Electrochemical Oxidation of Landfill Leachate after Biological Treatment by Electro-Fenton System with Corroding Electrode of Iron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Landfill Leachate Samples and Chemicals
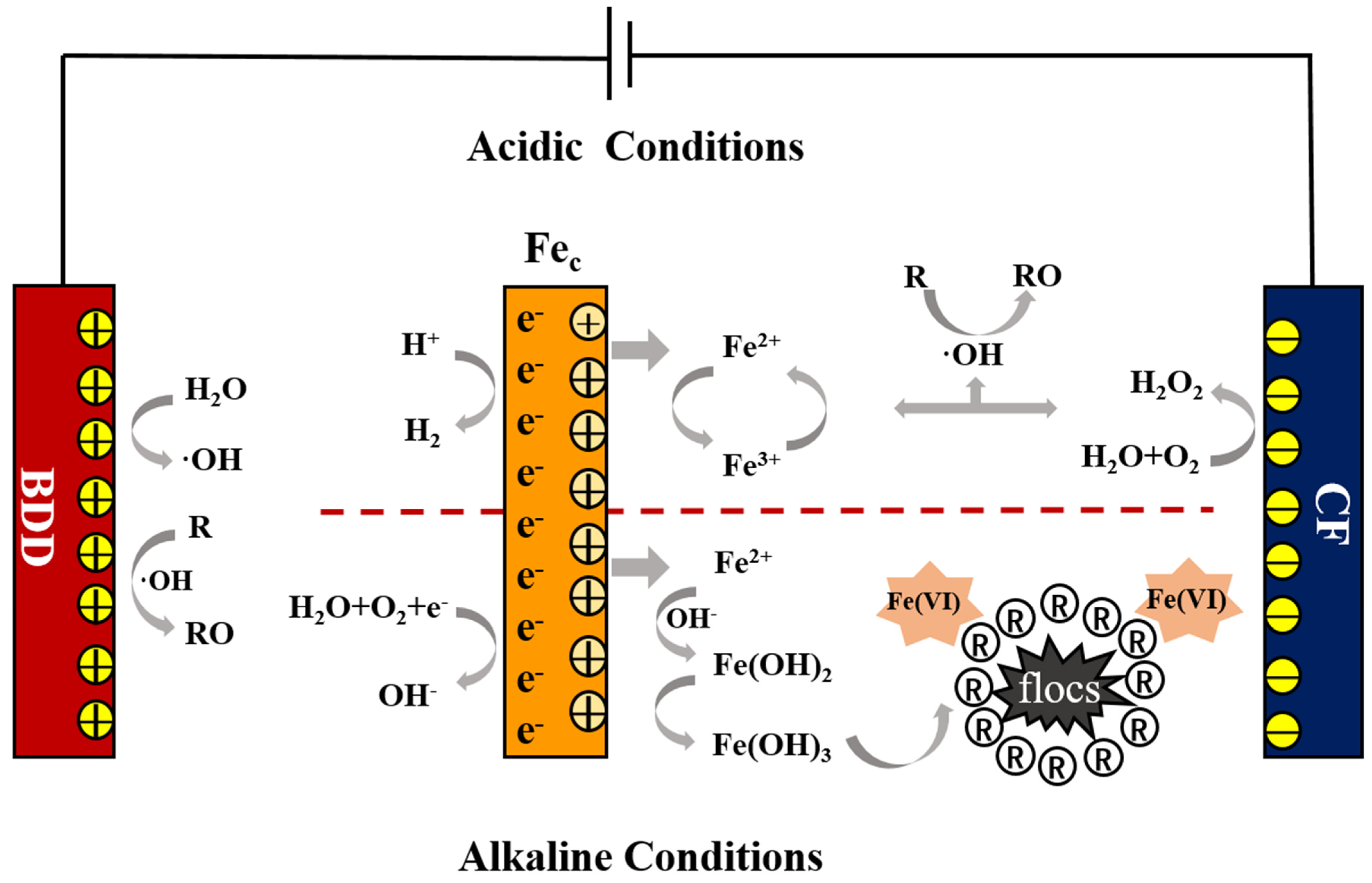
2.2. Electrochemical System
2.3. Analytical Methods
2.4. Response Surface Methodology
3. Results and Discussion
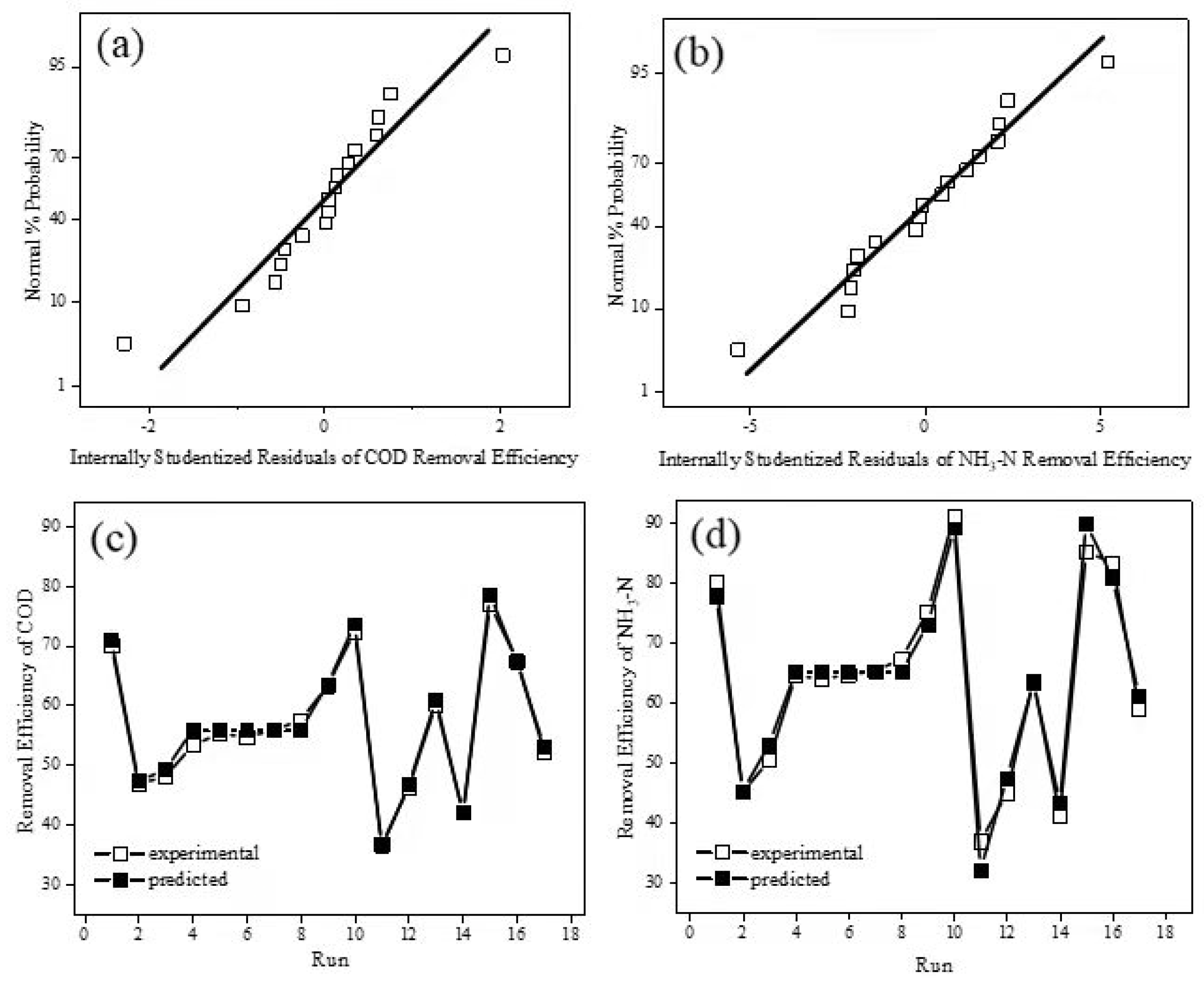
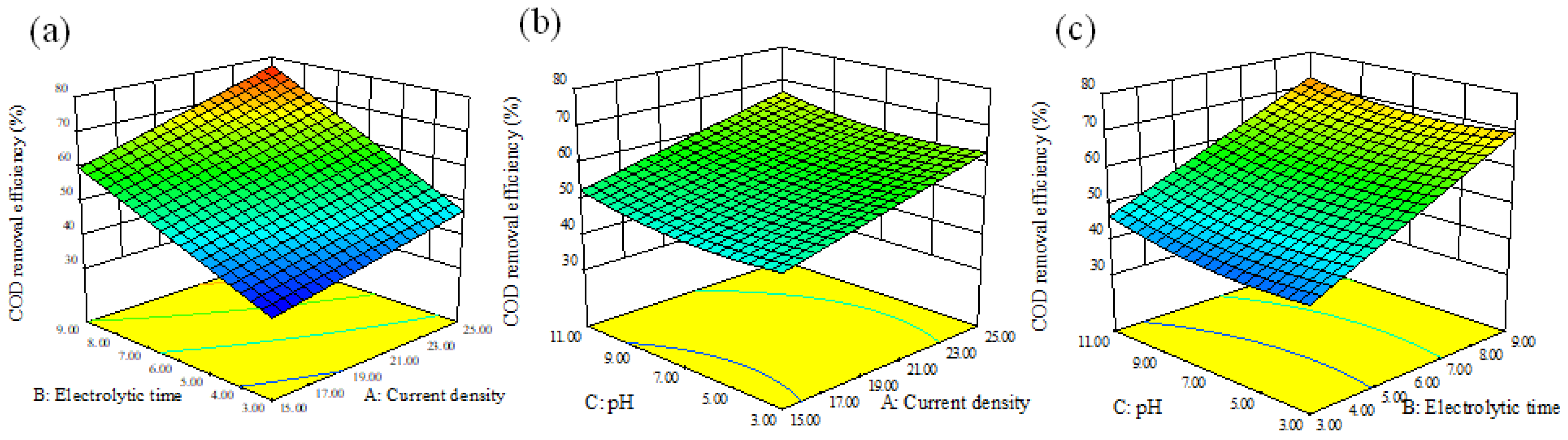
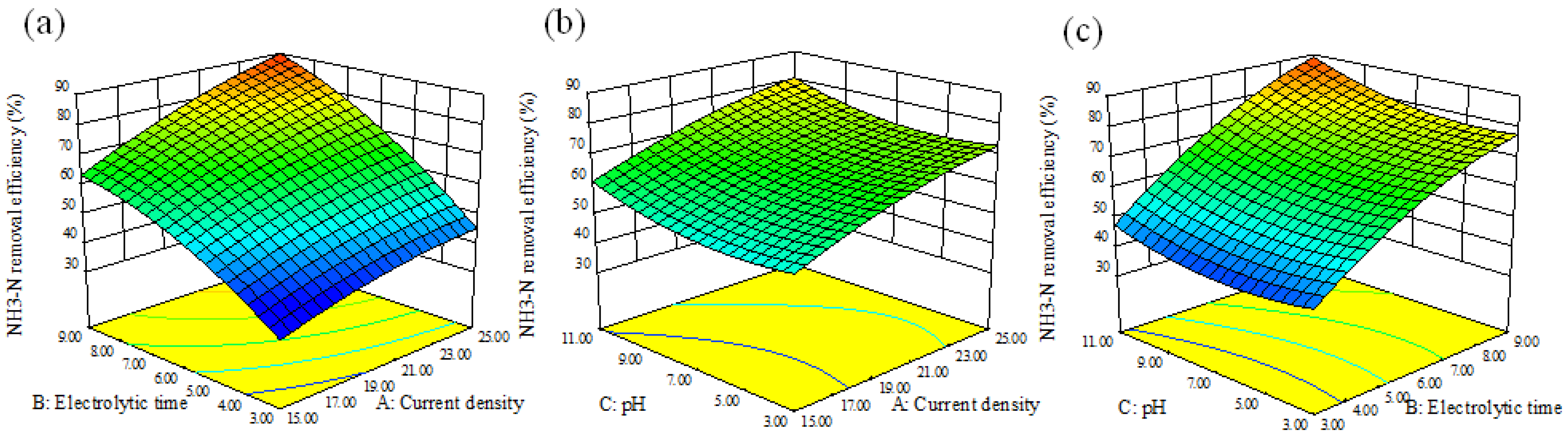
3.1. RSM Analysis
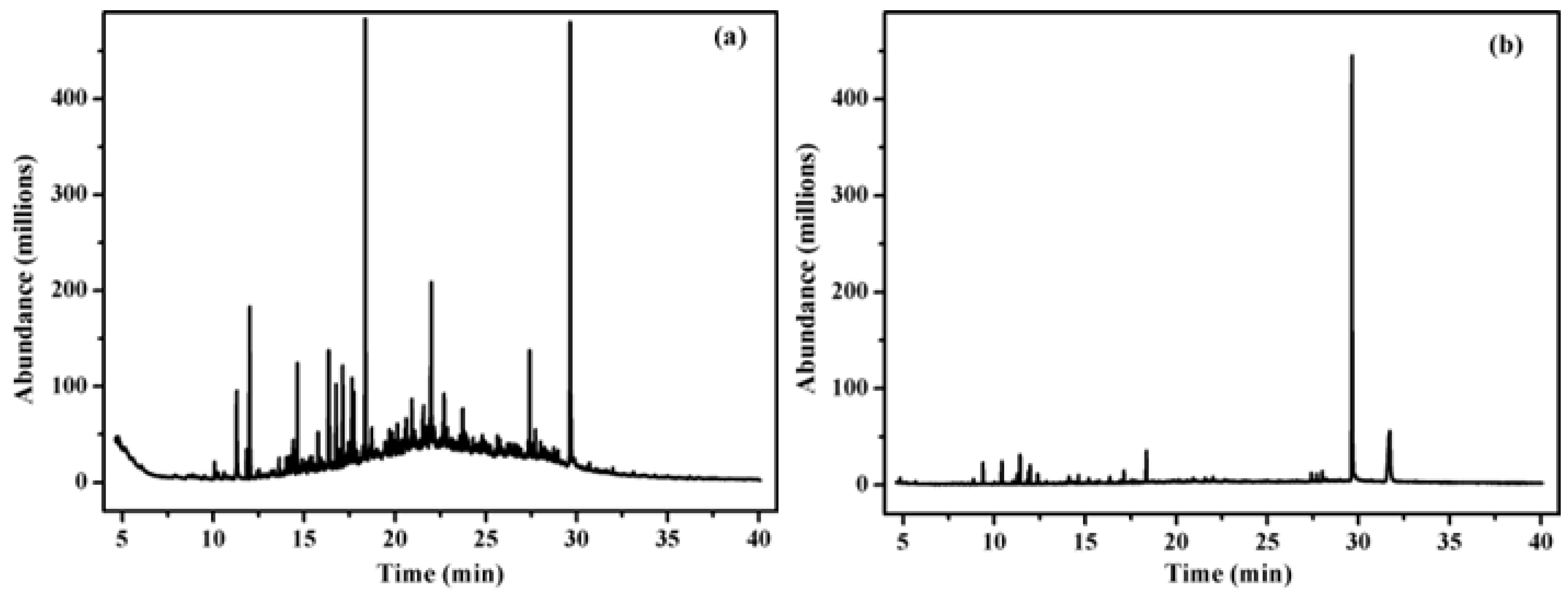
3.2. Analyzed by GC–MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on landfill leachate treatment by electrochemical oxidation: Drawbacks, challenges and future scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lee, S.H.; Kumar, P.; Kim, K.H.; Lee, S.S. Solid waste management: Scope and the challenge of sustainability. J. Clean. Prod. 2019, 228, 658–678. [Google Scholar]
- Ghahrchi, M.; Rezaee, A. Electro-catalytic ozonation for improving the biodegradability of mature landfill leachate. J. Environ. Manag. 2020, 254, 109811. [Google Scholar] [CrossRef]
- Reshadi, M.A.M.; Bazargan, A.; McKay, G. A review of the application of adsorbents for landfill leachate treatment: Focus on magnetic adsorption. Sci. Total Environ. 2020, 731, 138863. [Google Scholar] [CrossRef]
- Aziz, S.Q.; Aziz, H.A.; Bashir, M.J.K. Assessment of various tropical municipal landfill leachate characteristics and treatment opportunities. Glob. Nest J. 2015, 17, 439–450. [Google Scholar]
- Poblete, R.; Oller, I.; Maldonado, M.I.; Cortes, E. Improved landfill leachate quality using ozone, UV solar radiation, hydrogen peroxide, persulfate and adsorption processes. J. Environ. Manag. 2019, 232, 45–51. [Google Scholar] [CrossRef]
- Quan, X.; Hu, R.; Chang, H.; Tang, X.; Huang, X.; Cheng, C.; Yang, L. Enhancing microalgae growth and landfill leachate treatment through ozonization. J. Clean. Prod. 2020, 248, 119182. [Google Scholar] [CrossRef]
- Göde, J.N.; Souza, D.H.; Trevisan, V.; Skoronski, E. Application of the Fenton and Fenton-like processes in the landfill leachate tertiary treatment. J. Environ. Chem. Eng. 2019, 7, 103352. [Google Scholar] [CrossRef]
- Elleuch, L.; Messaoud, M.; Djebali, K.; Attafi, M.; Cherni, Y.; Kasmi, M. A new insight into highly contaminated landfill leachate treatment using Kefir grains pre-treatment combined with Ag-doped TiO2 photocatalytic process. J. Hazard. Mater. 2020, 382, 121119. [Google Scholar] [CrossRef]
- Giraldo, A.L.; Erazo-Erazo, E.D.; Flórez-Acosta, O.A.; Serna-Galvis, E.A.; Torres-Palma, R.A. Degradation of the antibiotic oxacillin in water by anodic oxidation with Ti/IrO2 anodes: Evaluation of degradation routes, organic by-products and effects of water matrix components. Chem. Eng. J. 2015, 279, 103–114. [Google Scholar] [CrossRef]
- Michele, M.; Vacca, A.; Palmas, S. Electrochemical treatment as a pre-oxidative step for algae removal using Chlorella vulgaris as a model organism and BDD anodes. Chem. Eng. J. 2013, 219, 512–519. [Google Scholar]
- Zhu, X.; Ni, J. The improvement of boron-doped diamond anode system in electrochemical degradation of p-nitrophenol by zero-valent iron. Electrochim. Acta 2011, 56, 10371–10377. [Google Scholar] [CrossRef]
- Fernandes, A.; Santos, D.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Nitrogen and organic load removal from sanitary landfill leachates by anodic oxidation at Ti/Pt/PbO2, Ti/Pt/SnO2-Sb2O4 and Si/BDD. Appl. Catal. B Environ. 2014, 148, 288–294. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Wang, X.; Huang, W.; Chen, R.; Li, H. A hydrophobic three-dimensionally networked boron-doped diamond electrode towards electrochemical oxidation. Chem. Commun. 2016, 52, 8026–8029. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.E.H.; Rollin, J.; Iourtchouk, T. The occurrence of perchlorate during drinking water electrolysis using BDD anodes. Electrochim. Acta 2009, 54, 2102–2107. [Google Scholar] [CrossRef]
- Miao, D.; Li, Z.; Chen, Y.; Liu, G.; Deng, Z.; Yu, Y.; Wei, Q. Preparation of macro-porous 3D boron-doped diamond electrode with surface micro structure regulation to enhance electrochemical degradation performance. Chem. Eng. J. 2022, 429, 132366. [Google Scholar] [CrossRef]
- Labiadh, L.; Oturan, M.A.; Panizza, M.; Hamadi, N.B.; Ammar, S. Complete removal of AHPS synthetic dye from water using new electro-fenton oxidation catalyzed by natural pyrite as heterogeneous catalyst. J. Hazard. Mater. 2015, 297, 34–41. [Google Scholar] [CrossRef]
- Fernandes, A.; Labiadh, L.; Ciríaco, L.; Pacheco, M.J.; Gadri, A.; Ammar, S.; Lopes, A. Electro-Fenton oxidation of reverse osmosis concentrate from sanitary landfill leachate: Evaluation of operational parameters. Chemosphere 2017, 184, 1223–1229. [Google Scholar] [CrossRef]
- Afanga, H.; Zazou, H.; Titchou, F.E.; El Gaayda, J.; Sopaj, F.; Akbour, R.A. Electrochemical oxidation of Naphthol Blue Black with different supporting electrolytes using a BDD/carbon felt cell. J. Environ. Chem. Eng. 2021, 9, 104498. [Google Scholar] [CrossRef]
- Yang, W.; Oturan, N.; Raffy, S.; Zhou, M. Electrocatalytic generation of homogeneous and heterogeneous hydroxyl radicals for cold mineralization of anti-cancer drug Imatinib. Chem. Eng. J. 2020, 383, 123155. [Google Scholar] [CrossRef]
- Zazou, H.; Afanga, H.; Akhouairi, S.; Ouchtak, H.; Addi, A.A.; Akbour, R.A. Treatment of textile industry wastewater by electrocoagulation coupled with electrochemical advanced oxidation process. J. Water Process Eng. 2019, 28, 214–221. [Google Scholar] [CrossRef]
- Li, H.; Xing, X.; Wang, K.; Zhu, X.; Jiang, Y.; Xia, J. Improved BDD anode system in electrochemical degradation of p-nitrophenol by corroding electrode of iron. Electrochim. Acta 2018, 291, 335–342. [Google Scholar] [CrossRef]
- Ghanbarzadeh, L.M.; Sabour, M.R.; Ghafari, E.; Amiri, A. Energy consumption and relative efficiency improvement of Photo-Fenton-Optimization by RSM for landfill leachate treatment, a case study. Waste Manage 2018, 79, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Samadder, S.R.; Prabhakar, R.; Khan, D.; Kishan, D.; Chauhan, M.S. Analysis of the contaminants released from municipal solid waste landfill site: A case study. Sci. Total Environ. 2017, 580, 593–601. [Google Scholar] [CrossRef]
- Yu, D.; Cui, J.; Li, X.; Zhang, H.; Pei, Y. Electrochemical treatment of organic pollutants in landfill leachate using a three-dimensional electrode system. Chemosphere 2020, 243, 125438. [Google Scholar] [CrossRef]
- Lei, Y.; Shen, Z.; Huang, R.; Wang, W. Treatment of landfill leachate by combined aged-refuse bioreactor and electro-oxidation. Water Res. 2007, 41, 2417–2426. [Google Scholar] [CrossRef]
- Bakraouy, H.; Souabi, S.; Digua, K.; Dkhissi, O.; Sabar, M.; Fadil, M. Optimization of the treatment of an anaerobic pretreated landfill leachate by a coagulation–flocculation process using experimental design methodology. Process Saf. Environ. 2017, 109, 621–630. [Google Scholar] [CrossRef]
- Sabour, M.R.; Amiri, A. Comparative study of ANN and RSM for simultaneous optimization of multiple targets in Fenton treatment of landfill leachate. Waste Manage 2017, 65, 54–62. [Google Scholar] [CrossRef]
- Zolfaghari, M.; Jardak, K.; Drogui, P.; Brar, S.K.; Buelna, G.; Dubé, R. Landfill leachate treatment by sequential membrane bioreactor and electro-oxidation processes. J. Environ. Manag. 2016, 184, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhou, S.; Qin, F.; Ye, X.; Zheng, K. Modeling physical and oxidative removal properties of Fenton process for treatment of landfill leachate using response surface methodology (RSM). J. Hazard. Mater. 2010, 180, 456–465. [Google Scholar] [CrossRef]
- Nakhate, P.H.; Patil, H.G.; Marathe, K.V. Intensification of landfill leachate treatment by advanced Fenton process using classical and statistical approach. Chem. Eng. Process. 2018, 133, 148–159. [Google Scholar] [CrossRef]
- Bianco, B.; De Michelis, I.; Vegliò, F. Fenton treatment of complex industrial wastewater: Optimization of process conditions by surface response method. J. Hazard. Mater. 2011, 186, 1733. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Bahrami, S.; Emadi, Z.; Shariatipor, H.; Nozari, M. Optimization of ammonium adsorption from landfill leachate using montmorillonite/hematite nanocomposite: Response surface method based on central composite design. Desalin. Water Treat. 2021, 232, 39–54. [Google Scholar] [CrossRef]
- Meng, G.; Jiang, N.; Wang, Y.; Zhang, H.; Tang, Y.; Lv, Y.; Bai, J. Treatment of coking wastewater in a heterogeneous electro-Fenton system: Optimization of treatment parameters, characterization, and removal mechanism. J. Water Process Eng. 2022, 45, 102482. [Google Scholar] [CrossRef]
- Gendel, Y.; Lahav, O. Revealing the mechanism of indirect ammonia electrooxidation. Electrochim. Acta 2012, 63, 209–219. [Google Scholar] [CrossRef]
- Anglada, Á.; Urtiaga, A.; Ortiz, I.; Mantzavinos, D.; Diamadopoulos, E. Boron-doped diamond anodic treatment of landfill leachate: Evaluation of operating variables and formation of oxidation by-products. Water Res. 2011, 45, 828–838. [Google Scholar] [CrossRef]
- Sandip, T.M.; Kanchan, C.K.; Ashok, H.B. Enhancement of methane production and bio-stabilisation of municipal solid waste in anaerobic bioreactor landfill. Bioresour. Technol. 2012, 110, 10–17. [Google Scholar] [CrossRef]
- Scandelai, A.P.J.; Zotesso, J.P.; Jegatheesan, V.; Cardozo-Filho, L.; Tavares, C.R.G. Intensification of supercritical water oxidation (ScWO) process for landfill leachate treatment through ion exchange with zeolite. Waste Manag. 2020, 101, 259–267. [Google Scholar] [CrossRef]
| Parameters | Unit | Value (Range) | Value (Average) |
|---|---|---|---|
| pH | - | 6.85~7.39 | 7.22 |
| COD | mg·L−1 | 2163~2602 | 2464 |
| NH3-N | mg·L−1 | 139.5~178.2 | 154.4 |
| Variables | Symbol | Units | Codes and Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Current density | x1 | mA·cm−2 | 15 | 20 | 25 |
| Electrolysis time | x2 | h | 3 | 6 | 9 |
| pH value | x3 | - | 3 | 7 | 11 |
| Number | Current Density (mA·cm−2) | Electrolytic Time (h) | pH | DCOD (%) | DNH3-N (%) |
|---|---|---|---|---|---|
| 1 | 20 | 9.00 | 3.00 | 70.01 | 80.14 |
| 2 | 25 | 3.00 | 7.00 | 46.8 | 45.09 |
| 3 | 15 | 6.00 | 3.00 | 48.09 | 50.42 |
| 4 | 20 | 6.00 | 7.00 | 53.29 | 64.53 |
| 5 | 20 | 6.00 | 7.00 | 55.24 | 63.98 |
| 6 | 20 | 6.00 | 7.00 | 54.7 | 64.7 |
| 7 | 20 | 6.00 | 7.00 | 55.83 | 65.29 |
| 8 | 20 | 6.00 | 7.00 | 57.52 | 67.3 |
| 9 | 25 | 6.00 | 3.00 | 63.29 | 75.26 |
| 10 | 20 | 9.00 | 11.00 | 72.38 | 91.2 |
| 11 | 15 | 3.00 | 7.00 | 36.67 | 36.89 |
| 12 | 20 | 3.00 | 11.00 | 46.33 | 45.02 |
| 13 | 15 | 9.00 | 7.00 | 60.17 | 63.58 |
| 14 | 20 | 3.00 | 3.00 | 41.95 | 41.27 |
| 15 | 25 | 9.00 | 7.00 | 76.9 | 85.2 |
| 16 | 25 | 6.00 | 11.00 | 67.42 | 83.4 |
| 17 | 15 | 6.00 | 11.00 | 52.28 | 58.9 |
| Model | R2 | Adj R2 | Pre R2 | AP | SD | CV(%) | PRESS |
|---|---|---|---|---|---|---|---|
| Y1 | 0.9941 | 0.9865 | 0.9769 | 42.000 | 1.28 | 2.27 | 44.69 |
| Y2 | 0.9768 | 0.95469 | 0.6513 | 20.305 | 3.71 | 5.83 | 1445.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Yao, S.; Xiao, F.; Xia, J.; Xing, X. Electrochemical Oxidation of Landfill Leachate after Biological Treatment by Electro-Fenton System with Corroding Electrode of Iron. Int. J. Environ. Res. Public Health 2022, 19, 7745. https://doi.org/10.3390/ijerph19137745
Tang J, Yao S, Xiao F, Xia J, Xing X. Electrochemical Oxidation of Landfill Leachate after Biological Treatment by Electro-Fenton System with Corroding Electrode of Iron. International Journal of Environmental Research and Public Health. 2022; 19(13):7745. https://doi.org/10.3390/ijerph19137745
Chicago/Turabian StyleTang, Juan, Shuo Yao, Fei Xiao, Jianxin Xia, and Xuan Xing. 2022. "Electrochemical Oxidation of Landfill Leachate after Biological Treatment by Electro-Fenton System with Corroding Electrode of Iron" International Journal of Environmental Research and Public Health 19, no. 13: 7745. https://doi.org/10.3390/ijerph19137745
APA StyleTang, J., Yao, S., Xiao, F., Xia, J., & Xing, X. (2022). Electrochemical Oxidation of Landfill Leachate after Biological Treatment by Electro-Fenton System with Corroding Electrode of Iron. International Journal of Environmental Research and Public Health, 19(13), 7745. https://doi.org/10.3390/ijerph19137745






