impairments 3–4 months after hospitalization. More than 80% of patients reported severe daily cognitive difficulties. Poorer pulmonary function and more respiratory symptoms after recovery were associated with more cognitive impairments, suggesting a potential link with brain hypoxia. | Good |
| Norrefalk et al., 2021 [35] | Questionnaire (Functional Compass COVID-19) | Persistent fatigue seems to be the most annoying symptom of post-COVID syndromes in mildly infected participants who developed pronounced impairments in functioning and disability. | Fair |
| Patel et al., 2021 [36] | MoCA | Cognitive improvement over time may reflect natural recovery and/or rehabilitation intervention effects | Fair |
| Poletti et al., 2021 [37] | Neuropsychological and psychiatric evaluations | Cognitive impairment in at least one cognitive function was observed in 1-,3-, and 6-month follow-up patients with no significant difference in cognitive performances between 1-,3-, and 6 months. COVID-19 patients performed the same as healthy control in working memory and verbal memory. Depressive psychopathology was the most predominant factor which, in turn, interacts with cognitive functions in determining the quality of life. Sequelae include signs of cognitive impairment, persist up to 6 months after hospital discharge, and affect the quality of life. | Good |
| Rousseau et al., 2021 [38] | MoCA | The burden of severe COVID-19 and prolonged ICU stay was considerable after 3 months, affecting both functional status and biological parameters. | Good |
| Solaro et al., 2021 [39] | MoCA | A significant cognitive impairment was observed in young sub-acute COVID-19 subjects at the time of hospital discharge. | Fair |
| Van den Borst et al., 2021 [40] | Questionnaires on mental, cognitive, health status, and QoL | Severe problems in several health domains were observed in a substantial number of COVID-19 patients. | Good |
| Vyas et al., 2021 [41] | Brain fog symptoms questionnaire (with a validated measure) | Brain fog was frequent in COVID-19 survivors and significantly higher with COVID-19 severity and in patients who received oxygen or who were placed under ventilator | Good |
| Zhou et al., 2021 [42] | Association analysis across 974 phenotypes and 30 blood biomarkers | Pre-existing Alzheimer’s disease and dementia were identified as top risk factors for hospital admission due to COVID-19, highlighting the necessity of providing adequate protective care for patients with cognitive disorders with this infection. | Good |
| Aiello et al., 2022 [43] | MoCA and MMSE | MMSE and MoCA are able to detect sequelae deficits in COVID-19-recovered individuals who were or were not at risk for cognitive deficits | Good |
| Bonizzato et al., 2022 [44] | MoCA and MMSE | Significant amelioration was found in neuropsychiatry inventory scores, a qualitative improvement has been detected at all tests, after discharge, and after 3 months. | Fair |
| Del Brutto et al., 2022 [45] | MoCA | Long COVID-related cognitive decline may spontaneously improve over time. | Good |
| Liu et al., 2022 [46] | Phone questionnaire (Telephone Interview of Cognitive Status-40 (TICS-40) and Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)) | COVID-19 survival was associated with an increase in the risk of longitudinal cognitive decline | Good |
| Tabacof et al., 2022 [47] | RedCap Survey (Neuro-Qol, EQ-5D-5L) | Persistent symptoms associated with post-acute COVID-19 syndrome seem to impact physical and cognitive function, health-related quality of life, and participation in society. | Fair |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houben, S.; Bonnechère, B. The Impact of COVID-19 Infection on Cognitive Function and the Implication for Rehabilitation: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 7748. https://doi.org/10.3390/ijerph19137748
Houben S, Bonnechère B. The Impact of COVID-19 Infection on Cognitive Function and the Implication for Rehabilitation: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(13):7748. https://doi.org/10.3390/ijerph19137748
Chicago/Turabian StyleHouben, Sarah, and Bruno Bonnechère. 2022. "The Impact of COVID-19 Infection on Cognitive Function and the Implication for Rehabilitation: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 13: 7748. https://doi.org/10.3390/ijerph19137748
APA StyleHouben, S., & Bonnechère, B. (2022). The Impact of COVID-19 Infection on Cognitive Function and the Implication for Rehabilitation: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(13), 7748. https://doi.org/10.3390/ijerph19137748







