Effort–Reward Imbalance at Work and Prescription Drug Misuse—Prospective Evidence from Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.2.1. Dependent Variable
2.2.2. Independent Variables
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Summary and Interpretation
4.2. Strengths, Limitations, and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Effort | −0.004 | −0.021 ** | ||
| [−0.009, 0.000] | [−0.034, −0.008] | |||
| Reward | −0.004 | −0.003 | ||
| [−0.010, 0.001] | [−0.020, 0.014] | |||
| Effort–reward ratio (ERR) | 0.002 | −0.026 * | ||
| [−0.005, 0.009] | [−0.046, −0.005] | |||
| Overcommitment | 0.012 *** | 0.011 *** | −0.007 | −0.001 |
| [0.008, 0.017] | [0.006, 0.015] | [−0.034, 0.020] | [−0.010, 0.008] | |
| Effort × Overcommitment | 0.007 ** | |||
| [0.002, 0.013] | ||||
| Reward × Overcommitment | −0.001 | |||
| [−0.007, 0.006] | ||||
| ERR × Overcommitment | 0.011 ** | |||
| [0.003, 0.018] | ||||
| Prior CE drug misuse | 0.160 *** | 0.161 *** | 0.159 *** | 0.160 *** |
| [0.147, 0.173] | [0.147, 0.174] | [0.146, 0.173] | [0.146, 0.173] | |
| Female | 0.000 | 0.000 | −0.000 | 0.000 |
| [−0.005, 0.006] | [−0.005, 0.006] | [−0.005, 0.005] | [−0.005, 0.006] | |
| Age | 0.000 | 0.000 | 0.000 | 0.000 |
| [−0.000, 0.000] | [−0.000, 0.000] | [−0.000, 0.000] | [−0.000, 0.000] | |
| Anonymity perceptions | −0.010 *** | −0.010 *** | −0.010 *** | −0.010 *** |
| [−0.013, −0.006] | [−0.013, −0.006] | [−0.013, −0.006] | [−0.014, −0.006] | |
| Constant | 0.742 *** | 0.719 *** | 0.784 *** | 0.748 *** |
| [0.713, 0.771] | [0.696, 0.741] | [0.715, 0.853] | [0.718, 0.779] | |
| F-Test | 95.190 *** | 110.107 *** | 75.067 *** | 95.565 *** |
| Dimension and Sub-dimension | Items |
|---|---|
| Effort | |
| Due to the high volume of work, there has often been a lot of time pressure. |
| I have often been interrupted and disturbed during my work. |
| My workload has become larger and larger. |
| Reward | |
| I get the recognition I have earned from my supervisor and/or equally important person. |
| The chances of getting a promotion in my field are poor. | |
| I am experiencing—or am expecting—my job situation to get worse. |
| My own job is at risk. | |
| When I think of all of my work and effort, I think I have received appropriate recognition. |
| When I think of all of my work and effort, I think my personal chances for professional advancement are appropriate. | |
| Overcommitment | |
| It often happens to me that I’m thinking about work problems as soon as I wake up. | |
| Those closest to me say that I sacrifice too much for my job. | |
| I rarely escape work completely, it is still in my head in the evenings. | |
| If I postpone something which I actually should have done today, I cannot sleep at night. |
References
- Siegrist, J. Adverse Health Effects of High-Effort/Low-Reward Conditions. J. Occup. Health Psychol. 1996, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, J. Effort-Reward Imbalance Model. In Stress: Concepts, Cognition, Emotion, and Behavior; Fink, G., Ed.; Elsevier: London, UK, 2016; pp. 81–86. [Google Scholar]
- Siegrist, J. Effort-Reward Imbalance at Work—Theory, Measurement and Evidence; Department of Medical Sociology, University Düsseldorf: Düsseldorf, Germany, 2012. [Google Scholar]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.P.; Stephens, P.M. Stress, Health, and the Social Environment: A Sociobiologic Approach to Medicine. In Topics in Environmental Physiology and Medicine, 1st ed.; Springer: New York, NY, USA, 1977; ISBN 978-0-387-90293-7. [Google Scholar]
- Nieuwenhuijsen, K.; Bruinvels, D.; Frings-Dresen, M. Psychosocial Work Environment and Stress-Related Disorders, a Systematic Review. Occup. Med. 2010, 60, 277–286. [Google Scholar] [CrossRef]
- Kivimäki, M.; Siegrist, J. Work Stress and Cardiovascular Disease: Reviewing Research Evidence with a Focus on Effort-Reward Imbalance at Work. In Work Stress and Health in a Globalized Economy; Springer: Berlin/Heidelberg, Germany, 2016; pp. 89–101. [Google Scholar]
- Rugulies, R.; Aust, B.; Madsen, I.E. Effort-Reward Imbalance and Affective Disorders. In Work Stress and Health in a Globalized Economy; Springer: Berlin/Heidelberg, Germany, 2016; pp. 103–143. [Google Scholar]
- Eddy, P.; Heckenberg, R.; Wertheim, E.H.; Kent, S.; Wright, B.J. A Systematic Review and Meta-Analysis of the Effort-Reward Imbalance Model of Workplace Stress with Indicators of Immune Function. J. Psychosom. Res. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Zhuo, L.-B.; Yao, W.; Yan, Z.; Giron, M.S.; Pei, J.-J.; Wang, H.-X. Impact of Effort Reward Imbalance at Work on Suicidal Ideation in Ten European Countries: The Role of Depressive Symptoms. J. Affect. Disord. 2020, 260, 214–221. [Google Scholar] [CrossRef]
- Kouvonen, A.; Kivimäki, M.; Virtanen, M.; Pentti, J.; Vahtera, J. Work Stress, Smoking Status, and Smoking Intensity: An Observational Study of 46,190 Employees. J. Epidemiol. Community Health 2005, 59, 63–69. [Google Scholar] [CrossRef]
- Head, J.; Stansfeld, S.A.; Siegrist, J. The Psychosocial Work Environment and Alcohol Dependence: A Prospective Study. Occup. Environ. Med. 2004, 61, 219–224. [Google Scholar] [CrossRef]
- Skogen, J.C.; Thørrisen, M.M.; Bonsaksen, T.; Vahtera, J.; Sivertsen, B.; Aas, R.W. Effort-Reward Imbalance is Associated with Alcohol-Related Problems. WIRUS-Screening Study. Front. Psychol. 2019, 10, 2079. [Google Scholar] [CrossRef]
- Ota, A.; Masue, T.; Yasuda, N.; Tsutsumi, A.; Mino, Y.; Ohara, H.; Ono, Y. Psychosocial Job Characteristics and Smoking Cessation: A Prospective Cohort Study Using the Demand-Control-Support and Effort-Reward Imbalance Job Stress Models. Nicotine Tob. Res. 2010, 12, 287–293. [Google Scholar] [CrossRef]
- Choi, B. Opioid Use Disorder, Job Strain, and High Physical Job Demands in US Workers. Int. Arch. Occup. Environ. Health 2020, 93, 577–588. [Google Scholar] [CrossRef]
- Wiesner, M.; Windle, M.; Freeman, A. Work Stress, Substance Use, and Depression among Young Adult Workers: An Examination of Main and Moderator Effect Model. J. Occup. Health Psychol. 2005, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Airagnes, G.; Lemogne, C.; Kab, S.; Hoertel, N.; Goldberg, M.; Wahrendorf, M.; Siegrist, J.; Roquelaure, Y.; Limosin, F.; Zins, M. Effort–Reward Imbalance and Long-Term Benzodiazepine Use: Longitudinal Findings from the CONSTANCES Cohort. J. Epidemiol. Community Health 2019, 73, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Matthews, T.A.; Chen, L.; Seamans, M.; Leineweber, C.; Siegrist, J. Effort–Reward Imbalance at Work and Drug Misuse: Evidence from a National Survey in the US. Int. J. Environ. Res. Public. Health 2021, 18, 13334. [Google Scholar] [CrossRef]
- Baum, M.; Sattler, S.; Reimann, M. Towards an Understanding of How Stress and Resources Affect the Nonmedical Use of Prescription Drugs for Performance Enhancement among Employees. Curr. Psychol. 2021. [Google Scholar] [CrossRef]
- Müller, C.; Schumann, G.; Müller, C.P.; Schumann, G. Drugs as Instruments: A New Framework for Non-Addictive Psychoactive Drug Use. Behav. Brain Sci. 2011, 34, 293–310. [Google Scholar] [CrossRef]
- Battleday, R.M.; Brem, A.-K. Modafinil for Cognitive Neuroenhancement in Healthy Non-Sleep-Deprived Subjects: A Systematic Review. Eur. Neuropsychopharmacol. 2015, 25, 1865–1881. [Google Scholar] [CrossRef]
- Roberts, C.A.; Jones, A.; Sumnall, H.; Gage, S.H.; Montgomery, C. How Effective are Pharmaceuticals for Cognitive Enhancement in Healthy Adults? A Series of Meta-Analyses of Cognitive Performance during Acute Administration of Modafinil, Methylphenidate and D-Amphetamine. Eur. Neuropsychopharmacol. 2020, 38, 40–62. [Google Scholar] [CrossRef]
- Greely, H.; Sahakian, B.; Harris, J.; Kessler, R.C.; Gazzaniga, M.; Campbell, P.; Farah, M.J. Towards Responsible Use of Cognitive-Enhancing Drugs by the Healthy. Nature 2008, 456, 702–705. [Google Scholar] [CrossRef]
- d’Angelo, L.C.; Savulich, G.; Sahakian, B.J. Lifestyle Use of Drugs by Healthy People for Enhancing Cognition, Creativity, Motivation and Pleasure. Br. J. Pharmacol. 2017, 174, 3257–3267. [Google Scholar] [CrossRef]
- Maier, L.J.; Ferris, J.A.; Winstock, A.R. Pharmacological Cognitive Enhancement among Non-ADHD Individuals—A Cross-Sectional Study in 15 Countries. Int. J. Drug Policy 2018, 58, 104–112. [Google Scholar] [CrossRef]
- Sattler, S.; Schunck, R. Associations between the Big Five Personality Traits and the Non-Medical Use of Prescription Drugs for Cognitive Enhancement. Front. Psychol. 2016, 6, 1971. [Google Scholar] [CrossRef] [PubMed]
- Bagusat, C.; Kunzler, A.; Schlecht, J.; Franke, A.G.; Chmitorz, A.; Lieb, K. Pharmacological Neuroenhancement and the Ability to Recover from Stress—A Representative Cross-Sectional Survey among the German Population. Subst. Abuse Treat. Prev. Policy 2018, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, C.; Sattler, S.; Göritz, A.S.; Diewald, M. Work-Related Stress and Cognitive Enhancement among University Teachers. Anxiety Stress Coping 2016, 29, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Köhler, T.; Knerr, P.; Kühne, S.; Moesgen, D.; Klein, M. Einfluss Psychischer Belastungen Am Arbeitsplatz Auf Das Neuroenhancement; Bundesanstalt für Arbeitsschutz und Arbeitsmedizin: Dortmund, Germany, 2015; ISBN 3-88261-052-2. [Google Scholar]
- Bavelier, D.; Savulescu, J.; Fried, L.P.; Friedmann, T.; Lathan, C.E.; Schürle, S.; Beard, J.R. Rethinking Human Enhancement as Collective Welfarism. Nat. Hum. Behav. 2019, 3, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, N.; Sandberg, A. Cognitive Enhancement: Methods, Ethics, Regulatory Challenges. Sci. Eng. Ethics 2009, 15, 311–341. [Google Scholar] [CrossRef] [PubMed]
- Racine, E.; Sattler, S.; Boehlen, W. Cognitive Enhancement: Unanswered Questions About Human Psychology and Social Behavior. Sci. Eng. Ethics 2021, 27, 19. [Google Scholar] [CrossRef]
- Jane, E.; Vincent, N. Cognitive Enhancement: A Social Experiment with Technology. In New Perspectives on Technology in Society Experimentation beyond the Laboratory; van de Poel, I., Asveld, L., Mehos, D.C., Eds.; Routlege: London, UK, 2017; pp. 141–164. [Google Scholar]
- Forsberg, E.-M.; Shelley-Egan, C.; Thorstensen, E.; Landeweerd, L.; Hofmann, B. Ethical Concerns in HCE: The Examples of Cognitive Enhancing Drugs and Noninvasive Brain Stimulation. In Evaluating Ethical Frameworks for the Assessment of Human Cognitive Enhancement Applications; SpringerBriefs in Ethics; Forsberg, E.-M., Shelley-Egan, C., Thorstensen, E., Landeweerd, L., Hofmann, B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 15–29. ISBN 978-3-319-53823-5. [Google Scholar]
- Maier, L.J.; Haug, S.; Schaub, M.P. The Importance of Stress, Self-Efficacy, and Self-Medication for Pharmacological Neuroenhancement among Employees and Students. Drug Alcohol Depend. 2015, 156, 221–227. [Google Scholar] [CrossRef]
- Sattler, S. Nonmedical Use of Prescription Drugs for Cognitive Enhancement as Response to Chronic Stress Especially When Social Support is Lacking. Stress Health 2019, 35, 127–137. [Google Scholar] [CrossRef]
- Lazarus, R.S.; Folkman, S. Stress, Appraisal, and Coping; Springer: New York, NY, USA, 1984. [Google Scholar]
- Statista Haushalte in Deutschland—Internetzugang bis 2019. Available online: https://de.statista.com/statistik/daten/studie/153257/umfrage/haushalte-mit-internetzugang-in-deutschland-seit-2002 (accessed on 8 July 2020).
- Johnston, L.D.; Miech, R.A.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E.; Patrick, M.E. Monitoring the Future National Survey Results on Drug Use, 1975–2018: Overview, Key Findings on Adolescent Drug Use. Inst. Soc. Res. 2019. Available online: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2018.pdf (accessed on 1 May 2022).
- Miech, R.; Johnston, L.; O’Malley, P.M.; Keyes, K.M.; Heard, K. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics 2015, 136, e1169–e1177. [Google Scholar] [CrossRef]
- Li, J.; Loerbroks, A.; Jarczok, M.N.; Schöllgen, I.; Bosch, J.A.; Mauss, D.; Siegrist, J.; Fischer, J.E. Psychometric Properties and Differential Explanation of a Short Measure of Effort–Reward Imbalance at Work: A Study of Industrial Workers in Germany. Am. J. Ind. Med. 2012, 55, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, A.; Iwata, N.; Wakita, T.; Kumagai, R.; Noguchi, H.; Kawakami, N. Improving the Measurement Accuracy of the Effort-Reward Imbalance Scales. Int. J. Behav. Med. 2008, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, J.; Li, J.; Montano, D. Psychometric Properties of the Effort-Reward Imbalance Questionnaire. Ger. Duesseldorf Univ. 2014. Available online: https://www.uniklinik-duesseldorf.de/fileadmin/Fuer-Patienten-und-Besucher/Kliniken-Zentren-Institute/Institute/Institut_fuer_Medizinische_Soziologie/Forschung/PsychometricProperties.pdf (accessed on 1 May 2022).
- Siegrist, J.; Wege, N.; Pühlhofer, F.; Wahrendorf, M. A Short Generic Measure of Work Stress in the Era of Globalization: Effort–Reward Imbalance. Int. Arch. Occup. Environ. Health 2009, 82, 1005–1013. [Google Scholar] [CrossRef]
- Bouffard, J.A.; Rice, S.K. The Influence of the Social Bond on Self-Control at the Moment of Decision: Testing Hirschi’s Redefinition of Self-Control. Am. J. Crim. Justice 2011, 36, 138–157. [Google Scholar] [CrossRef]
- Pickett, J.T.; Barnes, J.C.; Wilson, T.; Patrick Roche, S. Prospect Theory and Criminal Choice: Experiments Testing Framing, Reference Dependence, and Decision Weights. Justice Q. 2020, 37, 1140–1168. [Google Scholar] [CrossRef]
- Lange, C.; Hoebel, J.; Kamtsiuris, P.; Muters, S.; Schilling, R.; Lippe, E. KOLIBRI–Studie Zum Konsum Leistungsbeeinflussender Mittel in Alltag Und Freizeit; Robert Koch Institut: Berlin, Germany, 2011. [Google Scholar]
- Sattler, S. Cognitive Enhancement in Germany. Prevalence, Attitudes, Moral Acceptability, Terms, Legal Status, and the Ethics Debate. In Cognitive Enhancement: Ethical and Policy Implications in International Perspectives; Jotterand, F., Dubljevic, V., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 159–180. [Google Scholar]
- Dragano, N.; Siegrist, J.; Nyberg, S.T.; Lunau, T.; Fransson, E.I.; Alfredsson, L.; Bjorner, J.B.; Borritz, M.; Burr, H.; Erbel, R. Effort–Reward Imbalance at Work and Incident Coronary Heart Disease: A Multicohort Study of 90,164 Individuals. Epidemiology 2017, 28, 619. [Google Scholar] [CrossRef]
- Partridge, B.; Bell, S.; Lucke, J.; Hall, W. Australian University Students’ Attitudes towards the Use of Prescription Stimulants as Cognitive Enhancers: Perceived Patterns of Use, Efficacy and Safety. Drug Alcohol Rev. 2013, 32, 295–302. [Google Scholar] [CrossRef]
- Siegrist, J. A Theoretical Model in the Context of Economic Globalization. In Work Stress and Health in a Globalized Economy; Springer: Berlin/Heidelberg, Germany, 2016; pp. 3–19. [Google Scholar]
- Schepis, T.S.; Klare, D.L.; Ford, J.A.; McCabe, S.E. Prescription Drug Misuse: Taking a Lifespan Perspective. Subst. Abuse Res. Treat. 2020, 14, 1178221820909352. [Google Scholar] [CrossRef]
- Fuermaier, A.B.; Tucha, O.; Koerts, J.; Tucha, L.; Thome, J.; Faltraco, F. Feigning ADHD and Stimulant Misuse among Dutch University Students. J. Neural Transm. 2021, 128, 1079–1084. [Google Scholar] [CrossRef]
- van Veen, F.; Sattler, S.; Mehlkop, G.; Hasselhorn, F. Feigning Symptoms to Obtain Prescription Stimulants: A Vignette-Based Study on Its Conditions. J. Drug Issues 2021, 52, 225–249. [Google Scholar] [CrossRef]
- Bakker, A.B.; Demerouti, E. Job Demands–Resources Theory: Taking Stock and Looking Forward. J. Occup. Health Psychol. 2017, 22, 273. [Google Scholar] [CrossRef] [PubMed]
- Demerouti, E.; Bakker, A.B.; Nachreiner, F.; Schaufeli, W.B. The Job Demands-Resources Model of Burnout. J. Appl. Psychol. 2001, 86, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.G.; Bagusat, C.; Dietz, P.; Hoffmann, I.; Simon, P.; Ulrich, R.; Lieb, K. Use of Illicit and Prescription Drugs for Cognitive or Mood Enhancement among Surgeons. BMC Med. 2013, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Krumpal, I. Determinants of Social Desirability Bias in Sensitive Surveys: A Literature Review. Qual. Quant. Int. J. Methodol. 2013, 47, 2025–2047. [Google Scholar] [CrossRef]
- Patrzek, J.; Sattler, S.; van Veen, F.; Grunschel, C.; Fries, S. Investigating the Effect of Academic Procrastination on the Frequency and Variety of Academic Misconduct: A Panel Study. Stud. High. Educ. 2015, 40, 1014–1029. [Google Scholar] [CrossRef]
- Partridge, B.; Bell, S.K.; Lucke, J.C.; Yeates, S.; Hall, W.D. Smart Drugs “as Common as Coffee”: Media Hype about Neuroenhancement. PLoS ONE 2011, 6, e28416. [Google Scholar] [CrossRef]
- Schäfer, M. Medienhype’Hirndoping’? Nomos: Baden-Baden, Germany, 2018. [Google Scholar]
- Krämer, K. DAK-Gesundheitsreport. DAK. In Gesundheitsreport 2009. Analyse der Arbeitsunfähigkeitsdaten. Schwerpunktthema Doping am Arbeitsplatz; DAK/IGES: Berlin/Hamburg, Germany, 2009; p. 146. [Google Scholar]
- Caviola, L.; Mannino, A.; Savulescu, J.; Faulmüller, N. Cognitive Biases Can Affect Moral Intuitions about Cognitive Enhancement. Front. Syst. Neurosci. 2014, 8, 195. [Google Scholar] [CrossRef]
- Weyandt, L.L.; White, T.L.; Gudmundsdottir, B.G.; Nitenson, A.Z.; Rathkey, E.S.; De Leon, K.A.; Bjorn, S.A. Neurocognitive, Autonomic, and Mood Effects of Adderall: A Pilot Study of Healthy College Students. Pharmacy 2018, 6, 58. [Google Scholar] [CrossRef]
- Schepis, T.S.; Krishnan-Sarin, S. Characterizing Adolescent Prescription Misusers: A Population-Based Study. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 745–754. [Google Scholar] [CrossRef]
- Molloy, B.K.; Stock, M.L.; Dodge, T.; Aspelund, J.G. Predicting Future Academic Willingness, Intentions, and Nonmedical Prescription Stimulant (NPS) Use with the Theory of Reasoned Action and Prototype/Willingness Model. Subst. Use Misuse 2019, 54, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Sattler, S.; Guido, M. Mechanisms of Perceived Social Norms: The Mediating and Moderating Role of Morality and Outcome Expectations on Prescription Drug Misuse in the Working Population. Deviant Behav. 2022. [Google Scholar] [CrossRef]
- Ford, J.A.; Ong, J. Non-Medical Use of Prescription Stimulants for Academic Purposes among College Students: A Test of Social Learning Theory. Drug Alcohol Depend. 2014, 144, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.T.; Humphries, S.; Chatterjee, A. Public Opinion on Cognitive Enhancement Varies Across Different Situations. Am. J. Bioeth. Neurosci. 2020, 11, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.R.; Harms, P.D.; Gilmer, D.O. PCE Use in the Workplace: The Open Secret of Performance Enhancement. J. Manag. Inq. 2019, 28, 67–70. [Google Scholar] [CrossRef]
- Brühl, A.B.; Sahakian, B.J. Drugs, Games, and Devices for Enhancing Cognition: Implications for Work and Society. Ann. N. Y. Acad. Sci. 2016, 1369, 195–217. [Google Scholar] [CrossRef]
- Dragano, N.; Lunau, T. Technostress at Work and Mental Health: Concepts and Research Results. Curr. Opin. Psychiatry 2020, 33, 407–413. [Google Scholar] [CrossRef]
- Fink, G.; Fink, G. Stress, Definitions, Mechanisms, and Effects Outlined: Lessons from Anxiety. In Stress: Concepts, Cognition, Emotion, and Behavior, Volume 1 of the Handbook of Stress Series; Fink, G., Ed.; Elsevier Inc.: San Diego, CA, USA, 2016; pp. 3–11. [Google Scholar]
- Limm, H.; Gündel, H.; Heinmüller, M.; Marten-Mittag, B.; Nater, U.M.; Siegrist, J.; Angerer, P. Stress Management Interventions in the Workplace Improve Stress Reactivity: A Randomised Controlled Trial. Occup. Environ. Med. 2011, 68, 126–133. [Google Scholar] [CrossRef]
- Sattler, S.; von dem Knesebeck, O. Effort–Reward Imbalance at Work and Prescription Drug Misuse—Prospective Evidence from Germany; Bielefeld University: Bielefeld, Germany, 2022. [Google Scholar] [CrossRef]
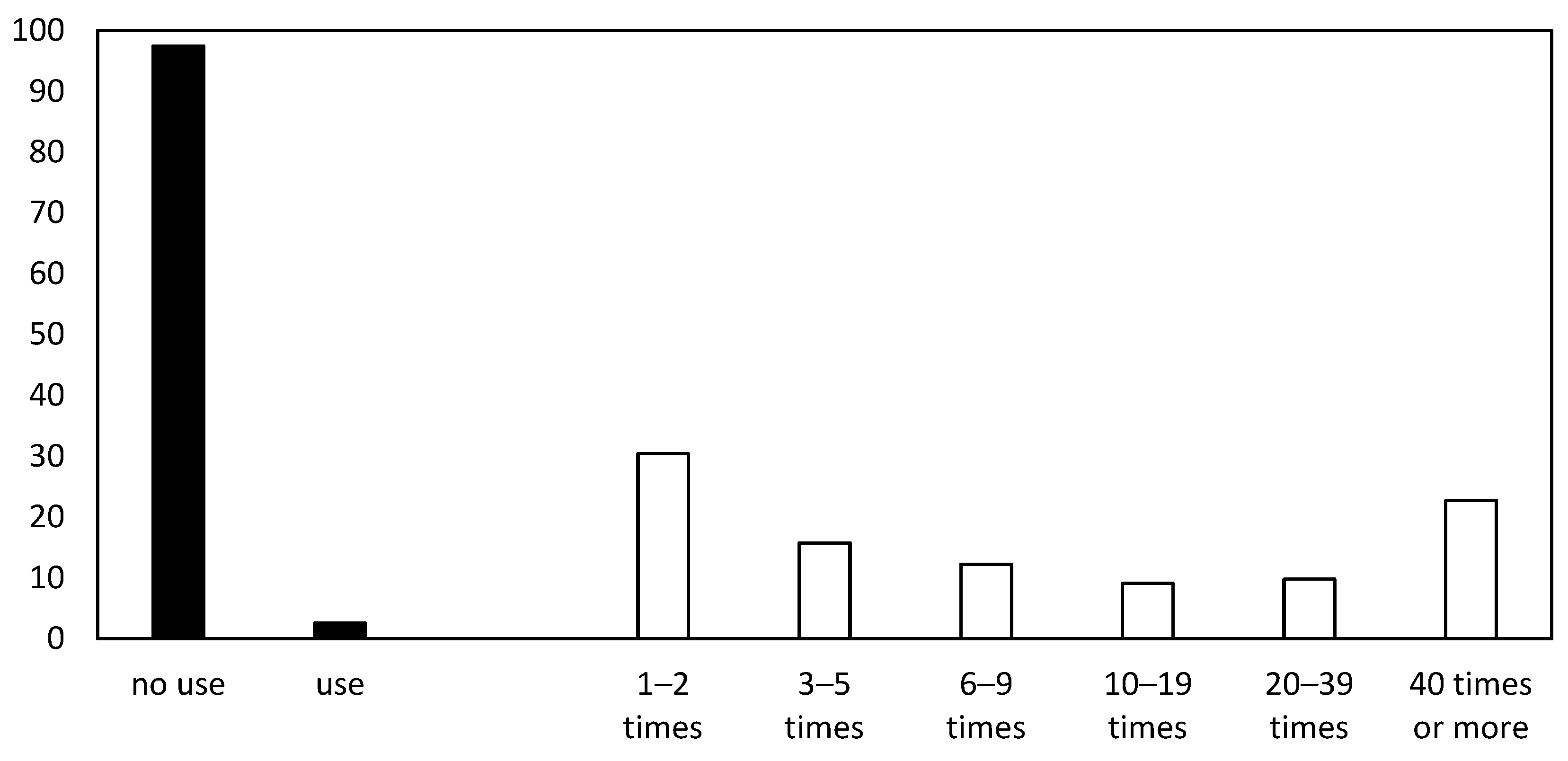
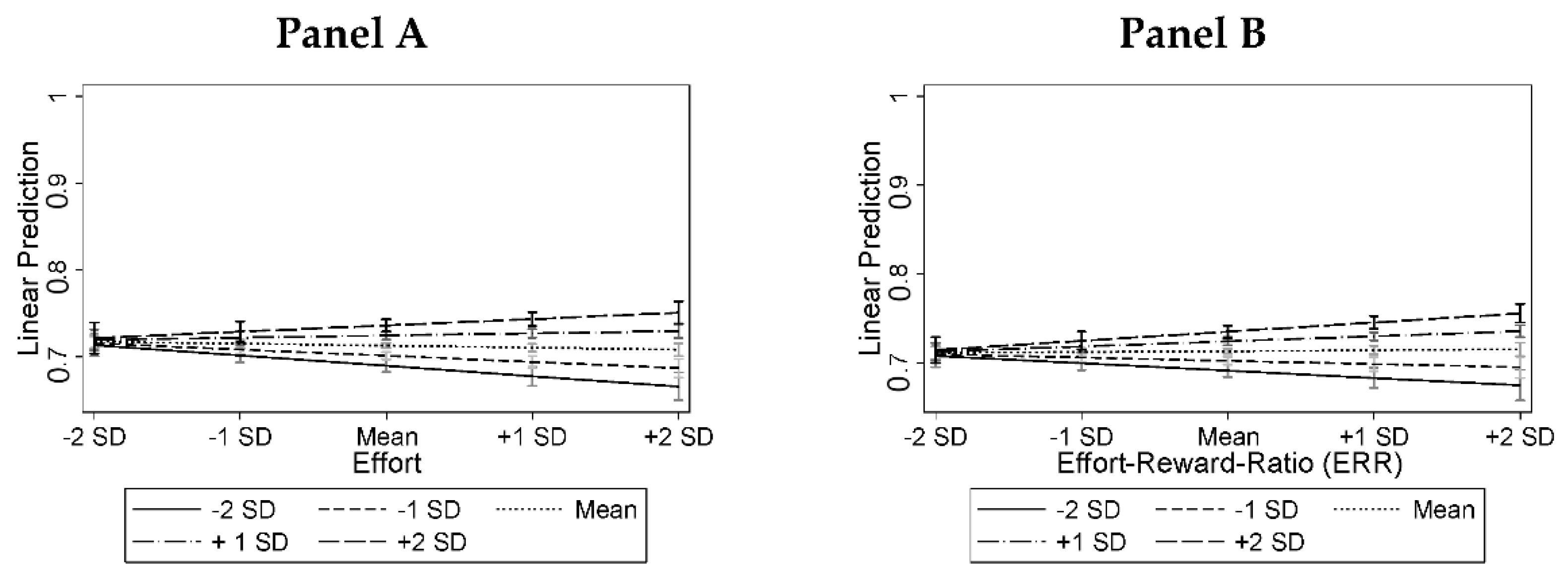
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| 12 month CE-drug misuse at t2 | 1.08 | 0.595 | 1.00 | 7.00 |
| 12 month CE-drug misuse at t2 (Log) | 0.72 | 0.152 | 0.69 | 2.08 |
| Effort | 2.79 | 0.716 | 1.00 | 4.00 |
| Reward | 2.69 | 0.561 | 1.00 | 4.00 |
| Effort–reward ratio (ERR) | 1.11 | 0.459 | 0.25 | 4.00 |
| Overcommitment | 2.35 | 0.735 | 1.00 | 4.00 |
| Prior CE-drug misuse (Dummy) | 0.04 | 0.207 | 0.00 | 1.00 |
| Female | 0.50 | 0.500 | 0.00 | 1.00 |
| Age | 48.16 | 11.590 | 18.00 | 86.00 |
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Effort | −0.003 | −0.022 *** | ||
| [−0.007, 0.001] | [−0.035, −0.009] | |||
| Reward | −0.005 | −0.005 | ||
| [−0.010, 0.001] | [−0.022, 0.012] | |||
| Effort–reward ratio (ERR) | 0.003 | −0.024 * | ||
| [−0.004, 0.010] | [−0.044, −0.003] | |||
| Overcommitment | 0.012 *** | 0.011 *** | −0.012 | −0.000 |
| [0.008, 0.017] | [0.007, 0.015] | [−0.039, 0.015] | [−0.009, 0.009] | |
| Effort × Overcommitment | 0.008 ** | |||
| [0.003, 0.014] | ||||
| Reward × Overcommitment | 0.000 | |||
| [−0.006, 0.007] | ||||
| ERR × Overcommitment | 0.010 ** | |||
| [0.003, 0176] | ||||
| Prior CE drug misuse | 0.165 *** | 0.165 *** | 0.164 *** | 0.165 *** |
| [0.151, 0.178] | [0.152, 0.179] | [0.151, 0.178] | [0.151, 0.178] | |
| Female | 0.000 | 0.000 | −0.000 | 0.000 |
| [−0.005, 0.006] | [−0.005, 0.006] | [−0.006, 0.005] | [−0.005, 0.006] | |
| Age | 0.000 | 0.000 | 0.000 | 0.000 |
| [−0.000, 0.000] | [−0.000, 0.000] | [−0.000, 0.000] | [−0.000, 0.000] | |
| Constant | 0.694 *** | 0.672 *** | 0.748 *** | 0.700 *** |
| [0.669, 0.719] | [0.657, 0.687] | [0.681, 0.815] | [0.675, 0.725] | |
| F-Test | 112.26 *** | 134.00 *** | 85.59 *** | 112.95 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattler, S.; von dem Knesebeck, O. Effort–Reward Imbalance at Work and Prescription Drug Misuse—Prospective Evidence from Germany. Int. J. Environ. Res. Public Health 2022, 19, 7632. https://doi.org/10.3390/ijerph19137632
Sattler S, von dem Knesebeck O. Effort–Reward Imbalance at Work and Prescription Drug Misuse—Prospective Evidence from Germany. International Journal of Environmental Research and Public Health. 2022; 19(13):7632. https://doi.org/10.3390/ijerph19137632
Chicago/Turabian StyleSattler, Sebastian, and Olaf von dem Knesebeck. 2022. "Effort–Reward Imbalance at Work and Prescription Drug Misuse—Prospective Evidence from Germany" International Journal of Environmental Research and Public Health 19, no. 13: 7632. https://doi.org/10.3390/ijerph19137632
APA StyleSattler, S., & von dem Knesebeck, O. (2022). Effort–Reward Imbalance at Work and Prescription Drug Misuse—Prospective Evidence from Germany. International Journal of Environmental Research and Public Health, 19(13), 7632. https://doi.org/10.3390/ijerph19137632






