Influence of Adding Low Concentration of Oxygenates in Mineral Diesel Oil and Biodiesel on the Concentration of NO, NO2 and Particulate Matter in the Exhaust Gas of a One-Cylinder Diesel Generator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Engine Specification
2.2. Exhaust Gas Emission
2.3. Measuring Protocol
2.4. Adding of Oxygenates
2.5. Statistical Testing
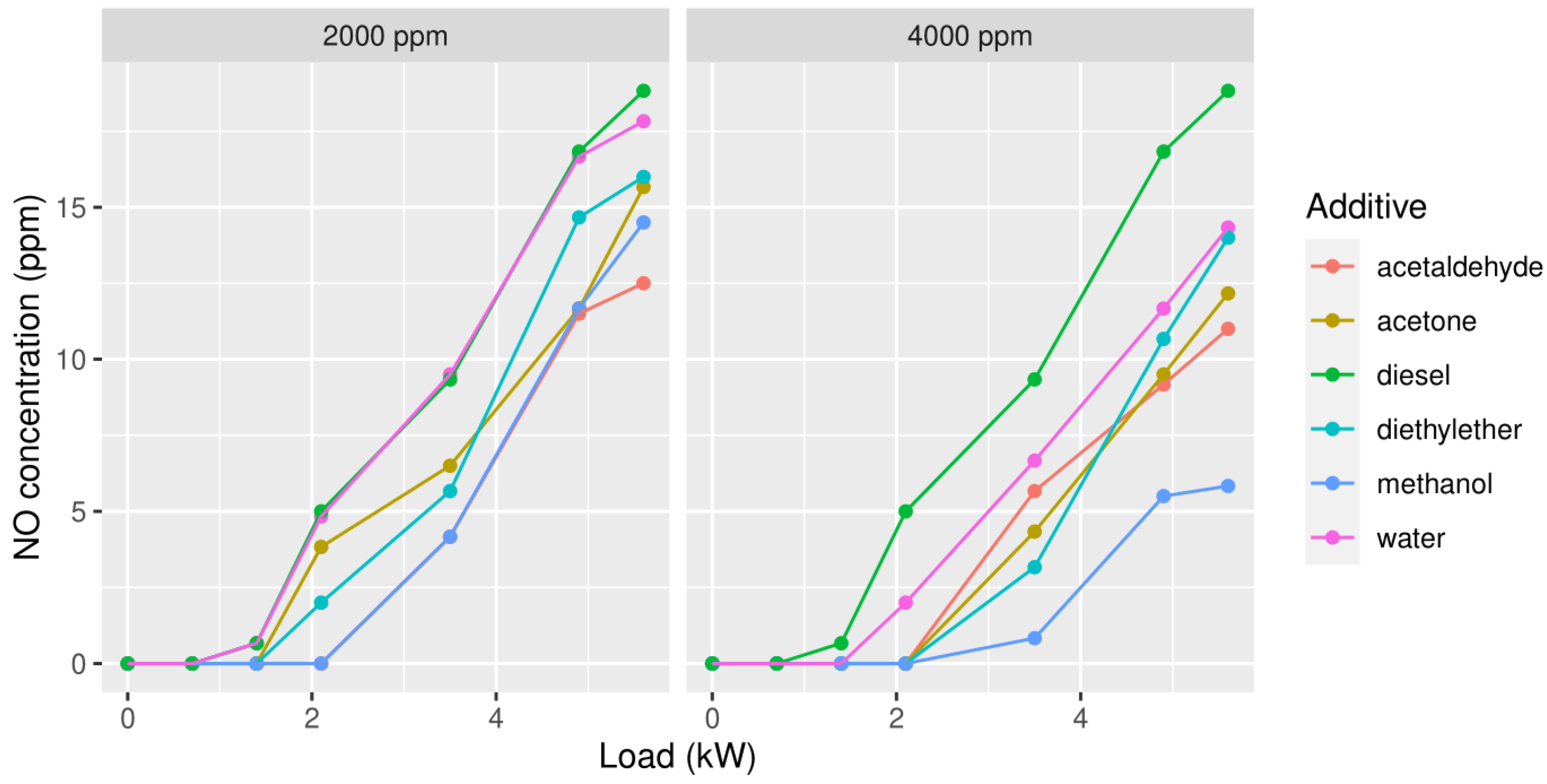
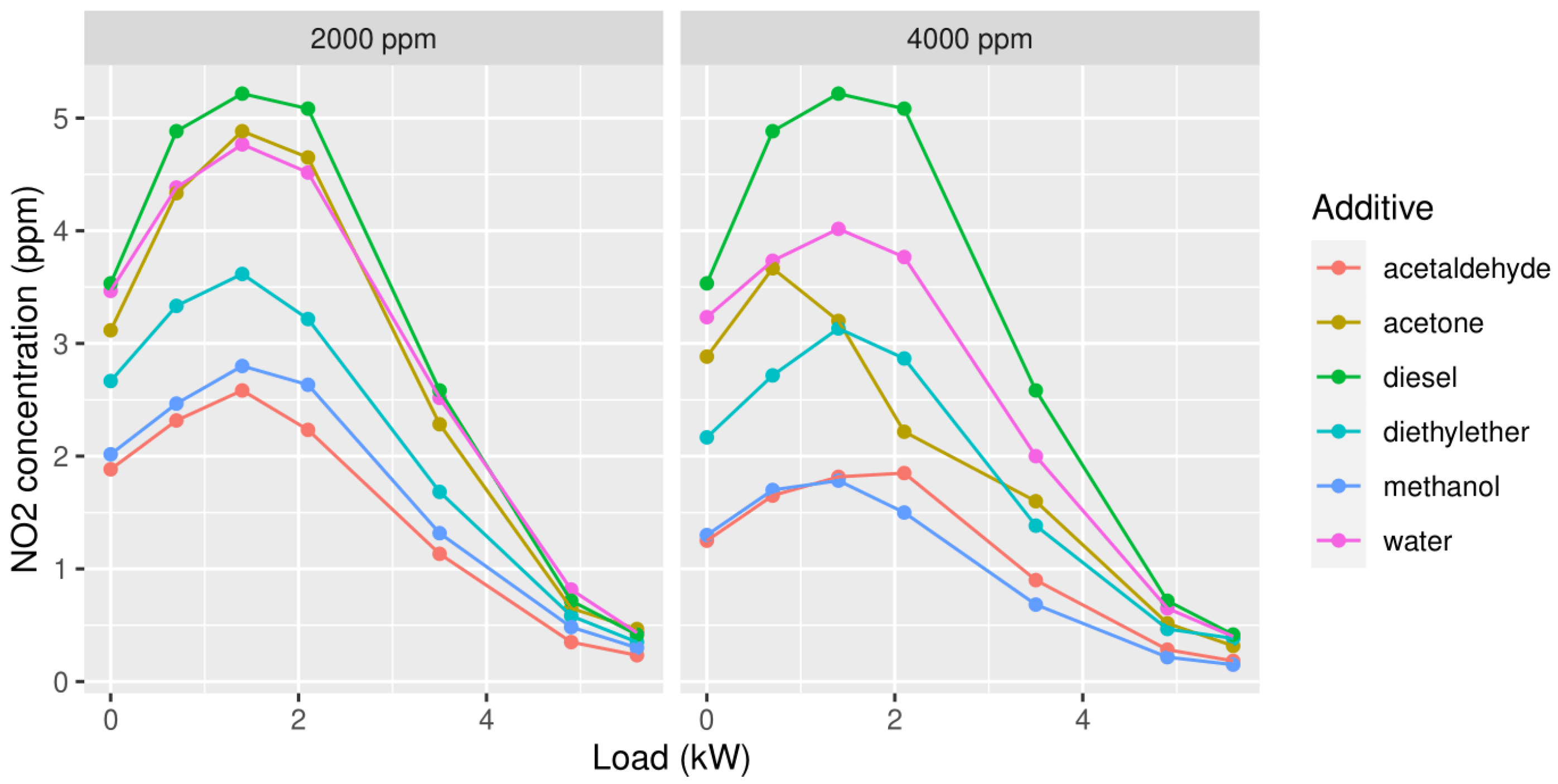
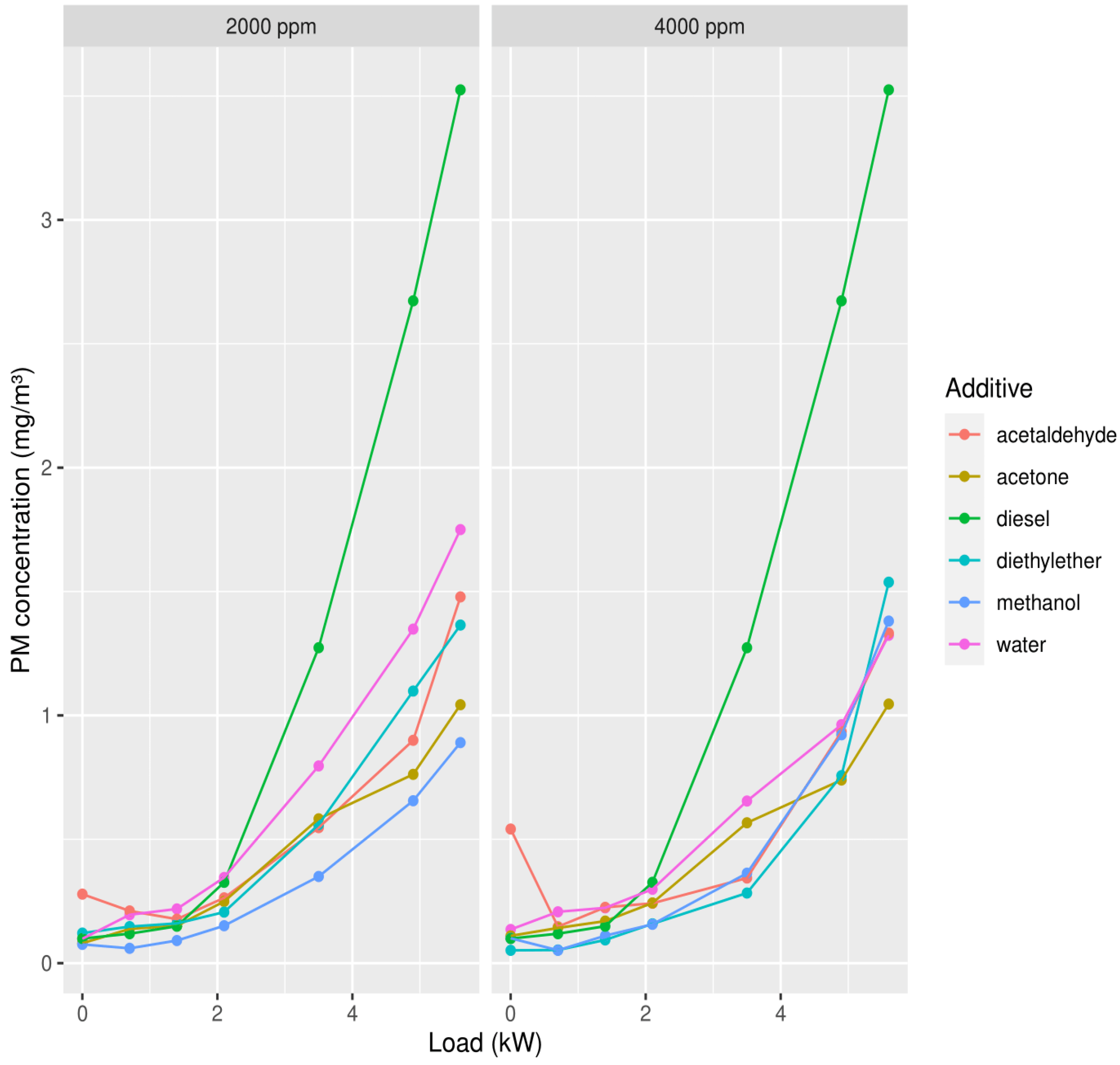
3. Results
3.1. First Measuring Campaign
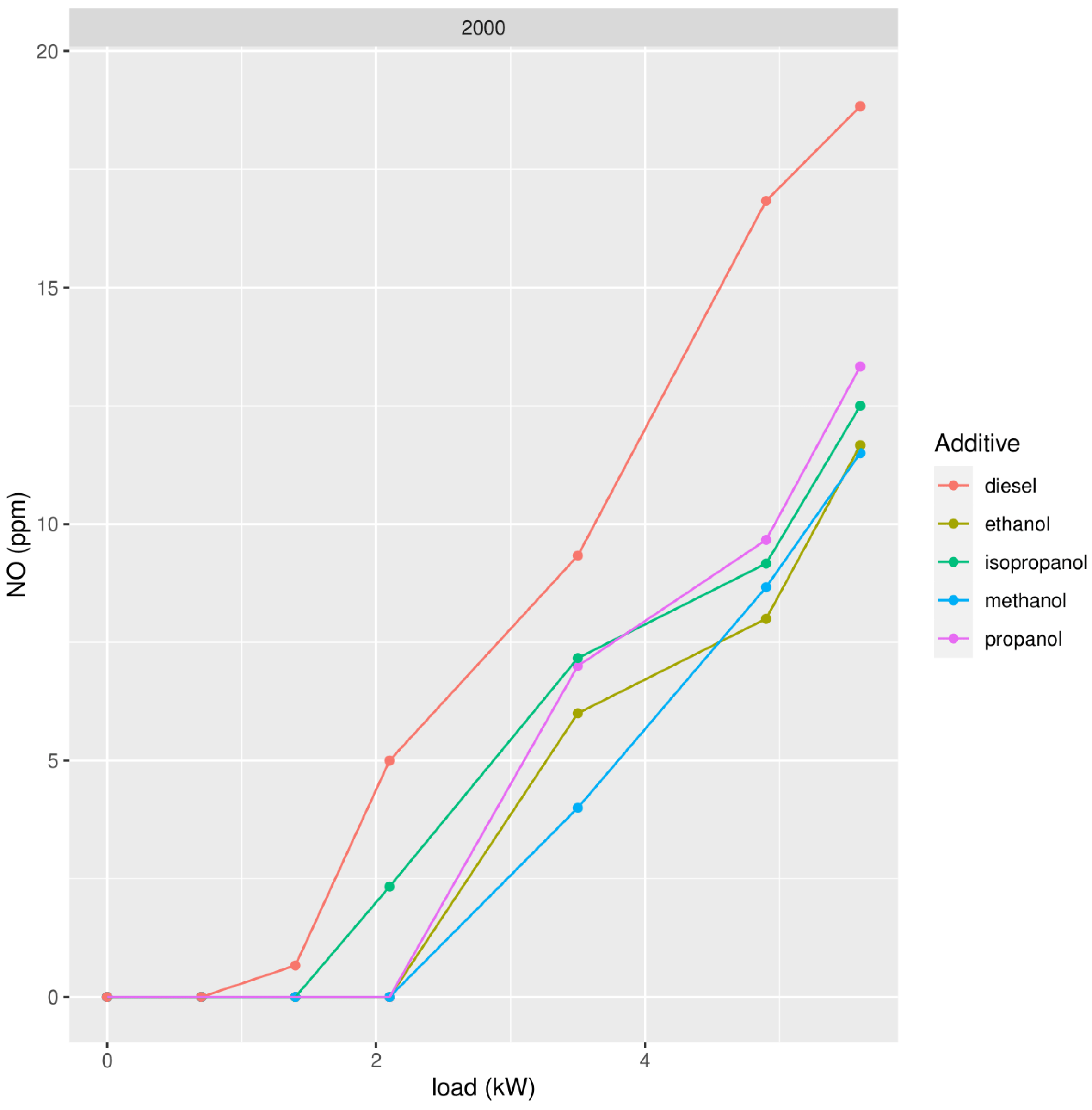
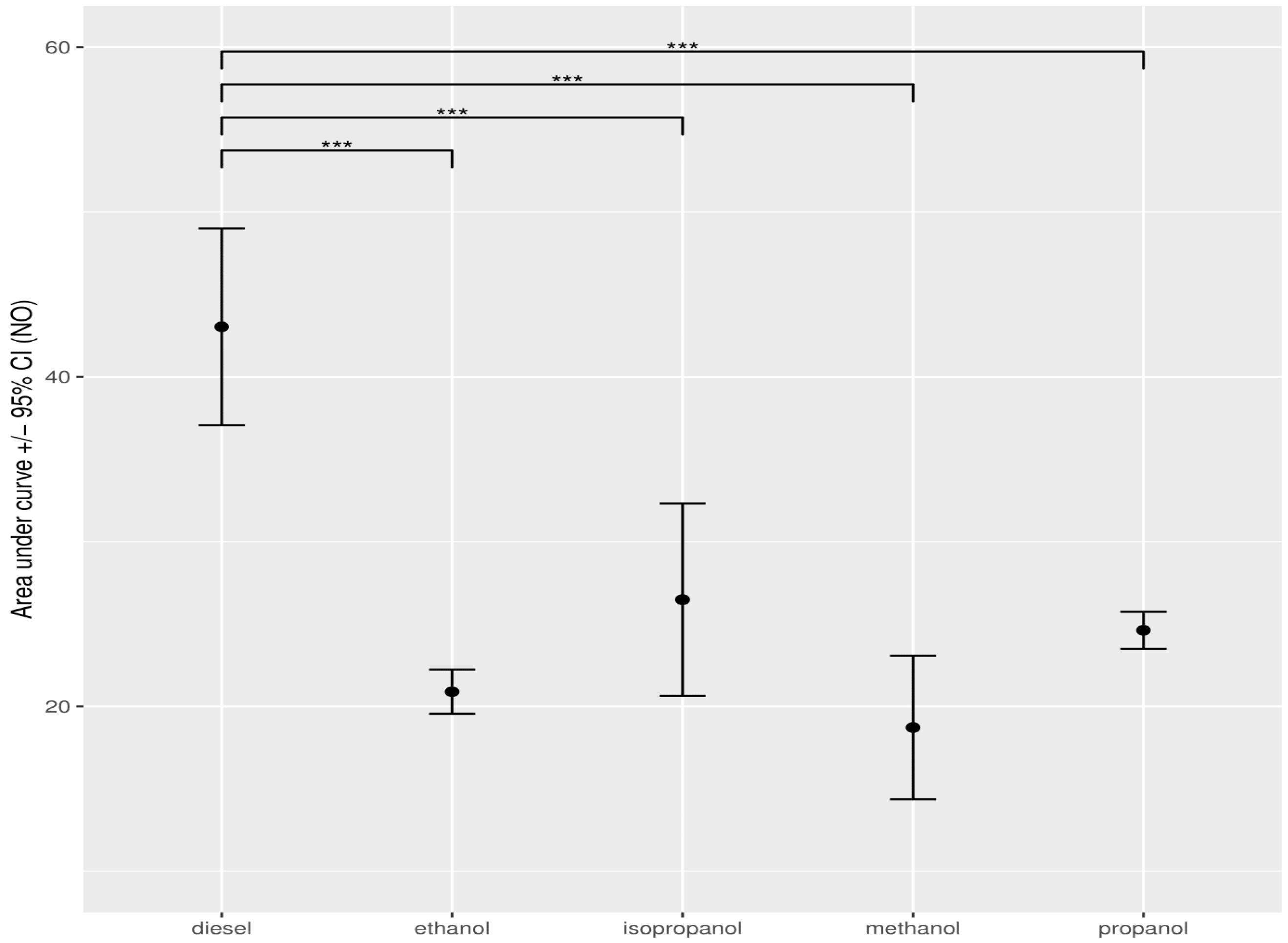
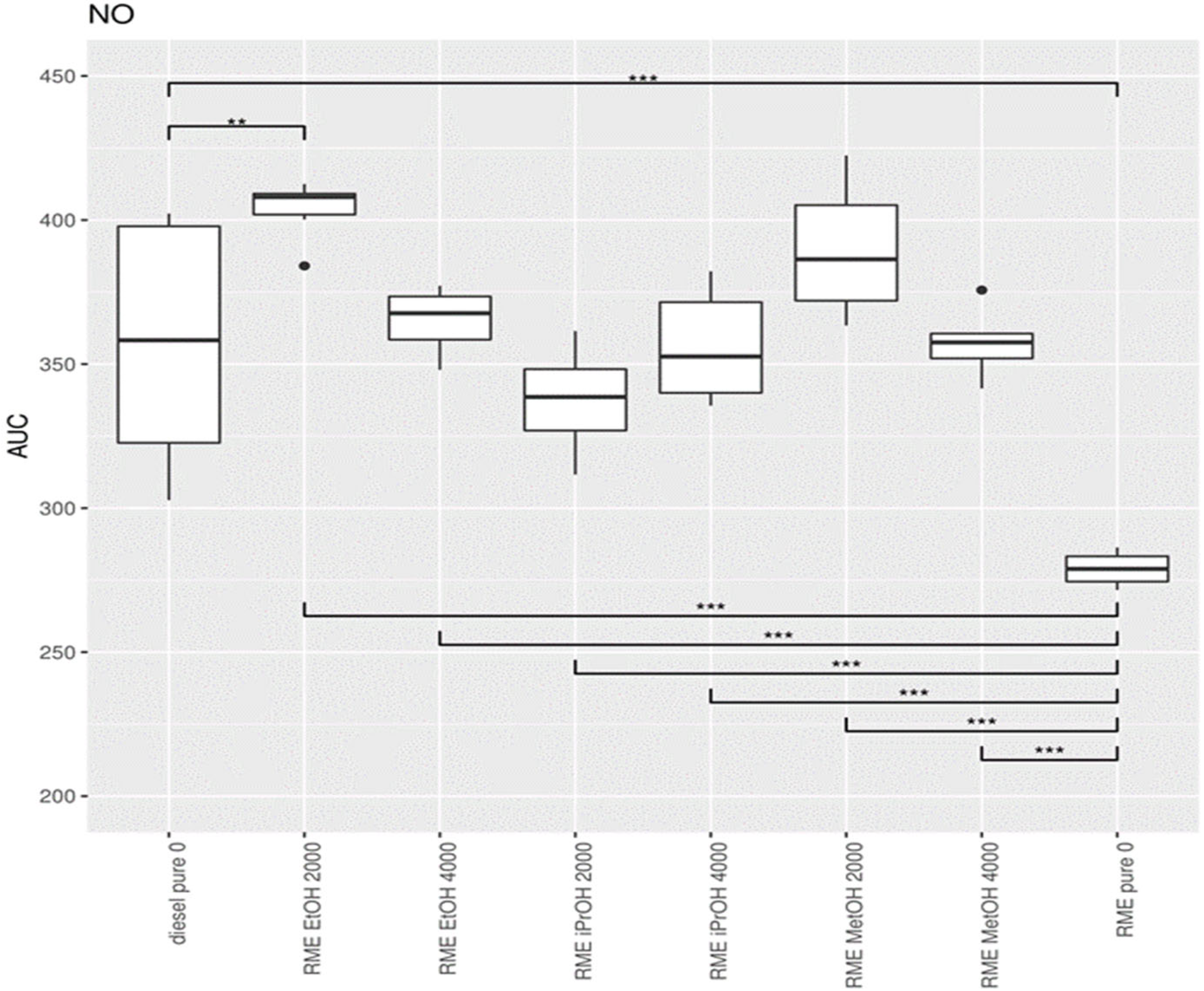
3.1.1. Nitrogen Oxide (NO)
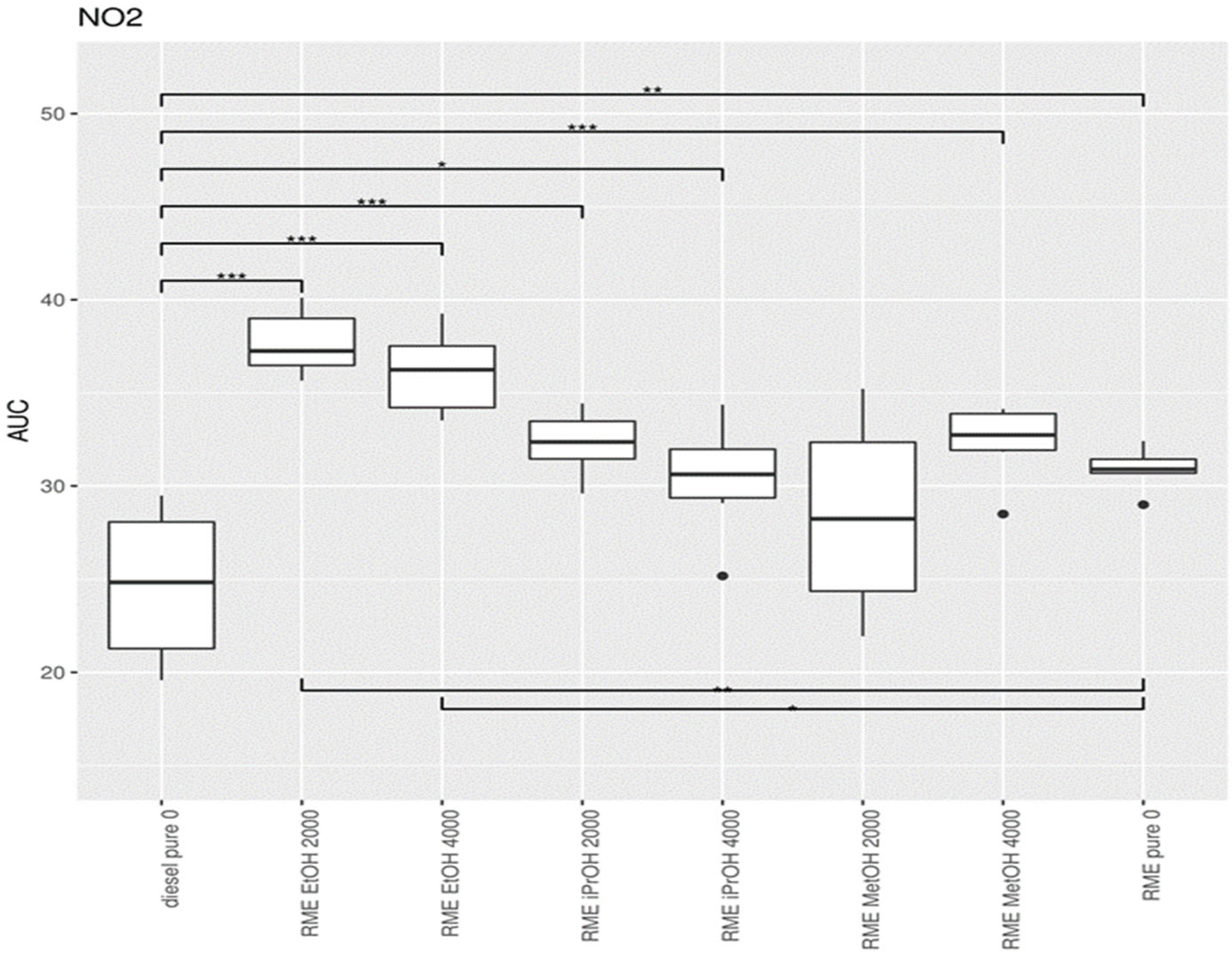
3.1.2. Nitrogen Dioxide (NO2)
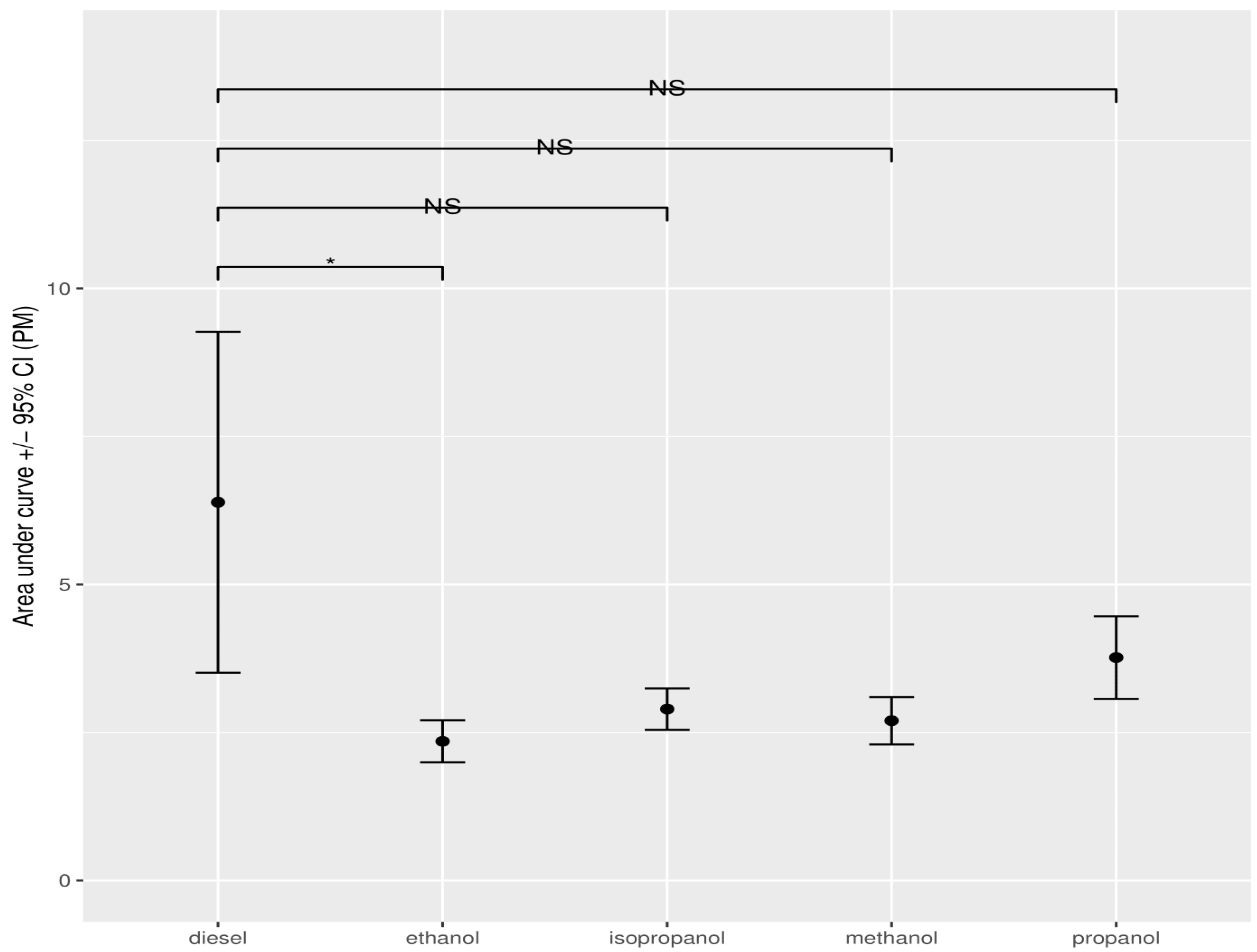
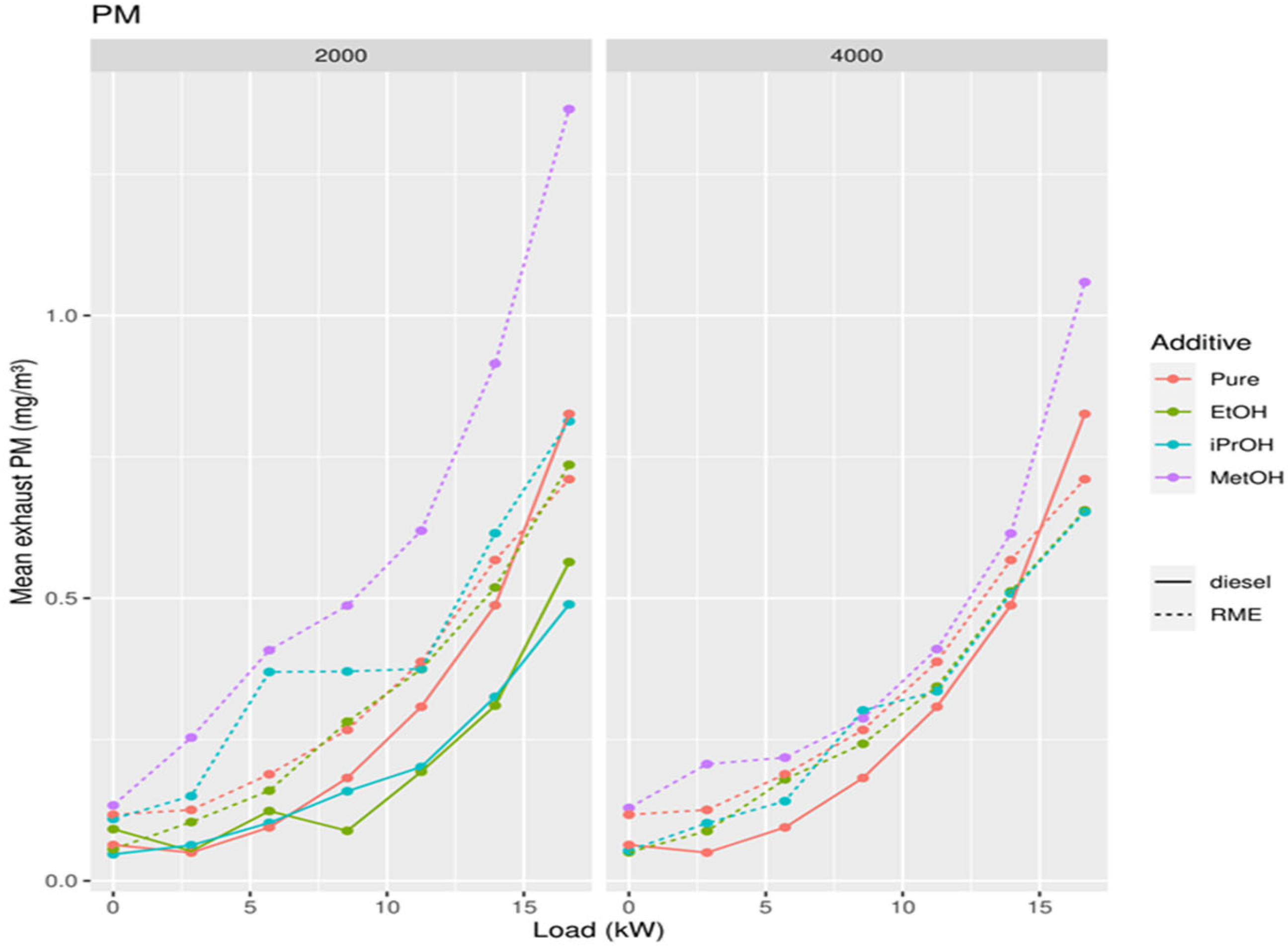
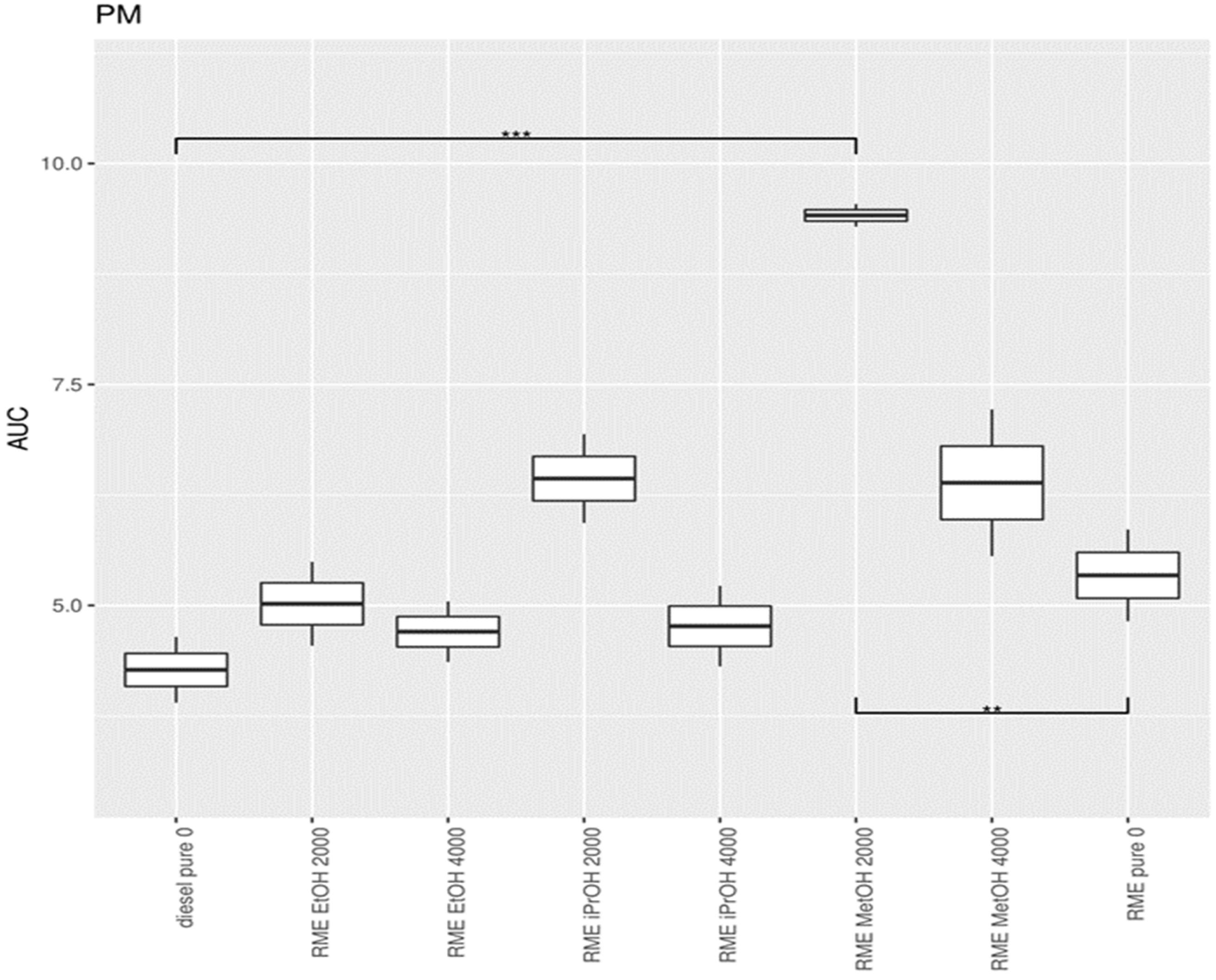
3.1.3. Particulate Matter (PM)
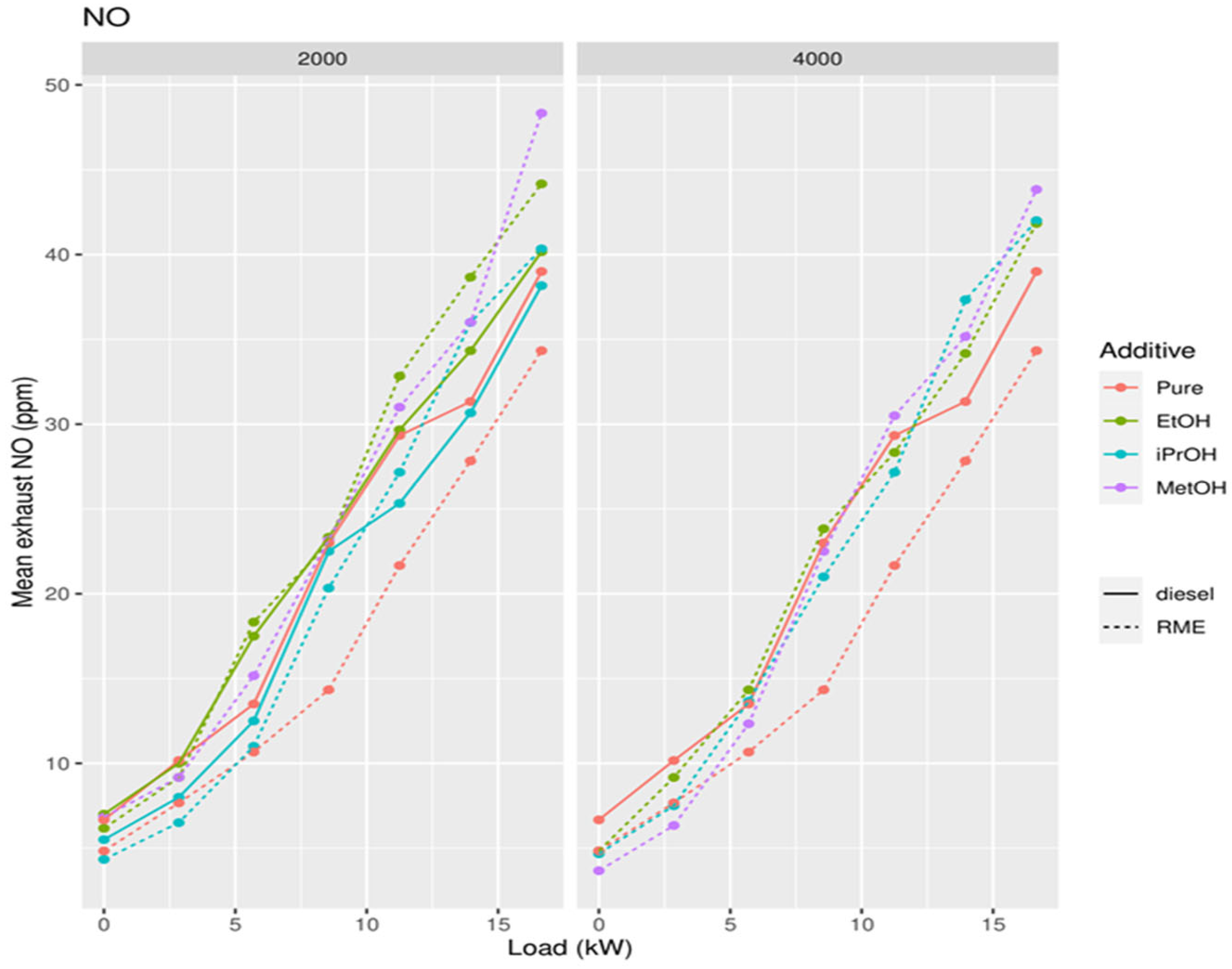
3.2. Second Measuring Campaign
3.3. Effect of Chain Length of Alcohols
3.4. Statistical Data Analysis When Adding Alcohol to Diesel
3.5. Addition of Oxygenates to Biodiesel
4. Discussion
4.1. Adding Oxygenates to Mineral Diesel
4.2. Effect of Alcohol Addition to Biodiesel and Diesel
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amin, A.; Altinoz, B.; Dogan, E. Analyzing the determinants of carbon emissions from transportation in European countries: The role of renewable energy and urbanization. Clean Technol. Environ. Policy 2020, 22, 1725–1734. [Google Scholar] [CrossRef]
- Ahmed, Z.; Ali, S.; Saud, S.; Shahzad, S.J.H. Transport CO2 emissions, drivers, and mitigation: An empirical investigation in India. Air Qual. Atmos. Health 2020, 13, 1367–1374. [Google Scholar] [CrossRef]
- Lajunen, A.; Kivekäs, K.; Vepsäläinen, J.; Tammi, K. Influence of increasing electrification of passenger vehicle fleet on carbon dioxide emissions in Finland. Sustainability 2020, 12, 5032. [Google Scholar] [CrossRef]
- Dér, A.; Erkisi-Arici, S.; Stachura, M.; Cerdas, F.; Böhme, S.; Herrmann, C. Life Cycle Assessment of Electric Vehicles in Fleet Applications. In Fleets Go Green; Springer: Cham, Switzerland, 2018; pp. 61–80. [Google Scholar]
- Ng, K.S.; Farooq, D.; Yang, A. Global biorenewable development strategies for sustainable aviation fuel production. Renew. Sustain. Energy Rev. 2021, 150, 111502. [Google Scholar] [CrossRef]
- Abrantes, I.; Ferreira, A.F.; Silva, A.; Costa, M. Sustainable aviation fuels and imminent technologies-CO2 emissions evolution towards 2050. J. Clean. Prod. 2021, 313, 127937. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Jalkanen, J.-P.; Johansson, L.; Kukkonen, J. A comprehensive inventory of ship traffic exhaust emissions in the European sea areas in 2011. Atmos. Chem. Phys. 2016, 16, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Lopatin, O.P. Phenomenology of nitrogen oxides formation in a gas-diesel engine. J. Phys. Conf. Ser. 2020, 1515, 042009. [Google Scholar] [CrossRef]
- Mao, G.; Shi, K.; Zhang, C.; Chen, S.; Wang, P. Experimental research on effects of biodiesel fuel combustion flame temperature on NOX formation based on endoscope high-speed photography. J. Energy Inst. 2020, 93, 1399–1410. [Google Scholar] [CrossRef]
- Asprion, J.; Chinellato, O.; Guzzella, L. A fast and accurate physics-based model for the NOx emissions of Diesel engines. Appl. Energy 2013, 103, 221–233. [Google Scholar] [CrossRef]
- Anufriev, I. Review of water/steam addition in liquid-fuel combustion systems for NOx reduction: Waste-to-energy trends. Renew. Sustain. Energy Rev. 2020, 138, 110665. [Google Scholar] [CrossRef]
- Zhang, Y.; Eastham, S.D.; Lau, A.K.; Fung, J.C.; Selin, N.E. Global air quality and health impacts of domestic and international shipping. Environ. Res. Lett. 2021, 16, 084055. [Google Scholar] [CrossRef]
- Bilsback, K.R.; Kerry, D.; Croft, B.; Ford, B.; Jathar, S.H.; Carter, E.; Martin, R.V.; Pierce, J.R. Beyond SOx reductions from shipping: Assessing the impact of NOx and carbonaceous-particle controls on human health and climate. Environ. Res. Lett. 2020, 15, 124046. [Google Scholar] [CrossRef]
- Contini, D.; Merico, E. Recent advances in studying air quality and health effects of shipping emissions. Atmosphere 2021, 12, 92. [Google Scholar] [CrossRef]
- Rebello, S.; Anoopkumar, A.; Aneesh, E.M.; Sindhu, R.; Binod, P.; Pandey, A. Sustainability and life cycle assessments of lignocellulosic and algal pretreatments. Bioresour. Technol. 2019, 301, 122678. [Google Scholar] [CrossRef]
- Parsons, S.; Allen, M.J.; Chuck, C.J. Coproducts of algae and yeast-derived single cell oils: A critical review of their role in improving biorefinery sustainability. Bioresour. Technol. 2020, 303, 122862. [Google Scholar] [CrossRef]
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a sustainable future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Koivisto, E.; Ladommatos, N.; Gold, M. The influence of various oxygenated functional groups in carbonyl and ether compounds on compression ignition and exhaust gas emissions. Fuel 2015, 159, 697–711. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Wang, Z.; Liu, H. Combustion and emissions of compression ignition in a direct injection diesel engine fueled with pentanol. Energy 2015, 80, 575–581. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Wang, Z.; Liu, H. Combustion and emission characteristics of diesel engine fueled with diesel/biodiesel/pentanol fuel blends. Fuel 2015, 156, 211–218. [Google Scholar] [CrossRef]
- Wei, L.; Cheung, C.S.; Huang, Z. Effect of n-pentanol addition on the combustion, performance and emission characteristics of a direct-injection diesel engine. Energy 2014, 70, 172–180. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G. Impact of properties of vegetable oil, biodiesel, ethanol and n-butanol on the combustion and emissions of turbocharged HDDI diesel engine operating under steafy and transient conditions. Fuel 2015, 156, 1–19. [Google Scholar] [CrossRef]
- Pepiot-Desjardins, P.; Pitsch, H.; Malhotra, R.; Kirby, S.R.; Boehman, A.L. Structural group analysis for soot reducion tendency of oxygenated fuels. Combust. Flame 2008, 154, 191–205. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.; Lee, P.; Chua, K.J.; Chou, S. Pine oil–biodiesel blends: A double biofuel strategy to completely eliminate the use of diesel in a diesel engine. Appl. Energy 2013, 130, 466–473. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Grab-Rogaliński, K. The effect of RME-1-butanol blends on combustion, performance and emission of a direct injection diesel engine. Energies 2021, 14, 2941. [Google Scholar] [CrossRef]
- Goodrum, J. Volatility and boiling points of biodiesel from vegetable oils and tallow. Biomass Bioenergy 2002, 22, 205–211. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 1993–1994, 74th ed.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Labeckas, G.; Slavinskas, S.; Lus, T.; Klyus, O.; Mažeika, M. Combustion and performance parameters of a diesel engine operating on ethanol-diesel blends. Sci. J. Marit. Univ. Szcecin 2013, 36, 102–109. [Google Scholar]
- Datta, A.; Mandal, B. Impact of alcohol addition to diesel on the performance combustion and emissions of a compression ignition engine. Appl. Therm. Eng. 2016, 98, 670–682. [Google Scholar] [CrossRef]
- Sayin, C. Engine performance and exhaust gas emissions of methanol and ethanol-diesel blends. Fuel 2010, 89, 3410–3415. [Google Scholar] [CrossRef]
- Nanthagopal, K.; Kishna, R.S.; Atabani, A.E.; Al-Muhtaseb, A.H.; Kumar, G.; Ashok, B. A compressive review on the effects of alcohols and nanoparticles as an oxygenated enhancer in compression ignition engine. Energy Convers. Manag. 2020, 203, 112244. [Google Scholar] [CrossRef]
- Atmanli, A.; Yilmaz, N. An experimental assessment on semi-low combustion using waste oil diesel/C3-C5 alcohol blends in a diesel engine. Fuel 2019, 260, 116357. [Google Scholar] [CrossRef]
- Yasin, M.H.M.; Mamat, R.; Yusop, A.F.; Rahim, R.; Aziz, A.; Shah, L.A. Fuel Physical Characteristics of Biodiesel Blend Fuels with Alcohol as Additives. Procedia Eng. 2013, 53, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Rajak, U.; Nashine, P.; Verma, T.N. Characteristics of microalgae spirulina biodiesel with the impact of n-butanol addition to a CI engine. Energy 2019, 189, 116311. [Google Scholar] [CrossRef]
- Tüccar, G.; Özgür, T.; Aydin, K. Effect of diesel- microalgae biodiesel-butanol blends on performance and emissions of diesel engine. Fuel 2014, 132, 47–52. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.; Nagappan, B.; Subbiah, G. Experimental assessment of performance and exhaust emission characterstics of a diesel engine fuelled with Punnai biodiesel/butanol fuel blends. Pet. Sci. 2019, 16, 1471–1478. [Google Scholar] [CrossRef] [Green Version]
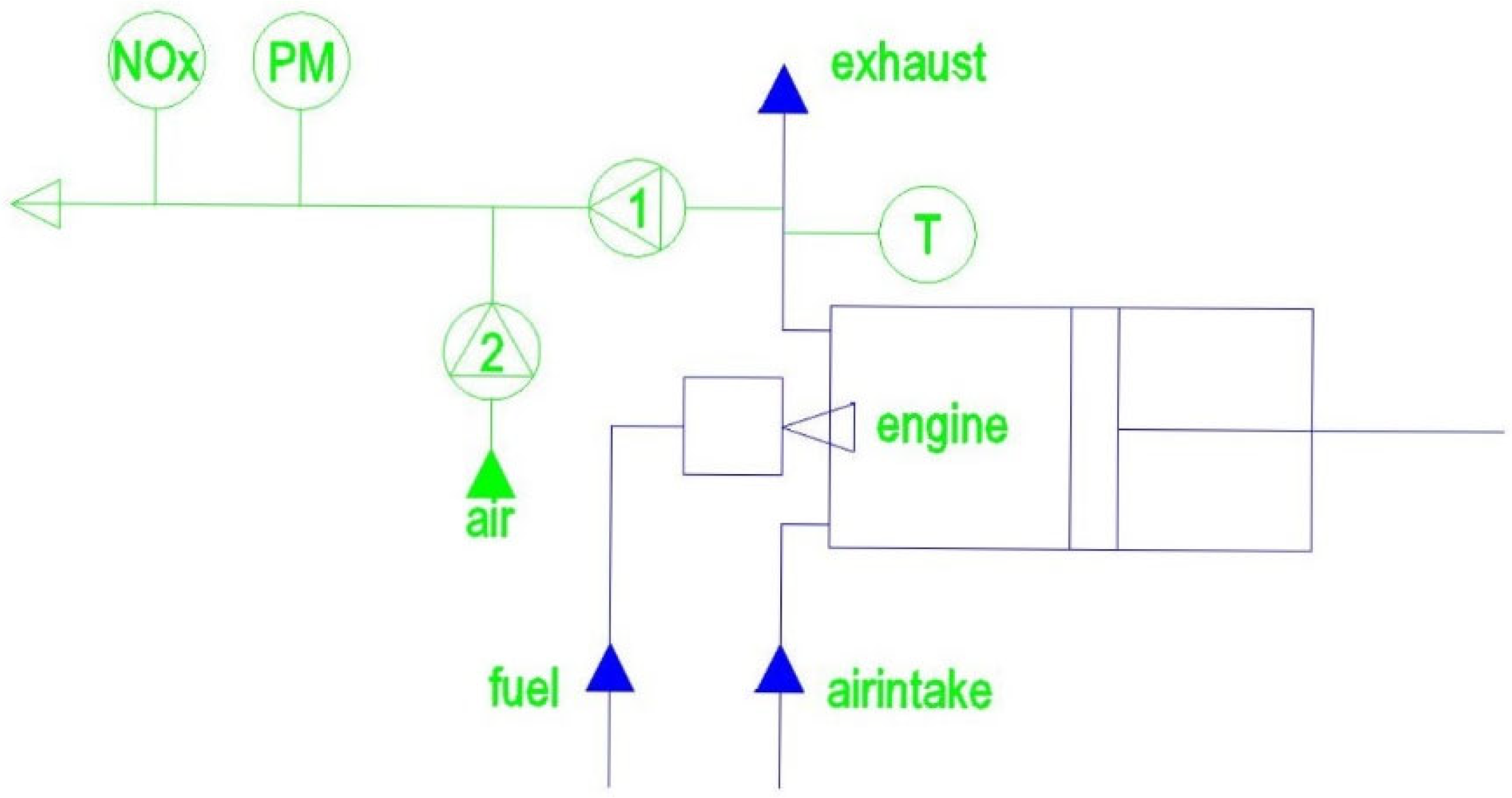




| Injection system | Direct injection |
| Type | Single cylinder |
| Cooling | Air cooled |
| Aspiration | Naturally aspirated |
| Bore (mm) | 86 |
| Stroke (mm) | 70 |
| Compression ratio | 19 |
| Boiling Point (°C) | Flash Point (°C) | Evaporation Heat * (kJ/mol at 25 °C) ** (kJ/kg at 25 °C) | |
|---|---|---|---|
| water | 100 | NA | 43.98 * |
| acetone | 56 | −20 | 30.99 * |
| acetaldehyde | 20.8 | −38 | 25.47 * |
| diethyl ether | 35 | −20 | 27.1 * |
| methanol | 64.96 | 11 | 37.43 * |
| ethanol | 78.37 | 12 | 42.32 * |
| propanol | 97.1 | 15 | 47.45 * |
| isopropanol | 82 | 12 | 45.39 * |
| diesel | 180–340 | 74 | 265 ** |
| RME | 369 | 91–135 | 250 ** |
| NO (Mean AUC) | NO2 (Mean AUC) | PM (Mean AUC) | |
|---|---|---|---|
| methanol | 18.71 | 8.11 | 2.70 |
| ethanol | 20.89 | 8.92 | 2.35 |
| propanol | 24.61 | 8.93 | 3.77 |
| isopropanol | 26.47 | 8.91 | 2.90 |
| pure diesel | 43.03 | 18.17 | 6.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maes, R.R.; Potters, G.; Fransen, E.; Van Schaeren, R.; Lenaerts, S. Influence of Adding Low Concentration of Oxygenates in Mineral Diesel Oil and Biodiesel on the Concentration of NO, NO2 and Particulate Matter in the Exhaust Gas of a One-Cylinder Diesel Generator. Int. J. Environ. Res. Public Health 2022, 19, 7637. https://doi.org/10.3390/ijerph19137637
Maes RR, Potters G, Fransen E, Van Schaeren R, Lenaerts S. Influence of Adding Low Concentration of Oxygenates in Mineral Diesel Oil and Biodiesel on the Concentration of NO, NO2 and Particulate Matter in the Exhaust Gas of a One-Cylinder Diesel Generator. International Journal of Environmental Research and Public Health. 2022; 19(13):7637. https://doi.org/10.3390/ijerph19137637
Chicago/Turabian StyleMaes, Rafael R., Geert Potters, Erik Fransen, Rowan Van Schaeren, and Silvia Lenaerts. 2022. "Influence of Adding Low Concentration of Oxygenates in Mineral Diesel Oil and Biodiesel on the Concentration of NO, NO2 and Particulate Matter in the Exhaust Gas of a One-Cylinder Diesel Generator" International Journal of Environmental Research and Public Health 19, no. 13: 7637. https://doi.org/10.3390/ijerph19137637
APA StyleMaes, R. R., Potters, G., Fransen, E., Van Schaeren, R., & Lenaerts, S. (2022). Influence of Adding Low Concentration of Oxygenates in Mineral Diesel Oil and Biodiesel on the Concentration of NO, NO2 and Particulate Matter in the Exhaust Gas of a One-Cylinder Diesel Generator. International Journal of Environmental Research and Public Health, 19(13), 7637. https://doi.org/10.3390/ijerph19137637






