Gait Speed and Sleep Duration Is Associated with Increased Risk of MCI in Older Community-Dwelling Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sleep Duration
2.3. Physical Activity, Gait Speed, and Physical Function
2.4. Statistical Analysis
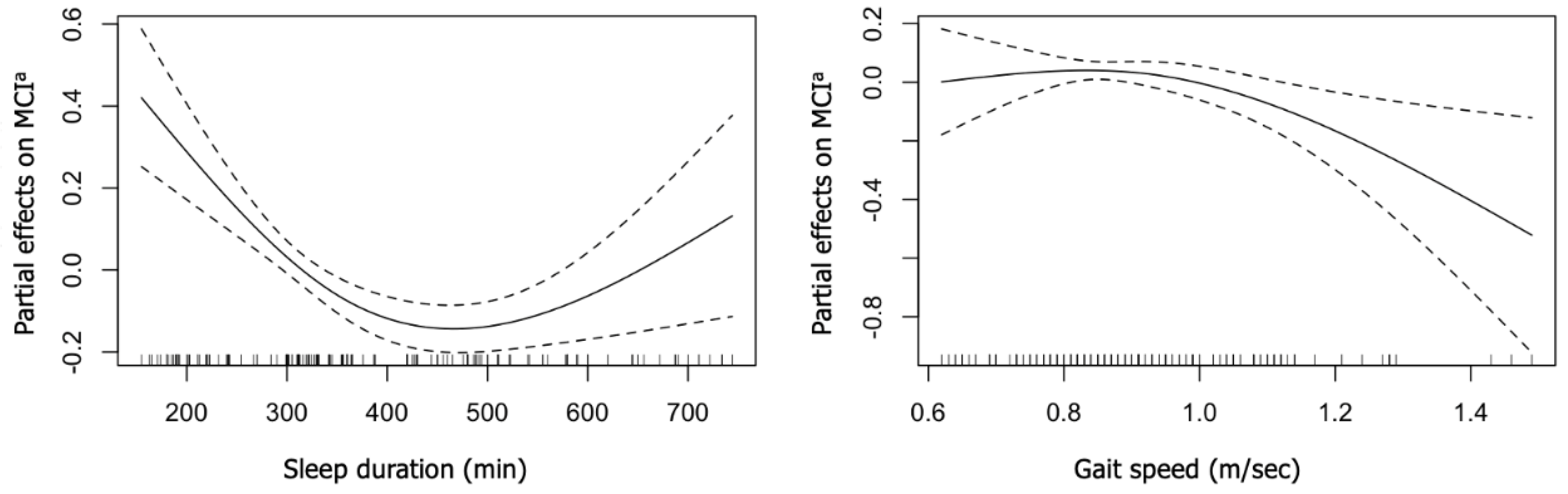
3. Results
Participant Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Yang, P.S.; Jin, M.N.; Yu, H.T.; Kim, T.H.; Jang, E.; Uhm, J.S.; Pak, H.N.; Lee, M.H.; Joung, B. Association of Physical Activity Level with Risk of Dementia in a Nationwide Cohort in Korea. JAMA Netw. Open 2021, 4, e2138526. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Kontogianni, M.; Scarmeas, N. Cognitive health and Mediterranean diet: Just diet or lifestyle pattern? Ageing Res. Rev. 2015, 20, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011, 306, 613–619. [Google Scholar] [CrossRef]
- Louis, E.D.; Schupf, N.; Manly, J.; Marder, K.; Tang, M.X.; Mayeux, R. Association between mild parkinsonian signs and mild cognitive impairment in a community. Neurology 2005, 64, 1157–1161. [Google Scholar] [CrossRef]
- Verghese, J.; Robbins, M.; Holtzer, R.; Zimmerman, M.; Wang, C.; Xue, X.; Lipton, R.B. Gait dysfunction in mild cognitive impairment syndromes. J. Am. Geriatr. Soc. 2008, 56, 1244–1251. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Wilson, R.S.; Beck, T.L.; Bienias, J.L.; Bennett, D.A. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch. Neurol. 2006, 63, 1763–1769. [Google Scholar] [CrossRef]
- Windham, B.G.; Parker, S.B.; Zhu, X.; Gabriel, K.P.; Palta, P.; Sullivan, K.J.; Parker, K.G.; Knopman, D.S.; Gottesman, R.F.; Griswold, M.E.; et al. Endurance and gait speed relationships with mild cognitive impairment and dementia. Alzheimers Dement. 2022, 14, e12281. [Google Scholar] [CrossRef]
- Waite, L.M.; Grayson, D.A.; Piguet, O.; Creasey, H.; Bennett, H.P.; Broe, G.A. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J. Neurol. Sci. 2005, 229–230, 89–93. [Google Scholar] [CrossRef]
- Camicioli, R.; Howieson, D.; Oken, B.; Sexton, G.; Kaye, J. Motor slowing precedes cognitive impairment in the oldest old. Neurology 1998, 50, 1496–1498. [Google Scholar] [CrossRef]
- Basta, M.; Simos, P.; Bertsias, A.; Duijker, G.; Zaganas, I.; Koutentaki, E.; Anastasaki, M.; Mavroidis, G.; Kalomoiri, G.; Panagiotakis, S.; et al. Association between insomnia symptoms and cognitive impairment in the Cretan Aging Cohort. Eur. Geriatr. Med. 2018, 9, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, S.J.; Ma, M.Y.; Bao, Y.P.; Han, Y.; Wang, Y.M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Jelicic, M.; Bosma, H.; Ponds, R.W.; Van Boxtel, M.P.; Houx, P.J.; Jolles, J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS). Int. J. Geriatr. Psychiatry 2002, 17, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.; Yaffe, K.; Laffan, A.; Ancoli-Israel, S.; Redline, S.; Ensrud, K.E.; Song, Y.; Stone, K.L.; Osteoporotic Fractures in Men Study, G. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: The MrOS sleep study. Sleep 2014, 37, 655–663. [Google Scholar] [PubMed]
- Wu, L.; Sun, D.; Tan, Y. A systematic review and dose-response meta-analysis of sleep duration and the occurrence of cognitive disorders. Sleep Breath. 2018, 22, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Benito-Leon, J.; Bermejo-Pareja, F.; Vega, S.; Louis, E.D. Total daily sleep duration and the risk of dementia: A prospective population-based study. Eur. J. Neurol. 2009, 16, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, J.H.; Na, H.R.; Hiroyuki, S.; Kim, G.M.; Jung, M.K.; Kim, W.K.; Park, K.W. Combined Intervention of Physical Activity, Aerobic Exercise, and Cognitive Exercise Intervention to Prevent Cognitive Decline for Patients with Mild Cognitive Impairment: A Randomized Controlled Clinical Study. J. Clin. Med. 2019, 8, 940. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Brachem, C.; Winkler, A.; Tebrugge, S.; Weimar, C.; Erbel, R.; Jockel, K.H.; Stang, A.; Dragano, N.; Moebus, S.; Kowall, B.; et al. Associations between self-reported sleep characteristics and incident mild cognitive impairment: The Heinz Nixdorf Recall Cohort Study. Sci. Rep. 2020, 10, 6542. [Google Scholar] [CrossRef]
- Chen, J.C.; Espeland, M.A.; Brunner, R.L.; Lovato, L.C.; Wallace, R.B.; Leng, X.; Phillips, L.S.; Robinson, J.G.; Kotchen, J.M.; Johnson, K.C.; et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement. 2016, 12, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Ito, T.; Lee, S.; Park, H.; et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J. Am. Med. Dir. Assoc. 2013, 14, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- EuroQol, G. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle; Petrov, B.N., Csaki, F., Eds.; Akad Emiai Kiado: Budapest, Hungary, 1973. [Google Scholar]
- Hastie, T.; Tibshirani, R. Generalized additive models for medical research. Stat. Methods Med. Res. 1995, 4, 187–196. [Google Scholar] [CrossRef]
- Devore, E.E.; Grodstein, F.; Schernhammer, E.S. Sleep Duration in Relation to Cognitive Function among Older Adults: A Systematic Review of Observational Studies. Neuroepidemiology 2016, 46, 57–78. [Google Scholar] [CrossRef]
- Lo, J.C.; Groeger, J.A.; Cheng, G.H.; Dijk, D.J.; Chee, M.W. Self-reported sleep duration and cognitive performance in older adults: A systematic review and meta-analysis. Sleep Med. 2016, 17, 87–98. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, C.Q.; Lam, T.H.; Liu, B.; Jin, Y.L.; Zhu, T.; Zhang, W.S.; Cheng, K.K.; Thomas, G.N. Short or long sleep duration is associated with memory impairment in older Chinese: The Guangzhou Biobank Cohort Study. Sleep 2011, 34, 575–580. [Google Scholar] [CrossRef]
- Kang, J.E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009, 326, 1005–1007. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Bin, Y.S. Is Sleep Quality More Important Than Sleep Duration for Public Health? Sleep 2016, 39, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Peigneux, P.; Cajochen, C. Age-related changes in sleep and circadian rhythms: Impact on cognitive performance and underlying neuroanatomical networks. Front. Neurol. 2012, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Kimiwada, T.; Sakurai, M.; Ohashi, H.; Aoki, S.; Tominaga, T.; Wada, K. Clock genes regulate neurogenic transcription factors, including NeuroD1, and the neuronal differentiation of adult neural stem/progenitor cells. Neurochem. Int. 2009, 54, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M. Chronobiological approaches to Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 93–98. [Google Scholar] [CrossRef]
- Kang, H.-J.; Lee, B.-K. Comparison of Gait variables and Relative Risk of Falls According to Walking Speed During Flat and Obstacles Walking of Fallers and Non-Fallers in Korean Elderly Women. Exerc. Sci. 2022, 31, 80–87. [Google Scholar] [CrossRef]
- Kyrdalen, I.L.; Thingstad, P.; Sandvik, L.; Ormstad, H. Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiother. Res. Int. 2019, 24, e1743. [Google Scholar] [CrossRef]
- Mielke, M.M.; Roberts, R.O.; Savica, R.; Cha, R.; Drubach, D.I.; Christianson, T.; Pankratz, V.S.; Geda, Y.E.; Machulda, M.M.; Ivnik, R.J.; et al. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 929–937. [Google Scholar] [CrossRef]
- Callisaya, M.L.; Launay, C.P.; Srikanth, V.K.; Verghese, J.; Allali, G.; Beauchet, O. Cognitive status, fast walking speed and walking speed reserve-the Gait and Alzheimer Interactions Tracking (GAIT) study. Geroscience 2017, 39, 231–239. [Google Scholar] [CrossRef]
- Doi, T.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Combined effects of mild cognitive impairment and slow gait on risk of dementia. Exp. Gerontol. 2018, 110, 146–150. [Google Scholar] [CrossRef]
- Taylor, M.E.; Lasschuit, D.A.; Lord, S.R.; Delbaere, K.; Kurrle, S.E.; Mikolaizak, A.S.; Kvelde, T.; Close, J.C.T. Slow gait speed is associated with executive function decline in older people with mild to moderate dementia: A one year longitudinal study. Arch. Gerontol. Geriatr. 2017, 73, 148–153. [Google Scholar] [CrossRef]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R. Motoric cognitive risk syndrome and the risk of dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Allali, G.; Ayers, E.I.; Verghese, J. Motoric Cognitive Risk Syndrome Subtypes and Cognitive Profiles. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 378–384. [Google Scholar] [CrossRef] [PubMed]
- De Laat, K.F.; Reid, A.T.; Grim, D.C.; Evans, A.C.; Kotter, R.; van Norden, A.G.; de Leeuw, F.E. Cortical thickness is associated with gait disturbances in cerebral small vessel disease. Neuroimage 2012, 59, 1478–1484. [Google Scholar] [CrossRef]
- Rosano, C.; Aizenstein, H.; Brach, J.; Longenberger, A.; Studenski, S.; Newman, A.B. Special article: Gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzoli, D.; Ortelli, P.; Madeo, G.; Giladi, N.; Petzinger, G.M.; Frazzitta, G. Basal ganglia and beyond: The interplay between motor and cognitive aspects in Parkinson’s disease rehabilitation. Neurosci. Biobehav. Rev. 2018, 90, 294–308. [Google Scholar] [CrossRef]
- Denison, H.J.; Jameson, K.A.; Sayer, A.A.; Patel, H.P.; Edwards, M.H.; Arora, T.; Dennison, E.M.; Cooper, C.; Baird, J. Poor sleep quality and physical performance in older adults. Sleep Health 2021, 7, 205–211. [Google Scholar] [CrossRef]
- Xu, W.; Bai, A.; Huang, X.; Gao, Y.; Liu, L. Association Between Sleep and Motoric Cognitive Risk Syndrome Among Community-Dwelling Older Adults: Results from the China Health and Retirement Longitudinal Study. Front. Aging Neurosci. 2021, 13, 774167. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, L.; Feng, B.; Li, H.; Wang, D.; Zheng, Z.; Zhang, Y.; Jiang, L.; Ye, H. Association between sleep disturbance with motoric cognitive risk syndrome in Chinese older adults. Eur. J. Neurol. 2021, 28, 1470–1478. [Google Scholar] [CrossRef]
- Kirshner, D.; Kizony, R.; Gil, E.; Asraf, K.; Krasovsky, T.; Haimov, I.; Shochat, T.; Agmon, M. Why Do They Fall? The Impact of Insomnia on Gait of Older Adults: A Case-Control Study. Nat. Sci. Sleep 2021, 13, 329–338. [Google Scholar] [CrossRef]
- Stenholm, S.; Kronholm, E.; Sainio, P.; Borodulin, K.; Era, P.; Fogelholm, M.; Partonen, T.; Porkka-Heiskanen, T.; Koskinen, S. Sleep-related factors and mobility in older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 649–657. [Google Scholar] [CrossRef]
- Goldman, S.E.; Ancoli-Israel, S.; Boudreau, R.; Cauley, J.A.; Hall, M.; Stone, K.L.; Rubin, S.M.; Satterfield, S.; Simonsick, E.M.; Newman, A.B.; et al. Sleep problems and associated daytime fatigue in community-dwelling older individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Kronholm, E.; Sallinen, M.; Suutama, T.; Sulkava, R.; Era, P.; Partonen, T. Self-reported sleep duration and cognitive functioning in the general population. J. Sleep Res. 2009, 18, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Elcombe, E.L.; Lagopoulos, J.; Duffy, S.L.; Lewis, S.J.; Norrie, L.; Hickie, I.B.; Naismith, S.L. Hippocampal volume in older adults at risk of cognitive decline: The role of sleep, vascular risk, and depression. J. Alzheimers Dis. 2015, 44, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, A.; Cohen, R.A.; Porges, E.C.; Nissim, N.R.; Woods, A.J. Cognitive Aging and the Hippocampus in Older Adults. Front. Aging Neurosci. 2016, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Allali, G.; van der Meulen, M.; Beauchet, O.; Rieger, S.W.; Vuilleumier, P.; Assal, F. The neural basis of age-related changes in motor imagery of gait: An fMRI study. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Atienza, M.; Ziontz, J.; Cantero, J.L. Low-grade inflammation in the relationship between sleep disruption, dysfunctional adiposity, and cognitive decline in aging. Sleep Med. Rev. 2018, 42, 171–183. [Google Scholar] [CrossRef]
- Legdeur, N.; Heymans, M.W.; Comijs, H.C.; Huisman, M.; Maier, A.B.; Visser, P.J. Age dependency of risk factors for cognitive decline. BMC Geriatr. 2018, 18, 187. [Google Scholar] [CrossRef]
- Verghese, J.; Holtzer, R.; Oh-Park, M.; Derby, C.A.; Lipton, R.B.; Wang, C. Inflammatory markers and gait speed decline in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1083–1089. [Google Scholar] [CrossRef]
- Biddle, D.J.; Robillard, R.; Hermens, D.F.; Hickie, I.B.; Glozier, N. Accuracy of self-reported sleep parameters compared with actigraphy in young people with mental ill-health. Sleep Health 2015, 1, 214–220. [Google Scholar] [CrossRef]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef]
- Kim, H.-J. Influence of Depression on Physical Activity, Symptoms of Chronic Pain and Sleep Disorders in the Female Elderly with Knee Osteoarthritis. Exerc. Sci. 2019, 28, 191–197. [Google Scholar] [CrossRef][Green Version]
- Roehrs, T.; Hyde, M.; Blaisdell, B.; Greenwald, M.; Roth, T. Sleep loss and REM sleep loss are hyperalgesic. Sleep 2006, 29, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, T.; Abrahamyan, L.; Niazi, A.; Piyasena, D.; Arif, A.A.; Wong, J. The prevalence of obstructive sleep apnea in mild cognitive impairment: A systematic review. BMC Neurol. 2019, 19, 195. [Google Scholar] [CrossRef]
- Ciavarella, D.; Campobasso, A.; Suriano, C.; Lo Muzio, E.; Guida, L.; Salcuni, F.; Laurenziello, M.; Illuzzi, G.; Tepedino, M. A new design of mandibular advancement device (IMYS) in the treatment of obstructive sleep apnea. Cranio 2022, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Swenor, B.K.; Bandeen-Roche, K.; Munoz, B.; West, S.K. Does walking speed mediate the association between visual impairment and self-report of mobility disability? The Salisbury Eye Evaluation Study. J. Am. Geriatr. Soc. 2014, 62, 1540–1545. [Google Scholar] [CrossRef]
- Makino, K.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Suzuki, T.; Shimada, H. Fear of falling and gait parameters in older adults with and without fall history. Geriatr. Gerontol. Int. 2017, 17, 2455–2459. [Google Scholar] [CrossRef]
| Variables | Total | Men | Women |
|---|---|---|---|
| Number, n | 233 | 92 | 141 |
| Age, years | 73.6 (4.3) | 74.1 (4.1) | 73.2 (4.2) |
| Weight, kg | 60.1 (6.5) | 67.9 (5.7) * | 52.2 (6.3) |
| Height, m | 1.61 (0.05) | 1.69 (0.06) * | 1.52 (0.05) |
| Body mass index, kg/m2 | 23.2 (3.3) | 23.8 (3.5) | 22.6 (3.0) |
| Tertiary education, n (%) | 52 (22) | 31 (33) * | 21 (14) |
| Living alone, n (%) | 21 (9.1) | 8 (8.6) | 13 (9.2) |
| Current smoker, n (%) | 24 (10.3) | 10 (10.8) | 14 (9.9) |
| Current full-time job, n (%) | 20 (8.6) | 9 (9.7) | 11 (7.8) |
| Alcohol drinker, n (%) | 28 (12.0) | 19 (20.5) * | 9 (6.4) |
| Mild cognitive impairment, n (%) | 39 (16.8) | 16 (17.3) | 23 (16.3) |
| Grip strength, kg | 25.0 (4.5) | 29.8 (4.9) * | 20.2 (4.1) |
| Moderate-intensity physical activity, min/day | 14.6 (4.6) | 15.2 (4.2) | 13.9 (5.1) |
| Mini-mental state examination, score | 25.9 (1.9) | 26.1 (2.0) | 25.9 (1.9) |
| Gait speed, m/sec | 1.15 (0.19) | 1.19 (0.21) | 1.12 (0.18) |
| Sleep duration, min | 361 (94) | 342 (94) | 379 (98) |
| EQ-5D index, score | 0.87 (0.02) | 0.86 (0.02) | 0.88 (0.01) |
| Variables | Non Adjusted | Multi Variable Adjusted |
|---|---|---|
| Gait speed | ||
| Optimal gait speed | Reference | Reference |
| Slower gait speed | 2.21 (1.13–4.14) | 1.84 (1.00–3.13) |
| Sleep duration | ||
| Optimal sleep | Reference | Reference |
| Short and long sleep (poor sleep) | 2.31 (1.03–3.54) | 1.76 (1.00–3.35) |
| Co-existence | ||
| Optimal sleep and gait speed | Reference | Reference |
| Slower gait or poor sleep | 2.23 (1.01–3.43) | 1.99 (0.83–3.47) |
| Slower gait and poor sleep | 5.23 (2.01–7.94) | 3.13 (1.93–5.14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, E.; Bae, S.; Park, H. Gait Speed and Sleep Duration Is Associated with Increased Risk of MCI in Older Community-Dwelling Adults. Int. J. Environ. Res. Public Health 2022, 19, 7625. https://doi.org/10.3390/ijerph19137625
Yoon E, Bae S, Park H. Gait Speed and Sleep Duration Is Associated with Increased Risk of MCI in Older Community-Dwelling Adults. International Journal of Environmental Research and Public Health. 2022; 19(13):7625. https://doi.org/10.3390/ijerph19137625
Chicago/Turabian StyleYoon, Eunju, Seongryu Bae, and Hyuntae Park. 2022. "Gait Speed and Sleep Duration Is Associated with Increased Risk of MCI in Older Community-Dwelling Adults" International Journal of Environmental Research and Public Health 19, no. 13: 7625. https://doi.org/10.3390/ijerph19137625
APA StyleYoon, E., Bae, S., & Park, H. (2022). Gait Speed and Sleep Duration Is Associated with Increased Risk of MCI in Older Community-Dwelling Adults. International Journal of Environmental Research and Public Health, 19(13), 7625. https://doi.org/10.3390/ijerph19137625







