Dental Care and Education Facing Highly Transmissible SARS-CoV-2 Variants: Prospective Biosafety Setting: Prospective, Single-Arm, Single-Center Study
Abstract
:1. Introduction
- (1)
- Planning and protocols;
- (2)
- Patient screening;
- (3)
- Preparation of facilities;
- (4)
- PPE and infection control;
- (5)
- Aerosol control.
2. Materials and Methods
2.1. Main Objective and Study Design
2.2. Participants and the Environment
2.3. Brief Description of the Protocol
- Efficient;
- Sustainable;
- Simple;
- Applicable in other dental specialties (despite orthodontic customization).
- Full room-volume air UVC sterilization for each patient ∗ total air volume per dental unit (separated room);
- Permanent air dispersed super small droplets of virucide oil—a protective puffer in recommended concentrations, created with disinfectant fogging machine (nebulizer).
- AI video-scan evaluation (common checkups rendered obsolete)—Dental Monitoring;
- custom made IOS/Android app coaching the proper habits—StrojCHECK [12];
- 3D printed aerosol vacuum pump ending, that supports aerosol dispersion during dental procedures and other customized 3D printing allowing more.
2.4. Comprehensive Biosafety Protocol Description
2.4.1. Introduction and the Focus of the Protocol
- Online dynamic anamnestic forms (effectively replacing part of 4D clinical examinations);
- Teledentistry Teleorthodontics—Dental Monitoring® (DM) (Dental Monitoring Co., Paris, France)/https://dental-monitoring.com/ (accessed on 1 December 2021) and (StrojCHECK®, Bratislava, Slovakia, 3Dent Medical, www.osim.sk (accessed on 1 December 2021) [12];
- Artificial intelligence in telediagnostics—active screening of patients in tandem with the doctor;
- Special medical devices—active FFP3 shield respirator, BioVYZR, Toronto USA-based Vyzr Technologies, a shield that covers the wearer’s face and protects against droplets and pathogens. Powered Air Purifying Respirator (PAPR), a device typically used only in industrial and healthcare settings;
- 3D printing of sterilizable devices (aerosol aspirators for surgical aspirators or individualized handles);
- Two-phase air sterilization (diffusers of biocidal oils with UVC, NewAroma.sk).
2.4.2. The Protocol Principles
- Minimization of time exposure (shortened duration of treatment, replacement of unnecessary physical visits of the patient via AV technologies, ordering for the exact time with preparation of everything necessary for the procedure in advance).
- Mechanical and physic-chemical prevention in the dispersion of the virus and its carriers into the space resp. decontamination (PPE, continuous sterilization of the air and virucide “puffer” in the air) et al.
- Preventive (patient input filter—symptoms, smart-App tracking, continuous testing of healthcare professionals).
2.4.3. Brief Description of Technologies Utilized in the Protocol
- Online dynamic anamnestic forms;
- Artificial intelligence in dental monitoring;
- Robotization of automatic surface disinfection;
- A.I. smart patient app for treatment coaching;
- 3D printing of sterilizable devices;
- Regular COVID-19 testing of personnel;
- UVC and diffused virucidal oil air treatment.
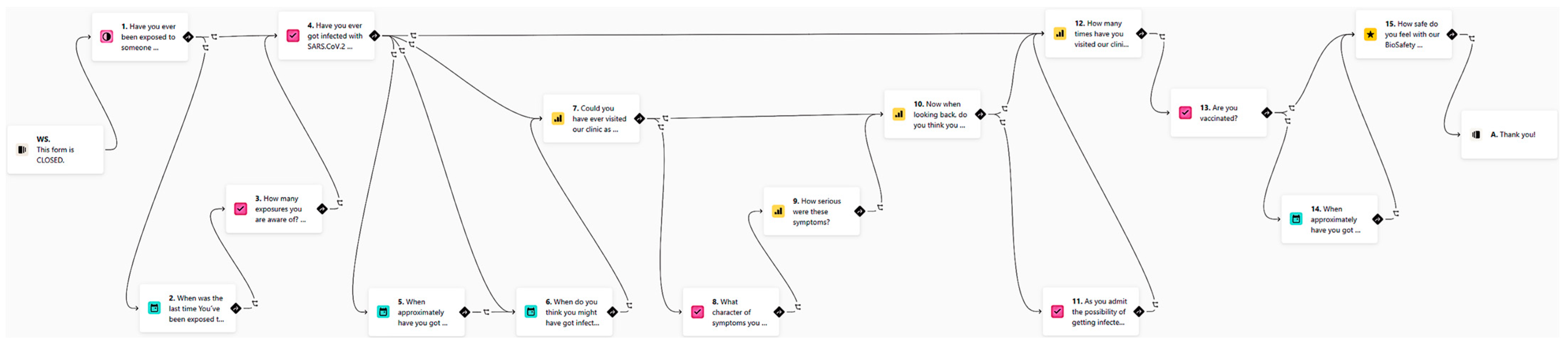
- The use of online dynamic anamnestic smart forms such as Typeform (www.typeform.com, accessed on 18 May 2022) brings interactivity and order to communication with patients at home. The idea of online dynamic anamnestic forms is to use the patient’s own mobile phone not only for video communication (WhatsApp, Facetime), but also for outsourcing part of the examination. For example, with the proper instructions, a short selfie/video sequence of a natural smile can be captured by the patient and provided within an online smart form. The anamnesis itself is very important in reducing the risk of transmission, the proportion of asymptomatic patients with COVID-19 was less than 30% in the number of children with the highest proportion of asymptomatic infection. The identification of symptomatic patients is the first and relatively most effective method of risk reduction. Since the anamnesis is taken remotely, neither staff nor co-patients are exposed to the risk of infection, nor is there any contamination of the surfaces in the waiting room. The use of dynamic forms from Typeform is a suitable choice for this protocol. Remote screening of COVID-19 symptoms by application has been identified as a suitable method for detecting COVID-19 infection. Even the survey form for this biosafety protocol clinical evaluation was created as a smart form. It asks a set of questions that differs according to the answers given. The logic behind it is shown in Figure 1.
- 2.
- Artificial intelligence (AI) technology implemented in the form of the Dental Monitoring software uses the patient’s mobile phone for regular “scanning”, either to assess the course of treatment or to screen a growing patient by monitoring their development or monitoring retention stability after cessation of treatment. A special holder allows the patient to record a video of their own teeth. Paradoxically, it allows more regular and even more thorough inspection, as using artificial intelligence allowed us to summon the patient to an appointment only when necessary (Figure 2). Each video scan first evaluates using AI and then it alerts the doctor only to the monitored situations. The form of our workflow has thus changed fundamentally, and healthcare professionals spend a large part of the day reviewing the outputs of artificial intelligence, which, in turn, extremely efficiently evaluates huge volumes of data, such as video scans of our patients’ oral cavities. It is not humanly possible to evaluate the hundreds of video scans that patients regularly make. The “brute force” of this technology is ideal for identifying situations requiring human intervention.
- 3.
- Robotized around-the-clock surface disinfection technology is also used. It should respect all existing guidelines with an emphasis on consistency and differentiation of surfaces and a higher frequency of cleaning (after each patient). After entering, the patient should only be in contact with the necessary surfaces, and disinfect her/his hands first. Frequent cleaning of surfaces in the clinic, after each procedure, should include frequently inspected surfaces such as door handles, keyboards, and mice. There is also the addition of floor cleaning by robotic vacuuming with a disinfection mop, in our case, the iRobot Braava jet m6, which is suitable for up to 100 m2. The CDC recommends applying standard virucidal disinfectants to potentially contaminated surfaces to prevent the spread of SARS-CoV-2 infection. The work on the susceptibility of human coronaviruses and SARS-CoV is expected to be highly effective in SARS-CoV-2, which is relatively resistant to environmental conditions and remains infectious on smooth surfaces such as metal and plastic for many days.
- 4.
- Self-developed smart mobile application technology is used for patient treatment coaching and remote discipline support. The authors of this paper have been gradually developing iterations of the free telehealth smart patient application for patients in orthodontic treatment with clear aligners, to support proper habits/stereotypes. This app allows for better remote management of the patient and possible reduction in the frequency of visits to the dental office. The application educates and motivates the patient to behave responsibly [12]. For example, the app provides motivation and coaching for more frequent cleaning of teeth and aligners and in general it improved patient compliance.
- 5.
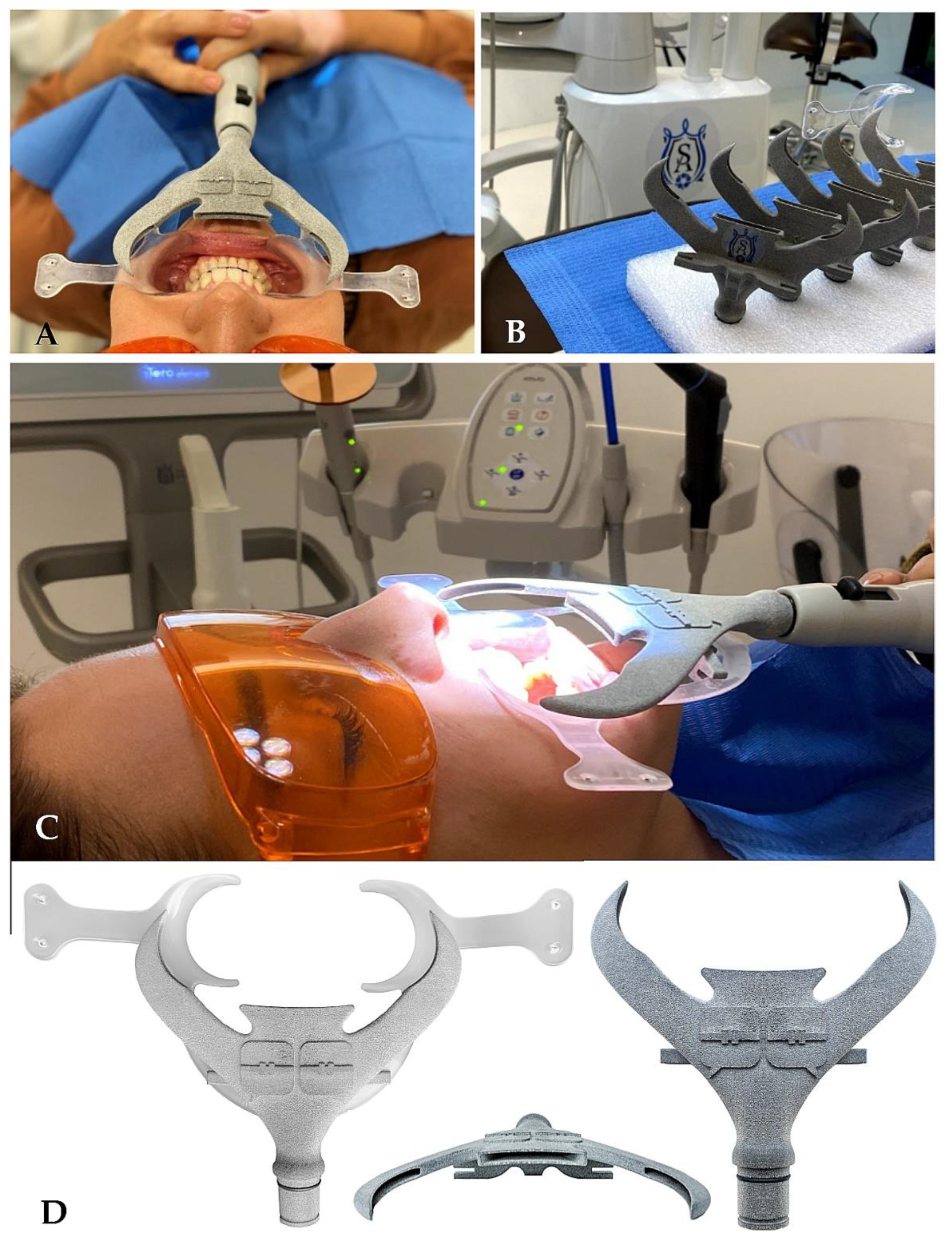
- 3D printing technology supports the presented biosafety protocol with specific sterilizable aerosol aspirators (Figure 3A–D). These devices are printed by a MultiJet Fusion 3D Pro Printer and are sterilizable at 121 °C in autoclave. This aerosol interception device is called “SUR-FACE” and was developed by an Italian orthodontist because of the COVID-19 pandemic. It connects to a conventional 16 mm suction device. The material is polyamide and is compatible with any retractor with thickness ≤ 2 mm. The material is recyclable and supplied with five additional rubber seals. The one-time use of suction cup attachments minimizes the risk of transmitting infection with this tool. The sterilizability of these handpieces is critical. From clinical experience, it is very effective at containing aerosols produced during clinical procedures.
- 6.
- Regular antigen testing from saliva or other convenient form of testing for possible infectiousness of everybody in the team shall be employed because, with the arrival of Omicron-like strains, it is even more likely that a vaccinated healthcare professional would become a “supercarrier”.
- 7.
- Air-processing technologies are key to the presented biosafety protocol with two-phase air treatment: room air is the most likely vector and therefore a key element in the stopping of transmission of infection. The transmission of SARS-CoV-2 infection occurs mainly through droplets that fall relatively rapidly to the ground. However, aerosolized particles smaller than 5 μm contaminate the air and can levitate indoors where the air is not exchanged and disinfected for several hours. In addition to PPE, aerosol extractors and other elements preventing significant air contamination in the clinic, it is therefore appropriate to include other forms of air conditioning, in our case hooded UVC emitters combined with diffusers of biocidal oils, suitable for combination with UVC sterilization.
2.4.4. Air-Processing Elements (UVC + Virucidal Diffusers)
- (A)
- Germicidal radiator PROLUX G30W A/SPH01:
- -
- UVC lamp life of 8000 h;
- -
- For two-shift operation endurance 550 days (2 × 7.5 h);
- -
- 1 emitter cycle is sufficient to sterilize a volume of 5.5 m × 4 m × 3.0 m;
- (B)
- Aroma Pro Mini—professional diffuser (aroma atomizer).
- -
- For air conditioning;
- -
- Capacity up to 1000 m3 (www.NewAroma.sk accessed on 15 December 2021);
- -
- Possibility to choose from several certified disinfectant oils;
- -
- Disinfectant is present in the mixture in 3 weight percent at an emission of 5 mL per hour (adjustable) to form an invisible aerosol dispersion in the air.
2.5. Protocol Development and Evaluation
2.6. Protocol strengths and Weaknesses
- -
- Low cost, affordable, effective, sustainable
- -
- Does not require specific rebuilding of the current dental set-ups
- -
- Dimensioned for highly transmissible strains coming after Omicron
- -
- Not dependable on vaccination status or unreliable testing results
- -
- Difficult to evaluate clinical reliability:
- ○
- The only possible way is feedback form (bias);
- ○
- Larger sample needed;
- ○
- Other studies for comparison are needed;
- -
- Requiring extra time gaps between patients in the same room;
- -
- Possible biosafety overkill;
- -
- Unknown performance under unexplored highly transmissible variants.
3. Results
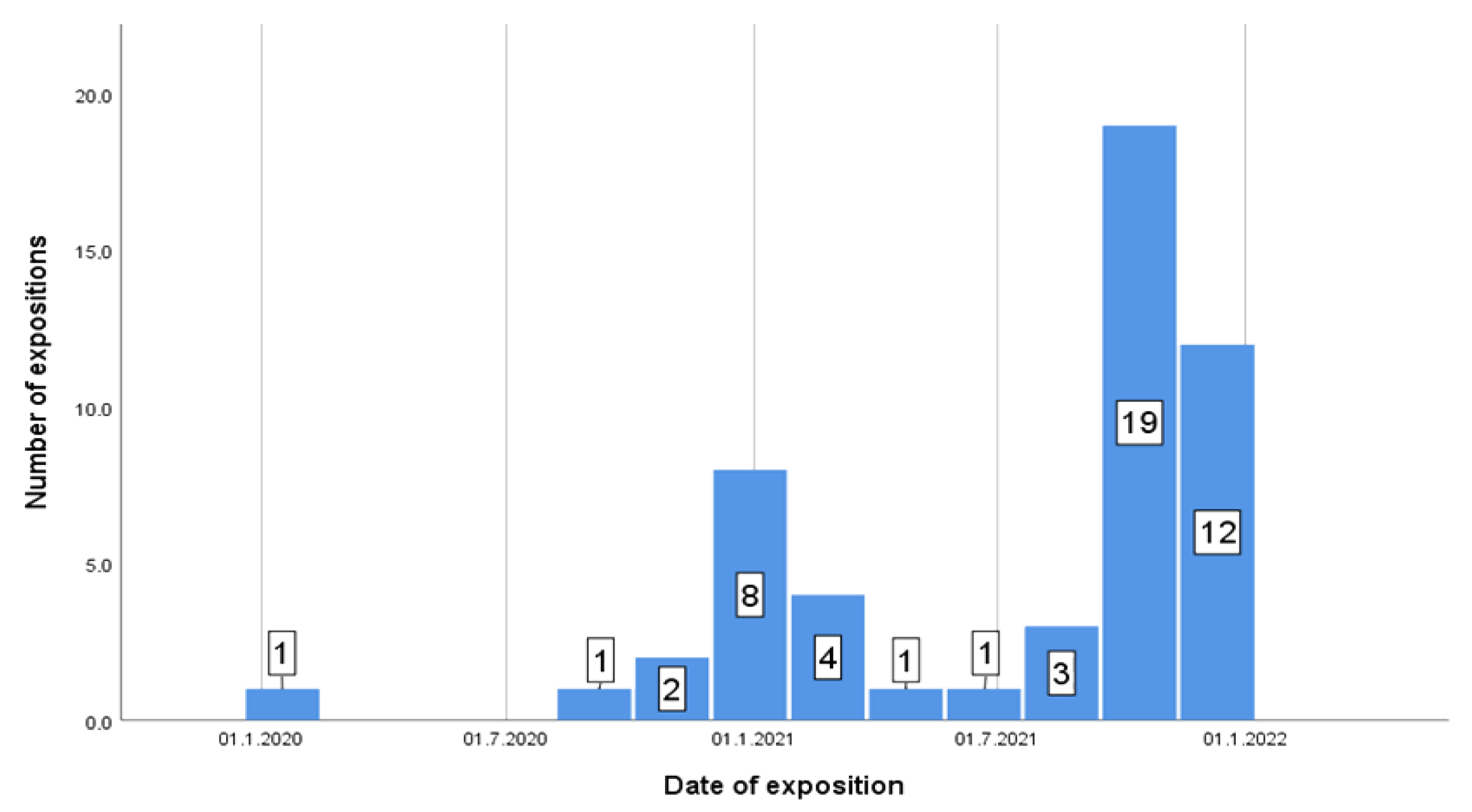
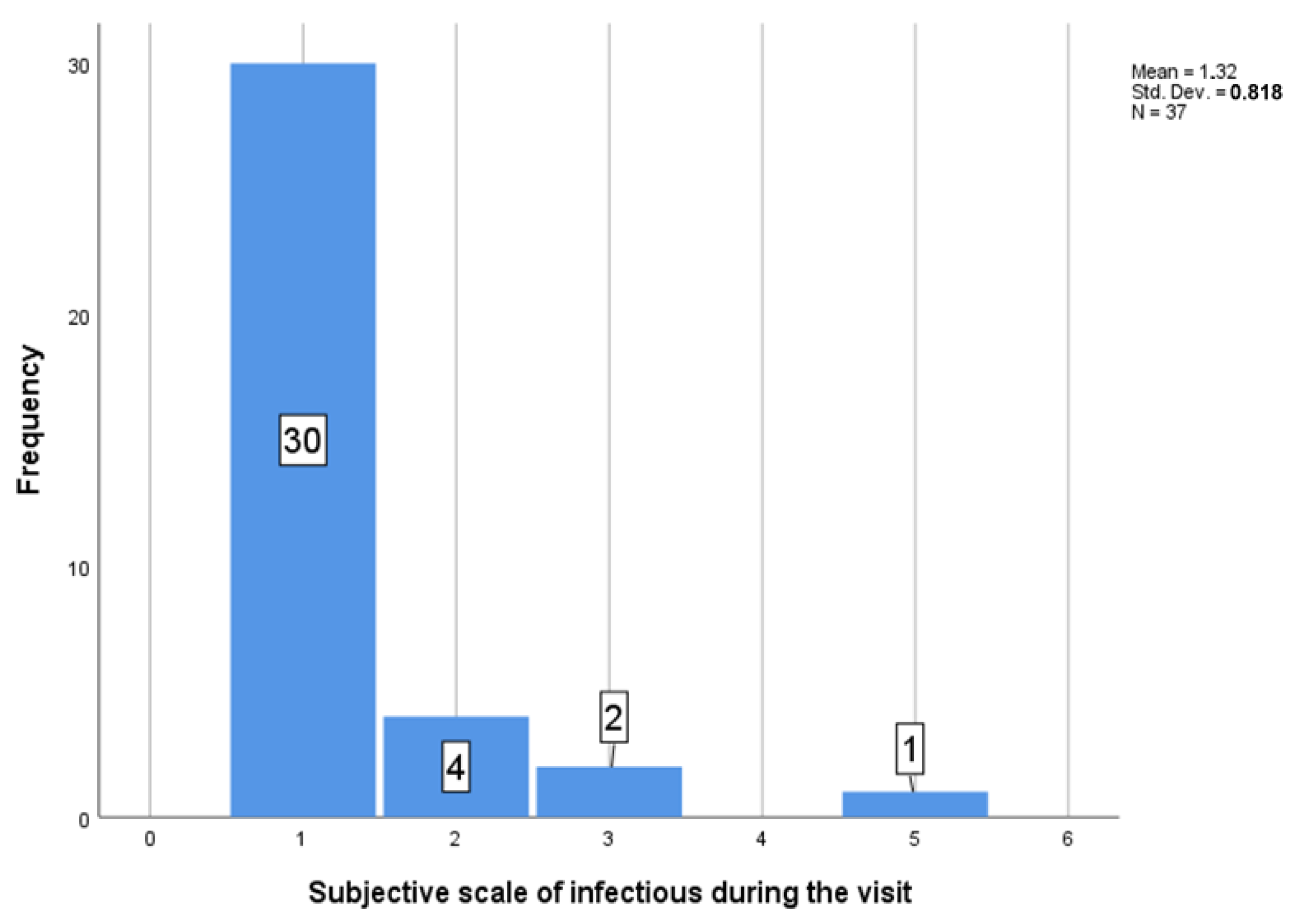
3.1. Descriptive Results
3.2. Graphical Interpretation of Clinical Evaluation of the Protocol
3.3. Biosafety Protocol
4. Discussion
- Certainly not! 0%;
- 25%;
- Maybe, 50%;
- 75%;
- I am sure I got it there 100%.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siles-Garcia, A.A.; Alzamora-Cepeda, A.G.; Atoche-Socola, K.J.; Peña-Soto, C.; Arriola-Guillén, L.E. Biosafety for Dental Patients During Dentistry Care After COVID-19: A Review of the Literature. Disaster Med. Public Health Prep. 2021, 15, e43–e48. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Tasayco, F.D.P.; Rivera-Carhuavilca, J.M.; Atoche-Socola, K.J.; Peña-Soto, C.; Arriola-Guillén, L.E. Biosafety Measures at the Dental Office After the Appearance of COVID-19: A Systematic Review. Disaster Med. Public Health Prep. 2020, 15, e34–e38. [Google Scholar] [CrossRef] [PubMed]
- Hocková, B.; Riad, A.; Valky, J.; Šulajová, Z.; Stebel, A.; Slávik, R.; Bečková, Z.; Pokorná, A.; Klugarová, J.; Klugar, M. Oral Complications of ICU Patients with COVID-19: Case-Series and Review of Two Hundred Ten Cases. J. Clin. Med. 2021, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Huth, K.C.; von Bronk, L.; Kollmuss, M.; Lindner, S.; Durner, J.; Hickel, R.; Draenert, M.E. Special Teaching Formats during the COVID-19 Pandemic—A Survey with Implications for a Crisis-Proof Education. J. Clin. Med. 2021, 10, 5099. [Google Scholar] [CrossRef] [PubMed]
- Feher, B.; Wieser, C.; Lukes, T.; Ulm, C.; Gruber, R.; Kuchler, U. The Effect of the COVID-19 Pandemic on Patient Selection, Surgical Procedures, and Postoperative Complications in a Specialized Dental Implant Clinic. J. Clin. Med. 2022, 11, 855. [Google Scholar] [CrossRef]
- Sycinska-Dziarnowska, M.; Maglitto, M.; Woźniak, K.; Spagnuolo, G. Oral Health and Teledentistry Interest during the COVID-19 Pandemic. J. Clin. Med. 2021, 10, 3532. [Google Scholar] [CrossRef]
- Sinjari, B.; Rexhepi, I.; Santilli, M.; D′addazio, G.; Chiacchiaretta, P.; Di Carlo, P.; Caputi, S. The Impact of COVID-19 Related Lockdown on Dental Practice in Central Italy—Outcomes of A Survey. Int. J. Environ. Res. Public Health 2020, 17, 5780. [Google Scholar] [CrossRef]
- Goriuc, A.; Sandu, D.; Tatarciuc, M.; Luchian, I. The Impact of the COVID-19 Pandemic on Dentistry and Dental Education: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 2537. [Google Scholar] [CrossRef]
- Cummins, C.P.; Ajayi, O.J.; Mehendale, F.V.; Gabl, R.; Viola, I.M. The dispersion of spherical droplets in source–sink flows and their relevance to the COVID-19 pandemic. Phys. Fluids 2020, 32, 083302. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. Dentistry 4.0 technologies applications for dentistry during COVID-19 pandemic. Sustain. Oper. Comput. 2021, 2, 87–96. [Google Scholar] [CrossRef]
- Pai, S.; Patil, V.; Kamath, R.; Mahendra, M.; Singhal, D.K.; Bhat, V. Work-life balance amongst dental professionals during the COVID-19 pandemic—A structural equation modelling approach. PLoS ONE 2021, 16, e0256663. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Kurilová, V.; Varga, I. Artificial Intelligence in Orthodontic Smart Application for Treatment Coaching and Its Impact on Clinical Performance of Patients Monitored with AI-TeleHealth System. Healthcare 2021, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, R.K.; Abdoun, H.A.E.; Koppolu, P.; Swapna, L.A. Infection Control Measures in Dental Clinics during Coronavirus Disease-19 Pandemic in Kingdom of Saudi Arabia: A Pilot Study. Open Access Maced. J. Med Sci. 2021, 9, 61–67. [Google Scholar] [CrossRef]
- Campus, G.; Diaz-Betancourt, M.; Cagetti, M.; Carvalho, J.; Carvalho, T.; Cortés-Martinicorena, J.; Deschner, J.; Douglas, G.; Giacaman, R.; Machiulskiene, V.; et al. Study Protocol for an Online Questionnaire Survey on Symptoms/Signs, Protective Measures, Level of Awareness and Perception Regarding COVID-19 Outbreak among Dentists. A Global Survey. Int. J. Environ. Res. Public Health 2020, 17, 5598. [Google Scholar] [CrossRef] [PubMed]
- Shihabi, S.; Al Nesser, S.; Hamadah, O. The preventive measures adopted during dental practice by the dentists in a low-income country to prevent the transmission of COVID-19: A questionnaire-based survey. Indian J. Med Sci. 2021, 73, 15–20. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, H.; Chu, C.; Li, X.; Liu, S.; Lu, S. Transmission Routes of SARS-CoV-2 and Protective Measures in Dental Clinics during the COVID-19 Pandemic. Am. J. Dent. 2020, 33, 129–134. [Google Scholar]
- Moraes, R.R.; Correa, M.B.; Queiroz, A.B.; Daneris, Â.; Lopes, J.P.; Pereira-Cenci, T.; D’Avila, O.; Cenci, M.; Lima, G.D.S.; Demarco, F. COVID-19 challenges to dentistry in the new pandemic epicenter: Brazil. PLoS ONE 2020, 15, e0242251. [Google Scholar] [CrossRef]
- Manuballa, S.; Abdelmaseh, M.; Tasgaonkar, N.; Frias, V.; Hess, M.; Crow, H.; Andreana, S.; Gupta, V.; Wooten, K.E.; Markiewicz, M.R.; et al. Managing the Oral Health of Cancer Patients during the COVID-19 Pandemic: Perspective of a Dental Clinic in a Cancer Center. J. Clin. Med. 2020, 9, 3138. [Google Scholar] [CrossRef]
- Meng, L.; Hua, F.; Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Froum, S.H.; Froum, S.J. Incidence of COVID-19 Virus Transmission in Three Dental Offices: A 6-Month Retrospective Study. Int. J. Periodontics Restor. Dent. 2020, 40, 853–859. [Google Scholar] [CrossRef]
- Hartig, M.; Stephens, C.; Foster, A.; Fontes, D.; Kinzel, M.; García-Godoy, F. Stopping the COVID-19 pandemic in dental offices: A review of SARS-CoV-2 transmission and cross-infection prevention. Exp. Biol. Med. 2021, 246, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.W.B.; Estrich, C.G.; Mikkelsen, M.; Morrissey, R.; Harrison, B.; Geisinger, M.L.; Ioannidou, E.; Vujicic, M. COVID-2019 among Dentists in the United States: A 6-Month Longitudinal Report of Accumulative Prevalence and Incidence. J. Am. Dent. Assoc. 2021, 152, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.-Y.; Yang, L.-M.; Xia, J.-J.; Fu, X.-H.; Zhang, Y.-Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ. Sci. B 2020, 21, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabiri, D.; Conti, S.R.; Pour, N.S.; Chong, A.; Dadjoo, S.; Dabiri, D.; Wiese, C.; Badal, J.; Hoogland, M.A.; Conti, H.R.; et al. A Multi-Disciplinary Review on the Aerobiology of COVID-19 in Dental Settings. Front. Dent. Med. 2021, 2, 66. [Google Scholar] [CrossRef] [PubMed]
- Veeraiyan, D.N.; Varghese, S.S.; Rajasekar, A.; Karobari, M.I.; Thangavelu, L.; Marya, A.; Messina, P.; Scardina, G.A. Comparison of Interactive Teaching in Online and Offline Platforms among Dental Undergraduates. Int. J. Environ. Res. Public Health 2022, 19, 3170. [Google Scholar] [CrossRef]
- Iqbal, A.; Ganji, K.K.; Khattak, O.; Shrivastava, D.; Srivastava, K.C.; Arjumand, B.; AlSharari, T.; A Alqahtani, A.M.; Hamza, M.O.; AbdelrahmanDafaalla, A.A.E.G. Enhancement of Skill Competencies in Operative Dentistry Using Procedure-Specific Educational Videos (E-Learning Tools) Post-COVID-19 Era—A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 4135. [Google Scholar] [CrossRef]
- Fazio, M.; Lombardo, C.; Marino, G.; Marya, A.; Messina, P.; Scardina, G.A.; Tocco, A.; Torregrossa, F.; Valenti, C. LinguAPP: An m-Health Application for Teledentistry Diagnostics. Int. J. Environ. Res. Public Health 2022, 19, 822. [Google Scholar] [CrossRef]
- Hamedani, S.; Farshidfar, N.; Ziaei, A.; Pakravan, H. The Dilemma of COVID-19 in Dental Practice Concerning the Role of Saliva in Transmission: A Brief Review of Current Evidence. Eur. Oral Res. 2020, 54, 92–100. [Google Scholar] [CrossRef]
- Meethil, A.; Saraswat, S.; Chaudhary, P.; Dabdoub, S.; Kumar, P. Sources of SARS-CoV-2 and Other Microorganisms in Dental Aerosols. J. Dent. Res. 2021, 100, 817–823. [Google Scholar] [CrossRef]
- Meister, T.L.; Brüggemann, Y.; Todt, D.; Conzelmann, C.; A Müller, J.; Groß, R.; Münch, J.; Krawczyk, A.; Steinmann, J.; Steinmann, J.; et al. Virucidal Efficacy of Different Oral Rinses against Severe Acute Respiratory Syndrome Coronavirus 2. J. Infect. Dis. 2020, 222, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Perry, E. How I Chose a Preprocedural Rinse—Registered Dental Hygienists. Available online: https://www.rdhmag.com/infection-control/article/14204889/how-i-chose-a-preprocedural-rinse (accessed on 15 December 2021).
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2008. [Google Scholar]
- Ortega, K.L.; Rech, B.O.; el Haje, G.L.C.; Gallo, C.B.; Pérez-Sayáns, M.; Braz-Silva, P.H. Do Hydrogen Peroxide Mouthwashes Have a Virucidal Effect? A Systematic Review. J. Hosp. Infect. 2020, 106, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Don’t Use Hydrogen Peroxide as a COVID-19 Pre-Procedural Rinse, Experts Say—Dental Tribune Canada. Available online: https://ca.dental-tribune.com/news/dont-use-hydrogen-peroxide-as-a-COVID-19-pre-procedural-rinse-experts-say/ (accessed on 15 December 2021).
- Moskowitz, H.; Mendenhall, M. Comparative Analysis of Antiviral Efficacy of Four Different Mouthwashes against Severe Acute Respiratory Syndrome Coronavirus 2: An in Vitro Study. Int. J. Exp. Dent. Sci. 2020, 9, 1–3. [Google Scholar] [CrossRef]
- Public Health Ontario. Rapid Review: Open Operatory Dental Setting Infection Control Practices and Risk of Transmission during Aerosol-Generating Dental Procedures; Public Health Ontario: Toronto, ON, Canada, 2020. [Google Scholar]
- Steinhauer, K.; Meister, T.; Todt, D.; Krawczyk, A.; Paßvogel, L.; Becker, B.; Paulmann, D.; Bischoff, B.; Pfaender, S.; Brill, F.; et al. Comparison of the in-vitro efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476. J. Hosp. Infect. 2021, 111, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.M.; De Almeida, A.C.; Rodrigues, C.M.d.C.; Sol, I.; Meneses-Santos, D. COVID-19—Mouthwash in dental clinical practice: Review. Arch. Health Investig. 2021, 10, 6–10. [Google Scholar] [CrossRef]
- Mohd-Said, S.; Mohd-Dom, T.N.; Suhaimi, N.; Rani, H.; McGrath, C. Effectiveness of Pre-procedural Mouth Rinses in Reducing Aerosol Contamination During Periodontal Prophylaxis: A Systematic Review. Front. Med. 2021, 8, 600769. [Google Scholar] [CrossRef]
- Kelly, N.; Nic Íomhair, A.; McKenna, G. Can oral rinses play a role in preventing transmission of COVID 19 infection? Evid.-Based Dent. 2020, 21, 42–43. [Google Scholar] [CrossRef]
- Mohebbi, S.Z.; Ebrahimi, T.; Shamshiri, A.R. Do Mouthwashes Reduce COVID-19 Viral Load during Dental Procedures and Oropharyngeal Examinations? A Systematic Review. Allergology 2021, 6, 249. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Natoli, V.; Bruni, A.; Coscione, C.; Magliano, G.; Giacobbo, G.; Morelli, A.; Moressa, S.; Scribante, A. Bio-Inspired Systems in Nonsurgical Periodontal Therapy to Reduce Contaminated Aerosol during COVID-19: A Comprehensive and Bibliometric Review. J. Clin. Med. 2020, 9, 3914. [Google Scholar] [CrossRef]
- Farshidfar, N.; Jafarpour, D.; Hamedani, S.; Dziedzic, A.; Tanasiewicz, M. Proposal for Tier-Based Resumption of Dental Practice Determined by COVID-19 Rate, Testing and COVID-19 Vaccination: A Narrative Perspective. J. Clin. Med. 2021, 10, 2116. [Google Scholar] [CrossRef]
- Shirazi, S.; Stanford, C.; Cooper, L. Characteristics and Detection Rate of SARS-CoV-2 in Alternative Sites and Specimens Pertaining to Dental Practice: An Evidence Summary. J. Clin. Med. 2021, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- López-Verdín, S.; Prieto-Correa, J.R.; Molina-Frechero, N.; Bologna-Molina, R. Screening Test for COVID-19 in Dentai Practice: Best Options. Am. J. Dent. 2021, 34, 127–131. [Google Scholar] [PubMed]
- Joshi, V.K. Dental treatment planning and management for the mouth cancer patient. Oral Oncol. 2010, 46, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, A.F.; Ciccarelli, M.; Marrari, A.; Gennaro, N.; Dipasquale, A.; Giordano, L.; Cariboni, U.; Quagliuolo, V.L.; Alloisio, M.; Santoro, A. Impact of Active Cancer on COVID-19 Survival: A Matched-Analysis on 557 Consecutive Patients at an Academic Hospital in Lombardy, Italy. Br. J. Cancer 2021, 125, 358–365. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Ardes, D.; Santilli, M.; Rexhepi, I.; D’Addazio, G.; Di Carlo, P.; Chiacchiaretta, P.; Caputi, S.; Cipollone, F. SARS-CoV-2 and Oral Manifestation: An Observational, Human Study. J. Clin. Med. 2020, 9, 3218. [Google Scholar] [CrossRef]
- dos Santos, J.A.; Normando, A.; da Silva, R.C.; Acevedo, A.; Canto, G.D.L.; Sugaya, N.; Santos-Silva, A.; Guerra, E. Oral Manifestations in Patients with COVID-19: A 6-Month Update. J. Dent. Res. 2021, 100, 1321–1329. [Google Scholar] [CrossRef]
- Aragoneses, J.; Suárez, A.; Algar, J.; Rodríguez, C.; López-Valverde, N.; Aragoneses, J.M. Oral Manifestations of COVID-19: Updated Systematic Review With Meta-Analysis. Front. Med. 2021, 8, 1423. [Google Scholar] [CrossRef]
- Orilisi, G.; Mascitti, M.; Togni, L.; Monterubbianesi, R.; Tosco, V.; Vitiello, F.; Santarelli, A.; Putignano, A.; Orsini, G. Oral Manifestations of COVID-19 in Hospitalized Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 12511. [Google Scholar] [CrossRef]
- Mekhemar, M.; Attia, S.; Dörfer, C.; Conrad, J. The Psychological Impact of the COVID-19 Pandemic on Dentists in Germany. J. Clin. Med. 2021, 10, 1008. [Google Scholar] [CrossRef]
- Pylińska-Dąbrowska, D.; Starzyńska, A.; Cubała, W.J.; Ragin, K.; Alterio, D.; Jereczek-Fossa, B.A. Psychological Functioning of Patients Undergoing Oral Surgery Procedures during the Regime Related with SARS-CoV-2 Pandemic. J. Clin. Med. 2020, 9, 3344. [Google Scholar] [CrossRef]
- Emodi-Perlman, A.; Eli, I.; Smardz, J.; Uziel, N.; Wieckiewicz, G.; Gilon, E.; Grychowska, N.; Wieckiewicz, M. Temporomandibular Disorders and Bruxism Outbreak as a Possible Factor of Orofacial Pain Worsening during the COVID-19 Pandemic—Concomitant Research in Two Countries. J. Clin. Med. 2020, 9, 3250. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, A.; Rzymski, P. Children’s Dental Anxiety during the COVID-19 Pandemic: Polish Experience. J. Clin. Med. 2020, 9, 2751. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Ebrahimi, A.; Ghorbani, F. The impact of COVID-19 pandemic on dental practice in Iran: A questionnaire-based report. BMC Oral Health 2020, 20, 354. [Google Scholar] [CrossRef] [PubMed]
- Faccini, M.; Ferruzzi, F.; Mori, A.A.; Santin, G.C.; Oliveira, R.C.; de Oliveira, R.C.G.; Queiroz, P.M.; Salmeron, S.; Pini, N.I.P.; Sundfeld, D.; et al. Dental Care during COVID-19 Outbreak: A Web-Based Survey. Eur. J. Dent. 2020, 14, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.N.; Vinothkumar, T.S.; Albar, N.H.M.; Karobari, M.I.; Renugalakshmi, A.; Bokhari, A.; Peeran, S.W.; Peeran, S.A.; Alhadri, L.M.; Tadakamadla, S.K. Knowledge of COVID-19 Infection Guidelines among the Dental Health Care Professionals of Jazan Region, Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 2034. [Google Scholar] [CrossRef] [PubMed]
- Derruau, S.; Bouchet, J.; Nassif, A.; Baudet, A.; Yasukawa, K.; Lorimier, S.; Prêcheur, I.; Bloch-Zupan, A.; Pellat, B.; Chardin, H.; et al. COVID-19 and Dentistry in 72 Questions: An Overview of the Literature. J. Clin. Med. 2021, 10, 779. [Google Scholar] [CrossRef]
- Cagetti, M.G.; Cairoli, J.L.; Senna, A. Guglielmo Campus COVID-19 Outbreak in North Italy: An Overview on Dentistry. A Questionnaire Survey. Int. J. Environ. Res. Public Health 2020, 17, 3835. [Google Scholar] [CrossRef]
- Sfikas, P.M. Teledentistry: Legal and Regulatory Issues Explored. J. Am. Dent. Assoc. 1997, 128, 1716–1718. [Google Scholar] [CrossRef]
- Kravitz, N.D.; Burris, B.; Butler, D.; Dabney, C.W. Teledentistry, Do-It-Yourself Orthodontics, and Remote Treatment Monitoring. J. Clin. Orthod. 2016, 50, 718–726. [Google Scholar]
- Park, J.H.; Rogowski, L.; Kim, J.H.; Al Shami, S.; Howell, S.E.I. Teledentistry Platforms for Orthodontics. J. Clin. Pediatr. Dent. 2021, 45, 48–53. [Google Scholar] [CrossRef]
- A Mandall, N.; O’Brien, K.; Brady, J.; Worthington, H.; Harvey, L. Teledentistry for screening new patient orthodontic referrals. Part 1: A randomised controlled trial. Br. Dent. J. 2005, 199, 659–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudice, A.; Barone, S.; Muraca, D.; Averta, F.; Diodati, F.; Antonelli, A.; Fortunato, L. Can Teledentistry Improve the Monitoring of Patients during the COVID-19 Dissemination? A Descriptive Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 3399. [Google Scholar] [CrossRef] [PubMed]
- Maspero, C.; Abate, A.; Cavagnetto, D.; El Morsi, M.; Fama, A.; Farronato, M. Available Technologies, Applications and Benefits of Teleorthodontics. A Literature Review and Possible Applications during the COVID-19 Pandemic. J. Clin. Med. 2020, 9, 1891. [Google Scholar] [CrossRef] [PubMed]
- Deana, N.F.; Seiffert, A.; Aravena-Rivas, Y.; Alonso-Coello, P.; Muñoz-Millán, P.; Espinoza-Espinoza, G.; Pineda, P.; Zaror, C. Recommendations for Safe Dental Care: A Systematic Review of Clinical Practice Guidelines in the First Year of the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 10059. [Google Scholar] [CrossRef]
- Statement on Omicron Sublineage BA.2. Available online: https://www.who.int/news/item/22-02-2022-statement-on-omicron-sublineage-ba.2 (accessed on 18 March 2022).
- Hoyte, T.; Kowlessar, A.; Mahabir, A.; Khemkaran, K.; Jagroo, P.; Jahoor, S. The Knowledge, Awareness, and Attitude Regarding COVID-19 among Trinidad and Tobago Dentists. A Cross-Sectional Survey. Oral 2021, 1, 250–260. [Google Scholar] [CrossRef]
- Estrich, C.G.; Mikkelsen, M.; Morrissey, R.; Geisinger, M.L.; Ioannidou, E.; Vujicic, M.; Araujo, M.W.B. Estimating COVID-19 prevalence and infection control practices among US dentists. J. Am. Dent. Assoc. 2020, 151, 815–824. [Google Scholar] [CrossRef]
- Gugnani, N.; Gugnani, S. Safety protocols for dental practices in the COVID-19 era. Evid.-Based Dent. 2020, 21, 56–57. [Google Scholar] [CrossRef]
- Souza, A.F.; de Arruda, J.A.A.; Costa, F.P.D.; Bemquerer, L.M.; Castro, W.H.; Campos, F.E.B.; Kakehasi, F.M.; Travassos, D.V.; Silva, T.A. Safety protocols for dental care during the COVID-19 pandemic: The experience of a Brazilian hospital service. Braz. Oral Res. 2021, 35, e070. [Google Scholar] [CrossRef]
- Bizzoca, M.E.; Campisi, G.; Muzio, L.L. An innovative risk-scoring system of dental procedures and safety protocols in the COVID-19 era. BMC Oral Health 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Alsaegh, A.; Belova, E.; Vasil’Ev, Y.; Zabroda, N.; Severova, L.; Timofeeva, M.; Dobrokhotov, D.; Leonova, A.; Mitrokhin, O. COVID-19 in Dental Settings: Novel Risk Assessment Approach. Int. J. Environ. Res. Public Health 2021, 18, 6093. [Google Scholar] [CrossRef]
- Falahchai, M.; Hemmati, Y.B.; Hasanzade, M. Dental care management during the COVID-19 outbreak. Spéc. Care Dent. 2020, 40, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Howaldt, H.-P. Impact of COVID-19 on the Dental Community: Part I before Vaccine (BV). J. Clin. Med. 2021, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.-W. Omicron (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. arXiv 2021, arXiv:2112.01318. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khan, K.; Khoury, D.; Moyo-Gwete, T.; Tegally, H.; Scheepers, C.; Amoako, D.; Karim, F.; Bernstein, M.; et al. SARS-CoV-2 Omicron Has Extensive but Incomplete Escape of Pfizer BNT162b2 Elicited Neutralization and Requires ACE2 for Infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Helfritz, F.A.; et al. Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and Monoclonal Antibodies. medRxiv 2021. [Google Scholar] [CrossRef]
- Gruell, H.; Vanshylla, K. MRNA Booster Immunization Elicits Potent Neutralizing Serum Activity against the SARS-CoV-2 Omicron Variant. Available online: https://drive.google.com/file/d/13iHMR6rk3MKRFhDZmNuH3AAjR1uT8mEU/view (accessed on 15 December 2021).
- HKUMed Finds Omicron SARS-CoV-2 Can Infect Faster and Better than Delta in Human Bronchus but with Less Severe Infection in Lung—News—HKUMed. Available online: http://www.med.hku.hk/en/news/press/20211215-omicron-SARS-COV-2-infection (accessed on 15 December 2021).
- Cameroni, E.; Saliba, C.; Bowen, J.E.; Rosen, L.E.; Culap, K.; Pinto, D.; de Marco, A.; Zepeda, S.K.; di Iulio, J.; Zatta, F.; et al. Broadly Neutralizing Antibodies Overcome SARS-CoV-2 Omicron Antigenic Shift. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Enhancing Readiness for Omicron (B.1.1.529): Technical Brief and Priority Actions for Member States. Available online: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states (accessed on 15 December 2021).
- Yamasoba, D.; Kimura, I.; Nasser, H.; Morioka, Y.; Nao, N.; Ito, J.; Uriu, K.; Tsuda, M.; Zahradnik, J.; Shirakawa, K.; et al. Virological Characteristics of SARS-CoV-2 BA.2 Variant. bioRxiv 2022. [Google Scholar] [CrossRef]
- Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 12 December 2021).
- Moderna—Moderna Announces Strategy to Address Omicron (B.1.1.529) SARS-CoV-2 Variant. Available online: https://investors.modernatx.com/news/news-details/2021/Moderna-Announces-Strategy-to-Address-Omicron-B.1.1.529-SARS-CoV-2-Variant/default.aspx (accessed on 12 December 2021).
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 Variant: A New Chapter in the COVID-19 Pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Pulliam, J.R.C.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medrxiv 2021. [Google Scholar] [CrossRef]
- Van Kasteren, P.B.; van Der Veer, B.; van den Brink, S.; Wijsman, L.; de Jonge, J.; van den Brandt, A.; Molenkamp, R.; Reusken, C.B.E.M.; Meijer, A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J. Clin. Virol. 2020, 128, 104412. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.Z.; Shakoor, S.; Kanji, A.; Shaheen, N.; Nasir, A.; Ansar, Z.; Ahmed, I.; Mahmood, S.F.; Hasan, R.; Hasan, Z. Discrepancy between PCR Based SARS-CoV-2 Tests Suggests the Need to Re-Evaluate Diagnostic Assays. BMC Res. Notes 2021, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.-H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable Escape of SARS-CoV-2 Variant Omicron to Antibody Neutralization. bioRxiv 2021. [Google Scholar] [CrossRef]
- Brandal, L.T.; MacDonald, E.; Veneti, L.; Ravlo, T.; Lange, H.; Naseer, U.; Feruglio, S.; Bragstad, K.; Hungnes, O.; Ødeskaug, L.E.; et al. Outbreak Caused by the SARS-CoV-2 Omicron Variant in Norway, November to December 2021. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2021, 26, 2101147. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.G.; Sutherland, A.D.; Suliman, T.; Shaw, M.; Preiser, W. Breakthrough Infections with SARS-CoV-2 Omicron Variant Despite Booster Dose of MRNA Vaccine. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust against Omicron. Viruses 2022, 14, 79. [Google Scholar] [CrossRef]
- SARS-CoV-2 Variants of Concern as of 13 January 2022. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 14 January 2022).
- Volgenant, C.M.C.; de Soet, J.J. Cross-transmission in the Dental Office: Does This Make You Ill? Curr. Oral Health Rep. 2018, 5, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Nemati, S. Impacts of COVID-19 Outbreak on Dentistry Dimensions. Indian J. Med Sci. 2021, 46, 149–150. [Google Scholar] [CrossRef]
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: From an acute to chronic disease? Potential long-term health consequences. Crit. Rev. Clin. Lab. Sci. 2020, 58, 297–310. [Google Scholar] [CrossRef]
- McDonald, L.T. Healing after COVID-19: Are survivors at risk for pulmonary fibrosis? Am. J. Physiol. Cell. Mol. Physiol. 2021, 320, L257–L265. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, T. One Year Update on the COVID-19 Pandemic: Where Are We Now? Acta Trop. 2021, 214, 105778. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M. Deleterious Outcomes in Long-Hauler COVID-19: The Effects of SARS-CoV-2 on the CNS in Chronic COVID Syndrome. ACS Chem. Neurosci. 2020, 11, 4017–4020. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, A.; Scarinci, A.; Monda, V.; Sessa, F.; Messina, A.; Monda, M.; Precenzano, F.; Mollica, M.; Carotenuto, M.; Messina, G.; et al. The Social Brain and Emotional Contagion: COVID-19 Effects. Medicina 2020, 56, 640. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Madjunkov, M.; Dviri, M.; Librach, C. A comprehensive review of the impact of COVID-19 on human reproductive biology, assisted reproduction care and pregnancy: A Canadian perspective. J. Ovarian Res. 2020, 13, 1–18. [Google Scholar] [CrossRef]
- Wang, N.; Qin, L.; Ma, L.; Yan, H. Effect of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on reproductive system. Stem Cell Res. 2021, 52, 102189. [Google Scholar] [CrossRef]
- Thurzo, A.; Kočiš, F.; Novák, B.; Czako, L.; Varga, I. Three-Dimensional Modeling and 3D Printing of Biocompatible Orthodontic Power-Arm Design with Clinical Application. Appl. Sci. 2021, 11, 9693. [Google Scholar] [CrossRef]
- Thurzo, A.; Urbanová, W.; Novák, B.; Waczulíková, I.; Varga, I. Utilization of a 3D Printed Orthodontic Distalizer for Tooth-Borne Hybrid Treatment in Class II Unilateral Malocclusions. Materials 2022, 15, 1740. [Google Scholar] [CrossRef]
- Takanabe, Y.; Maruoka, Y.; Kondo, J.; Yagi, S.; Chikazu, D.; Okamoto, R.; Saitoh, M. Dispersion of Aerosols Generated during Dental Therapy. Int. J. Environ. Res. Public Health 2021, 18, 11279. [Google Scholar] [CrossRef]
- Rexhepi, I.; Mangifesta, R.; Santilli, M.; Guri, S.; Di Carlo, P.; D’Addazio, G.; Caputi, S.; Sinjari, B. Effects of Natural Ventilation and Saliva Standard Ejectors during the COVID-19 Pandemic: A Quantitative Analysis of Aerosol Produced during Dental Procedures. Int. J. Environ. Res. Public Health 2021, 18, 7472. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv-Baran, T.; Arbel, Y.S.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—14 December 2021. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---14-december-2021 (accessed on 16 December 2021).
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Berger, N.A.; Kaelber, D.C.; Davis, P.B.; Volkow, N.D.; Xu, R. Comparison of Outcomes from COVID Infection in Pediatric and Adult Patients before and after the Emergence of Omicron. medRxiv 2022. [Google Scholar] [CrossRef]

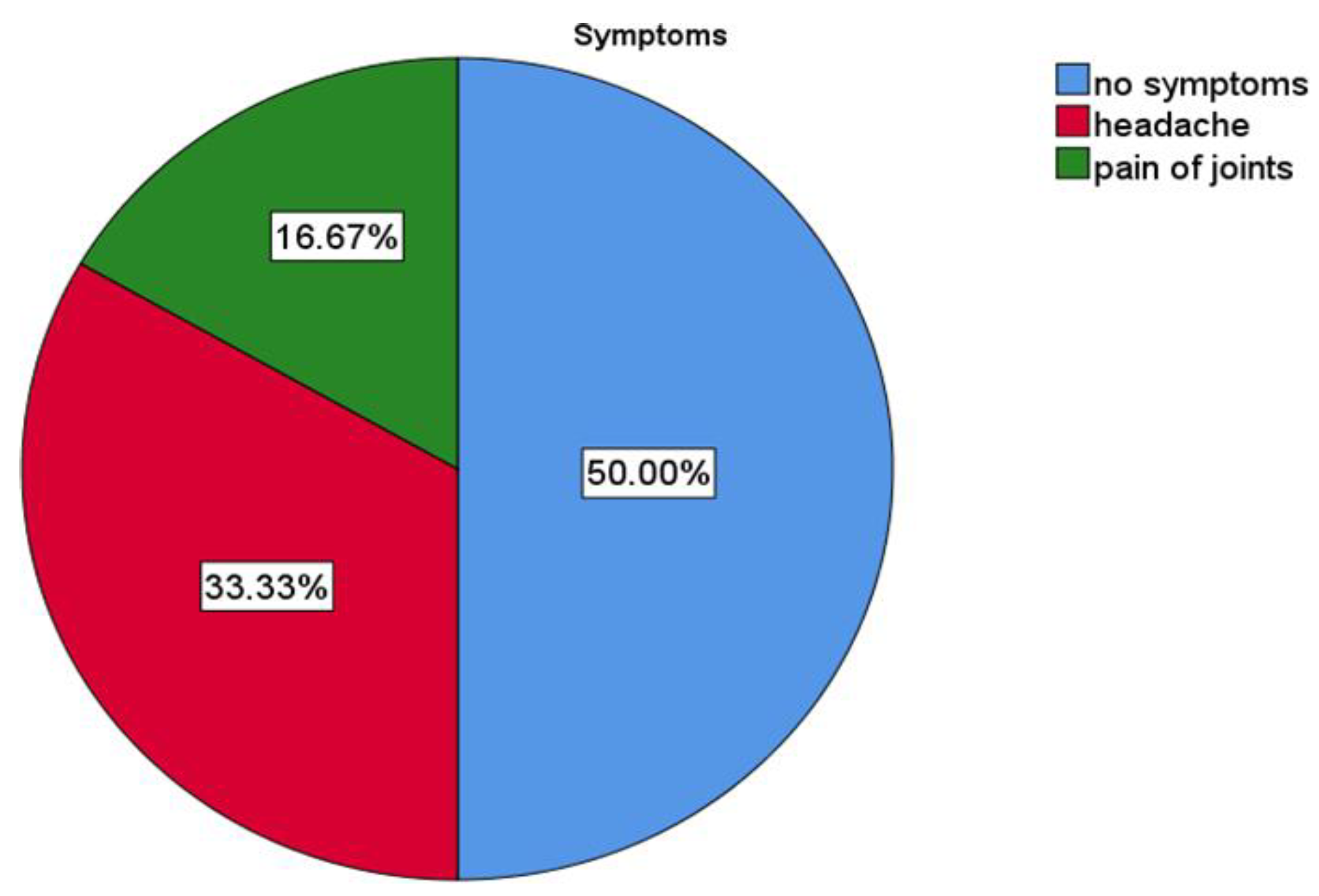
| Status | Frequency | Percent |
|---|---|---|
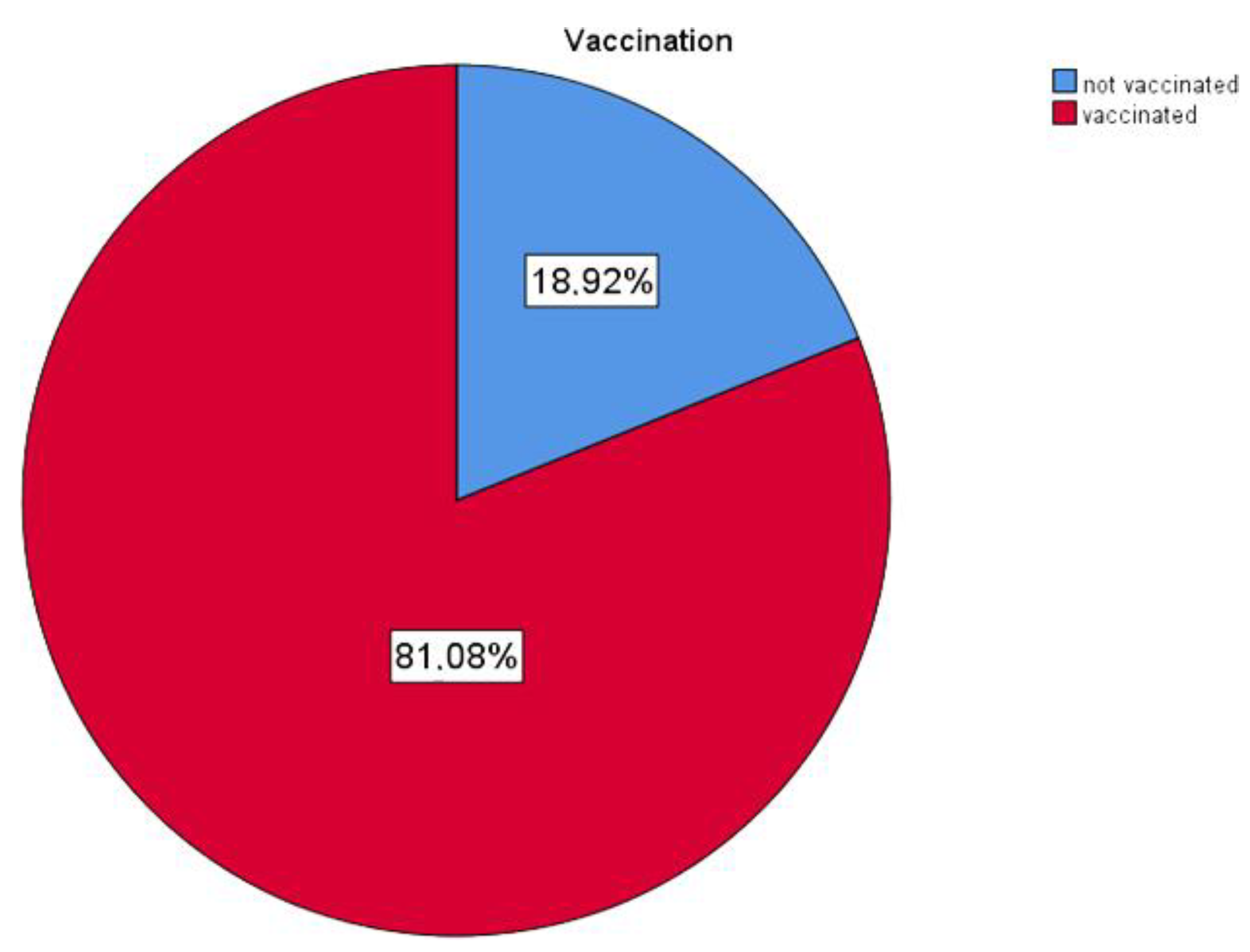
| not vaccinated | 21 | 18 9 |
| vaccinated | 90 | 81 1 |
| Total | 111 | 100 0 |
| 95% Confidence Interval for Mean | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | Std. Error | Lower Bound | Upper Bound | Minimum | Maximum | |
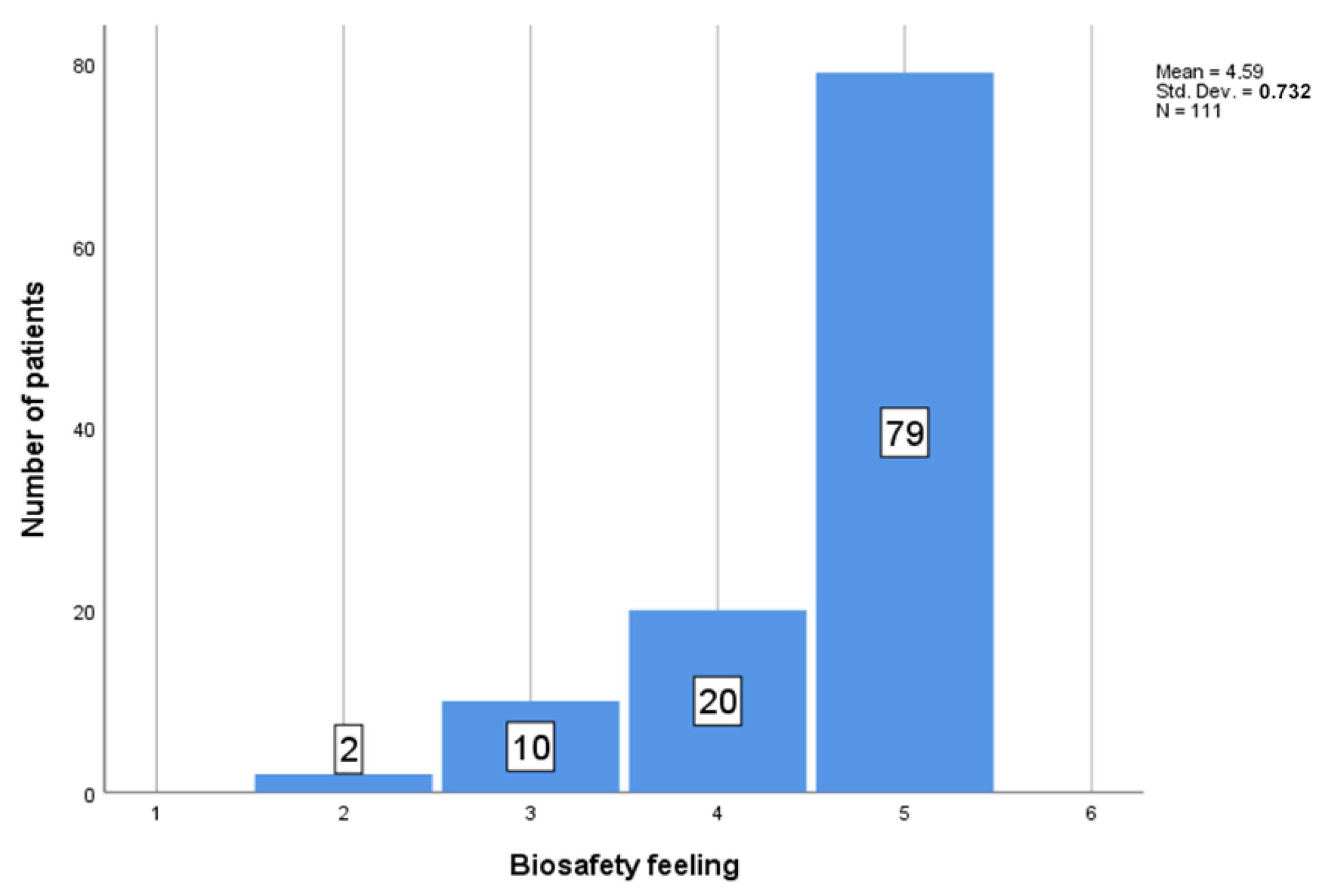
| disease free | 74 | 4.55 | 0.743 | 0.086 | 4.38 | 4.73 | 2 | 5 |
| Ab + | 3 | 4.67 | 0.577 | 0.333 | 3.23 | 6.10 | 4 | 5 |
| PCR/Ag + | 30 | 4.73 | 0.583 | 0.106 | 4.52 | 4.95 | 3 | 5 |
| multiple | 3 | 3.67 | 1.528 | 0.882 | −0.13 | 7.46 | 2 | 5 |
| other | 1 | 5.00 | 0.0 | 0.0 | 0.0 | 0.0 | 5 | 5 |
| Total | 111 | 4.59 | 0.732 | 0.069 | 4.45 | 4.72 | 2 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thurzo, A.; Urbanová, W.; Waczulíková, I.; Kurilová, V.; Mriňáková, B.; Kosnáčová, H.; Gális, B.; Varga, I.; Matajs, M.; Novák, B. Dental Care and Education Facing Highly Transmissible SARS-CoV-2 Variants: Prospective Biosafety Setting: Prospective, Single-Arm, Single-Center Study. Int. J. Environ. Res. Public Health 2022, 19, 7693. https://doi.org/10.3390/ijerph19137693
Thurzo A, Urbanová W, Waczulíková I, Kurilová V, Mriňáková B, Kosnáčová H, Gális B, Varga I, Matajs M, Novák B. Dental Care and Education Facing Highly Transmissible SARS-CoV-2 Variants: Prospective Biosafety Setting: Prospective, Single-Arm, Single-Center Study. International Journal of Environmental Research and Public Health. 2022; 19(13):7693. https://doi.org/10.3390/ijerph19137693
Chicago/Turabian StyleThurzo, Andrej, Wanda Urbanová, Iveta Waczulíková, Veronika Kurilová, Bela Mriňáková, Helena Kosnáčová, Branislav Gális, Ivan Varga, Marek Matajs, and Bohuslav Novák. 2022. "Dental Care and Education Facing Highly Transmissible SARS-CoV-2 Variants: Prospective Biosafety Setting: Prospective, Single-Arm, Single-Center Study" International Journal of Environmental Research and Public Health 19, no. 13: 7693. https://doi.org/10.3390/ijerph19137693
APA StyleThurzo, A., Urbanová, W., Waczulíková, I., Kurilová, V., Mriňáková, B., Kosnáčová, H., Gális, B., Varga, I., Matajs, M., & Novák, B. (2022). Dental Care and Education Facing Highly Transmissible SARS-CoV-2 Variants: Prospective Biosafety Setting: Prospective, Single-Arm, Single-Center Study. International Journal of Environmental Research and Public Health, 19(13), 7693. https://doi.org/10.3390/ijerph19137693