Skin Toxicity of Selected Hair Cosmetic Ingredients: A Review Focusing on Hairdressers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Registration and Protocol
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Selection Process
2.6. Data Collection Process and Data Items
2.7. Effect Measures
2.8. Synthesis Methods
3. Results
3.1. Study Selection and Study Characteristics
3.2. Results of Individual Studies
3.2.1. Cysteamine Hydrochloride
3.2.2. Polyvinylpyrrolidone
3.2.3. Polyvinylpyrrolidone Copolymers
3.2.4. Sodium Laureth Sulfate
3.2.5. Cocamide Diethanolamine
3.2.6. Cocamidopropyl Betaine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Study | Year (Range) | Country | Study Design | Patch Testing | Patch Testing Context | Population Tested | Female (%) | Age (Years) | No. of Hairdresser Tested | No. of Hairdressers with a Positive Result | No. of Others Tested | No. of Others Tested with a Positive Result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ito et al. [73] | 2012–2014 | Japan | epidemiological sample | yes | patch test special series | all patch-tested patients | 87 | 58 (mean) | n/a | n/a | 192 | 26 |
| Schwensen et al. [56] | 2002–2011 | Denmark | epidemiological sample | yes | patch test special series | hairdressers | 83 | 30.8 (mean) | 12 | 1 | n/a | n/a |
| Study | Year (Range) | Country | Sex | Age | Occupation | Working Years | Own Products Tested |
|---|---|---|---|---|---|---|---|
| Isaakson and van der Walle [85] | 2007 | Sweden | 1 female | 53 | hairdresser | 27 | permanent-wave solution |
| Landers, Law, and Storrs [86] | 2002 | USA | 1 female | 38 | hairdresser | n/a | permanent-wave solution |
| Nishioka, Koizumi, and Takita [87] | 2012–2017 | Japan | 4 females 3 males | 22 to 73 | hairdresser | n/a | n/a |
| Study | Year (Range) | Country | Sex | Age | Occupation | Working Years | Own Product Tested |
|---|---|---|---|---|---|---|---|
| Buonomo and Warshaw [88] | 2021 | USA | 1 female | 25 | n/a | n/a | moisturizer |
| Pastor et al. [89] | 2008 | Spain | 1 female | 20 | n/a | n/a | lipstick |
| Quartier et al. [90] | 2006 | Belgium | 1 female | 28 | n/a | n/a | lipstick |
| Scheman and Cummins [91] | 1998 | USA | 1 female | 53 | n/a | n/a | skin care products |
| Study | Year (Range) | Country | Study Design | Patch Testing | Patch Testing Context | Population Tested | Female (%) | Age (years) | No. of Hairdresser Tested | No. of Hairdressers with a Positive Result | No. of Others Tested | No. of Others Tested with a Positive Result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mertens, Gilissen, and Goossens [71] | 1990–2015 | Belgium | monocentric retrospective study | yes | epidemiological sample | all patch-tested patients | n/a | n/a | n/a | 6 | 1767 | 18 |
| Aalto-Korte et al. [69] | 1993–2011 | Finland | monocentric retrospective study | yes | epidemiological sample | occupational patch-tested patients | n/a | n/a | n/a | n/a | 2572 | 25 |
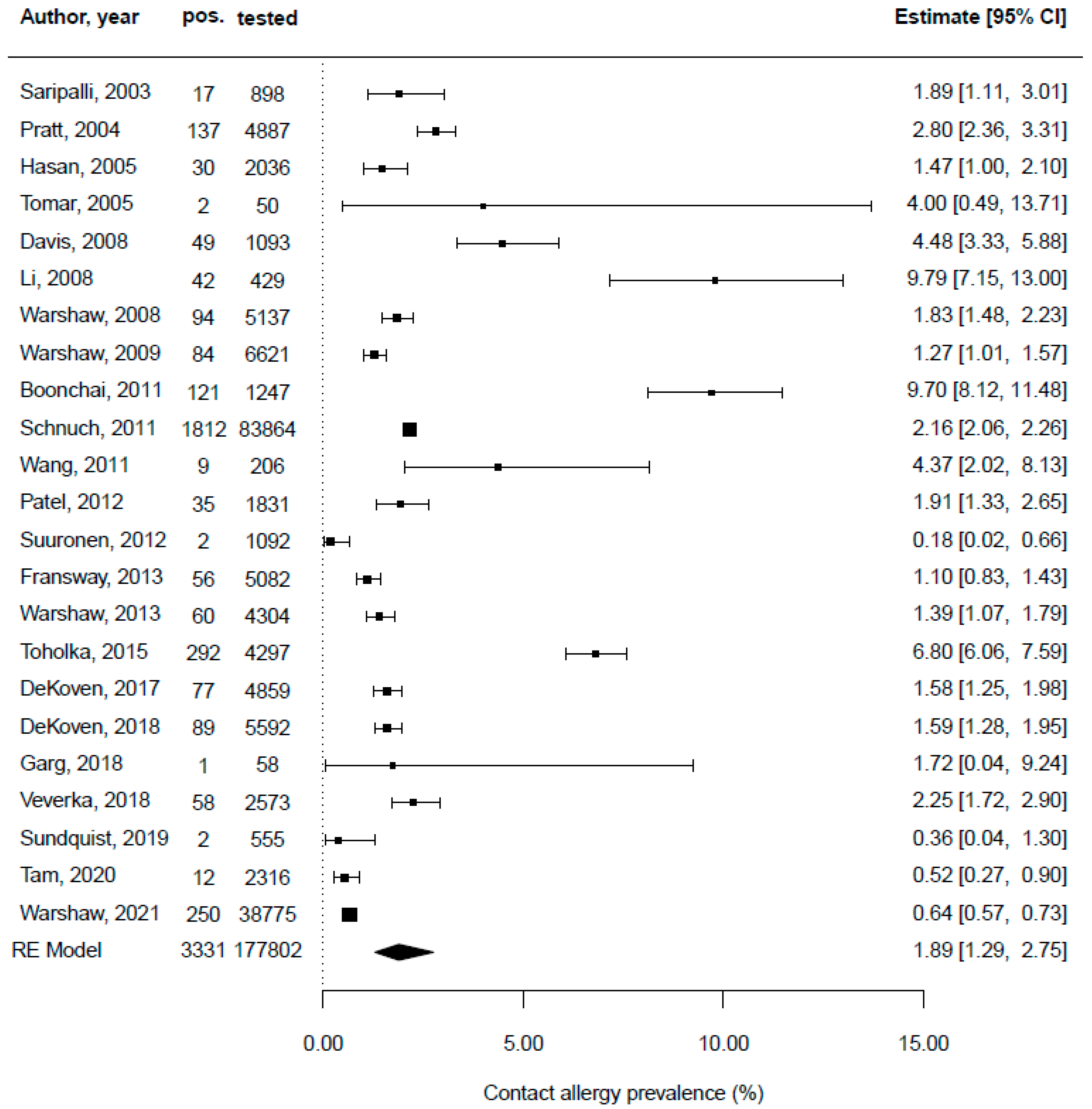
| Warshaw et al. [67] | 2001–2004 | USA | multicentric retrospective study | yes | consecutive patient | all patch-tested patients | 66 | n/a | n/a | n/a | 609 | 28 |
| Davis et al. [40] | 2001–2005 | USA | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | 66.8 | 55.1 (mean) | n/a | n/a | 410 | 1 |
| Toholka et al. [60] | 2001–2010 | Australia | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | 65 | 40 (mean) | n/a | n/a | 4297 | 1 |
| Warshaw et al. [65] | 2003–2004 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 65.2 | 47.5 (mean) | n/a | n/a | 5137 | 56 |
| Fransway et al. [43] | 2007–2008 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 64.3 | n/a | n/a | n/a | 5082 | 4 |
| Warshaw et al. [66] | 2009–2010 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 77.9 | 48.4 (mean) | n/a | n/a | 4304 | 1 |
| Sundquist, Yang, and Pasha [57] | 2010–2016 | Canada | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | 71.1 | 4 to 92 | n/a | n/a | 385 | 3 |
| Warshaw et al. [72] | 2011–2012 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 68.6 | 50 (mean) | n/a | n/a | 4230 | 4 |
| Veverka et al. [63] | 2011–2015 | USA | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | 67.4 | 53.4 (mean) | n/a | n/a | 2573 | 2 |
| DeKoven et al. [41] | 2013–2014 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 70 | 50 (mean) | n/a | n/a | 4859 | 1 |
| DeKoven et al. [42] | 2015–2016 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 72 | 50 (mean) | n/a | n/a | 5594 | 2 |
| Grey et al. [70] | 2015–2016 | USA | others | yes | patch test special series | all patch-tested patients | 78.7 | 55.2 (mean) | n/a | n/a | 47 | 4 |
| Study | Year (Range) | Country | Sex | Age | Occupation | Working Years | Own Product Tested |
|---|---|---|---|---|---|---|---|
| Fowler [84] | 1997 | USA | 1 female 2 males | 40 47, 28 | n/a manufacturing, mechanics | n/a | personal care products |
| Dejobert et al. [83] | 2005 | France | 1 female | 27 | n/a | n/a | shampoo |
| Badaoui et al. [82] | 2012–2014 | France | 4 females 2 males | 51.6 (mean) | n/a | n/a | mycoster cream, cyteal solution |
| Study | Year (Range) | Country | Study Design | Patch Testing | Patch Testing Context | Population Tested | Female (%) | Age (years) | No. of Hairdresser Tested | No. of Hairdressers with a Positive Result | No. of Others Tested | No. of Others Tested with a Positive Result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
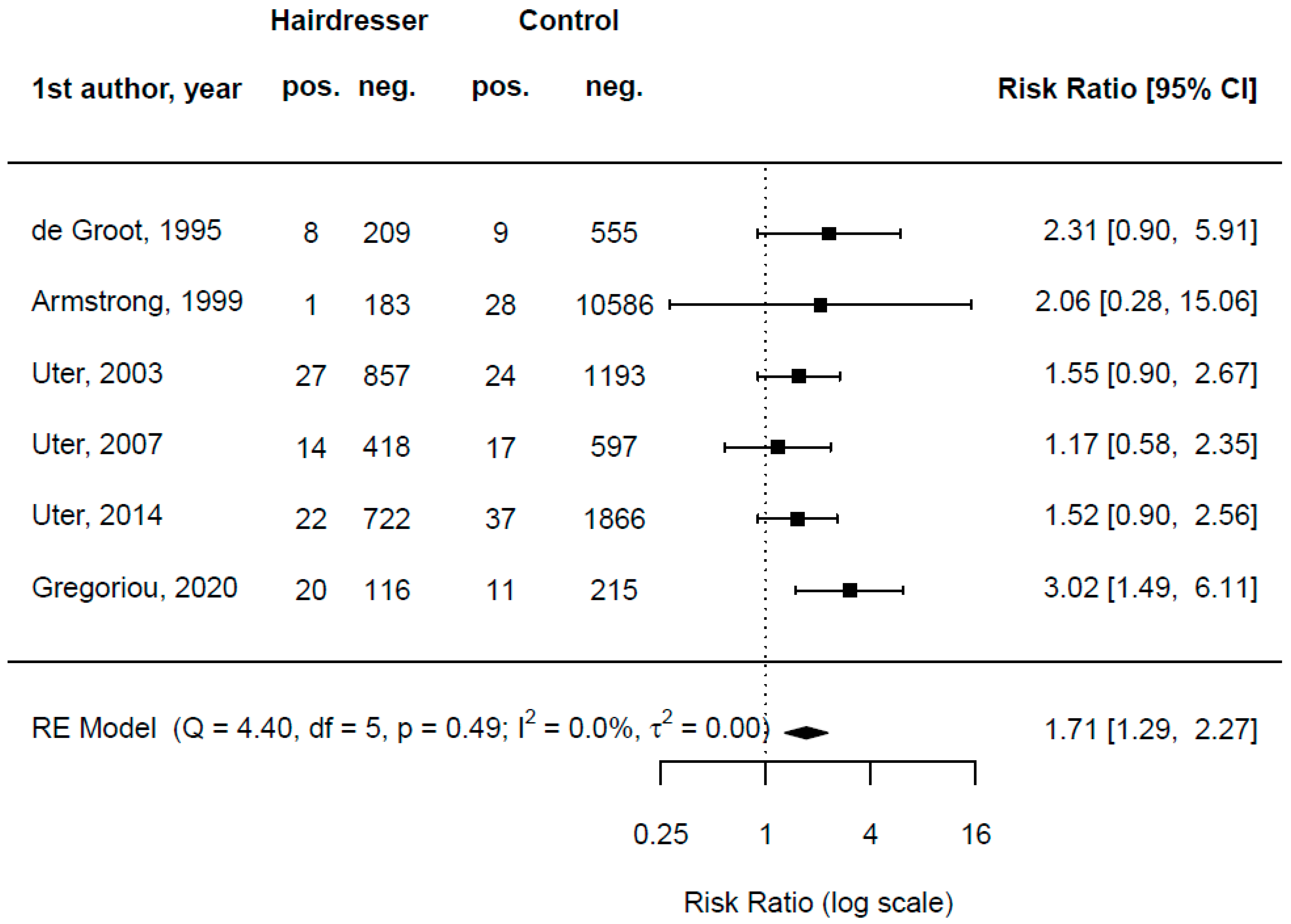
| De Groot, van der Walle, and Weyland [26] | 1991–1994 | Netherlands | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | 217 | 8 | 564 | 9 |
| Armstrong et al. [25] | 1991–1998 | UK | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | 184 | 1 | 10,614 | 28 |
| Uter et al. [24] | 1995–2002 | Austria, Germany, Switzerland | multicentric retrospective study | yes | epidemiological sample | hairdressers | 100 | 24 (mean) | 884 | 27 | 1217 | 24 |
| Uter et al. [23] | 2003–2006 | Germany | multicentric retrospective study | yes | epidemiological sample | hairdressers | 100 | 26 (mean) | 432 | 14 | 614 | 17 |
| Uter et al. [22] | 2007–2012 | Austria, Germany, Switzerland | multicentric retrospective study | yes | epidemiological sample | hairdressers | 100 | 24 (mean) | 744 | 22 | 1903 | 37 |
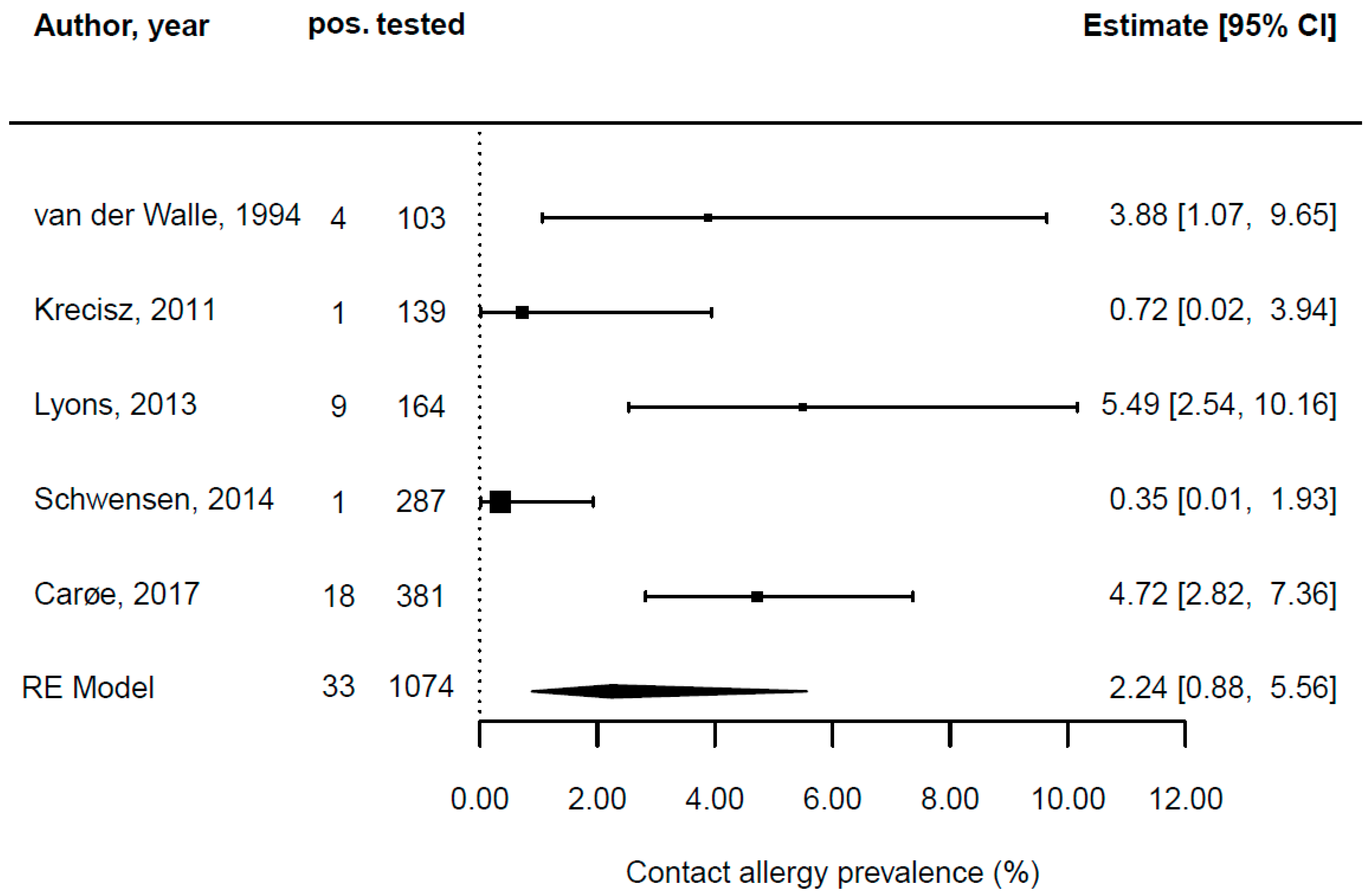
| Van der Walle and Brunsveld [62] | 1989–1992 | Netherlands | monocentric retrospective study | yes | epidemiological sample | hairdressers | 92.2 | 16 to 52 | 103 | 4 | n/a | n/a |
| Lyons et al. [50] | 1993–2010 | Australia | monocentric retrospective study | yes | epidemiological sample | hairdressers | 96 | 23 (mean) | 164 | 1 | n/a | n/a |
| Schwensen et al. [56] | 2002–2011 | Denmark | epidemiological sample | yes | epidemiological sample | hairdressers | n/a | 16 to 79 | 287 | 1 | 1995 | n/a |
| Krecisz, Kiec-Swierczynska, and Chomiczewska [48] | 2011 | Poland | epidemiological sample | yes | patch test special series | hairdressers | 96 | 18 (mean) | 139 | 1 | n/a | n/a |
| Carøe, Ebbehøj, and Agner [6] | 2006–2011 | Denmark | epidemiological sample | yes | epidemiological sample | hairdressers | 99.7 | 25 (mean) | 381 | 18 | n/a | n/a |
| Hillen, Grabbe, and Uter [47] | 1993–2003 | Germany | multicentric retrospective study | yes | epidemiological sample | all patch-tested patients | n/a | n/a | n/a | n/a | 1021 | 48 |
| Patel and Belsito [51] | 1995–2005 2005–2010 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | n/a | n/a | 1831 | 35 |
| Hasan et al. [46] | 1995–1997 | Finland | multicentric retrospective study | yes | patch test special series | all patch-tested patients | n/a | n/a | n/a | n/a | 2036 | 30 |
| Saripalli, Achen, and Belsito [54] | 1995–2001 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | n/a | n/a | 898 | 17 |
| Schnuch et al. [55] | 1996–2009 | Germany | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | n/a | n/a | 83,864 | 1.812 |
| Boonchai, Desomchoke, and Iamtharachai [39] | 1999–2008 | Thailand | monocentric retrospective study | yes | epidemiological sample | all patch-tested patients | 81 | 8 to 84 | n/a | n/a | 1247 | 121 |
| Wang et al. [64] | 2000–2008 | USA | multicentric retrospective study | yes | patch test special series | all patch-tested patients | 94.8 | 53.8 (mean) | n/a | n/a | 206 | 9 |
| Pratt et al. [52] | 2001–2002 | USA | Multicentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | n/a | n/a | 4887 | 137 |
| Warshaw et al. [67] | 2001–2004 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 66 | 49.3 (mean) | n/a | n/a | 6621 | 84 |
| Davis et al. [40] | 2001–2005 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 66.8 | 55.1 (mean) | n/a | n/a | 1093 | 49 |
| Toholka et al. [60] | 2001–2010 | Australia | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | 65 | 41 (mean) | n/a | n/a | 4297 | 292 |
| Warshaw et al. [68] | 2001–2016 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | n/a | n/a | 38,775 | 250 |
| Suuronen, Pesonen, and Aalto-Korte [58] | 2002–2009 | Finland | monocentric retrospective study | yes | epidemiological sample | all patch-tested patients | n/a | n/a | n/a | n/a | 1092 | 2 |
| Warshaw et al. [65] | 2003–2004 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 65.2 | n/a | n/a | n/a | 5137 | 94 |
| Li [49] | 2005–2006 | China | monocentric retrospective study | yes | patch test special series | all patch-tested patients | 75 | 9 to 81 | n/a | n/a | 429 | 42 |
| Tomar et al. [61] | 2005 | India | monocentric retrospective study | yes | epidemiological sample | all patch-tested patients | 70 | 16 to 55 | n/a | n/a | 50 | 2 |
| Fransway et al. [43] | 2007–2008 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | n/a | n/a | 5082 | 56 |
| Tam et al. [59] | 2007–2016 | USA | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | 73.4 | 47.7 (mean) | n/a | n/a | 2316 | 12 |
| Warshaw et al. [66] | 2009–2010 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | 67.9 | 48.4 (mean) | n/a | n/a | 4304 | 4 |
| Salverda et al. CAPB [53] | 2009–2011 | Netherlands | other (cosmetovigilance) | yes | consecutive patients | n/a | n/a | 41 (mean) | n/a | n/a | n/a | n/a |
| Sundquist, Yang, and Pasha [57] | 2010–2016 | Canada | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | 71.1 | 4 to 92 | n/a | n/a | 555 | 2 |
| Gregoriou et al. [45] | 2010–2019 | Greece | monocentric retrospective study | yes | patch test special series | all patch-tested patients | 89.5 | 13 to 87 | 136 | 20 | 226 | 11 |
| Veverka et al. [63] | 2011–2015 | USA | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | 67.4 | 53.4 (mean) | n/a | n/a | 2573 | 58 |
| DeKoven et al. exposure [41] | 2013–2014 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | n/a | n/a | 4859 | 77 |
| Garg et al. [44] | 2013–2015 | India | monocentric retrospective study | yes | consecutive patients | all patch-tested patients | 86.2 | 36.3 (mean) | n/a | n/a | 58 | 1 |
| DeKoven et al. [42] | 2015–2016 | USA | multicentric retrospective study | yes | consecutive patients | all patch-tested patients | n/a | n/a | n/a | n/a | 5592 | 89 |
| Study | Year (Range) | Country | Sex | Age | Occupation | Working Years | Own Product Tested |
|---|---|---|---|---|---|---|---|
| Korting et al. [75] | 1992 | Germany | 2 females | n/a | hairdresser | n/a | shampoos |
| Taniguchi et al. [81] | 1992 | Japan | male | 22 | hairdresser | n/a | shampoos |
| Lin-Hui and Sun [76] | 1998 | Taiwan | female | 47 | hairdresser | 30 | shampoo, hair dye |
| Ross and White [80] | 1991 | UK | female | 60 | n/a | n/a | eye make-up remover |
| Brand and Delaney [74] | 1998 | Australia | female | 50 | n/a | n/a | shampoos |
| Mowad [79] | 2001 | USA | male | 75 | n/a | n/a | shampoo |
| McFadden [77] | 2001 | UK | 6 females 1 male | 26–69 | n/a | n/a | eye make-up remover, liquid soap |
| Moreau and Sasseville [78] | 2004 | Canada | female | 39 | n/a | n/a | facial creams |
References and Note
- Dickel, H.; Kuss, O.; Blesius, C.R.; Schmidt, A.; Diepgen, T.L. Occupational skin diseases in Northern Bavaria between 1990 and 1999: A population-based study. Br. J. Dermatol. 2001, 145, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Babić, Ž.; Samardžić, T.; Macan, J. Comparison of beautician and hairdressing apprentices with regard to skin health and skin barrier function. Arh. Hig. Rada Toksikol. 2020, 71, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Havmose, M.S.; Kezic, S.; Uter, W.; Symanzik, C.; Hallmann, S.; Strahwald, J.; Weinert, P.; Macan, M.; Turk, R.; van der Molen, H.F.; et al. Prevalence and incidence of hand eczema in hairdressers—A systematic review and meta-analysis of the published literature from 2000–2021. Contact Dermat. 2022, 86, 254–265. [Google Scholar] [CrossRef]
- Antonov, D.; Schliemann, S.; Elsner, P. Hand dermatitis: A review of clinical features, prevention and treatment. Am. J. Clin. Dermatol. 2015, 16, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Kieć-Swierczyńska, M.; Chomiczewska, D.; Krecisz, B. Wet work–praca w środowisku mokrym. Med. Pr. 2010, 61, 65–77. [Google Scholar]
- Carøe, T.K.; Ebbehøj, N.E.; Agner, T. Occupational dermatitis in hairdressers - influence of individual and environmental factors. Contact Dermat. 2017, 76, 146–150. [Google Scholar] [CrossRef]
- Uter, W.; Bensefa-Colas, L.; Frosch, P.; Giménez-Arnau, A.; John, S.M.; Lepoittevin, J.P.; Lidén, C.; White, I.R.; Duus Johansen, J. Patch testing with hair cosmetic series in Europe: A critical review and recommendation. Contact Dermat. 2015, 73, 69–81. [Google Scholar] [CrossRef]
- Pesonen, M.; Jolanki, R.; Larese Filon, F.; Wilkinson, M.; Kręcisz, B.; Kieć-Świerczyńska, M.; Bauer, A.; Mahler, V.; John, S.M.; Schnuch, A.; et al. Patch test results of the European baseline series among patients with occupational contact dermatitis across Europe - analyses of the European Surveillance System on Contact Allergy network, 2002–2010. Contact Dermat. 2015, 72, 154–163. [Google Scholar] [CrossRef]
- Carøe, T.K.; Ebbehøj, N.E.; Bonde, J.P.; Agner, T. Occupational hand eczema and/or contact urticaria: Factors associated with change of profession or not remaining in the workforce. Contact Dermat. 2018, 78, 55–63. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Scheidt, R.; Weisshaar, E.; John, S.M.; Hieke, K. Cost of illness from occupational hand eczema in Germany. Contact Dermat. 2013, 69, 99–106. [Google Scholar] [CrossRef]
- Politiek, K.; Oosterhaven, J.A.; Vermeulen, K.M.; Schuttelaar, M.L. Systematic review of cost-of-illness studies in hand eczema. Contact Dermat. 2016, 75, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uter, W.; Werfel, T.; Lepoittevin, J.P.; White, I.R. Contact Allergy—Emerging Allergens and Public Health Impact. Int. J. Environ. Res. Public Health 2020, 17, 2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SCCS (Scientific Committee on Consumer Safety), 2018. SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 10th Revision. October 2018. SCCS/1602/18. pp. 24–25. Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_224.pdf (accessed on 22 June 2021).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast). Available online: http://data.europa.eu/eli/reg/2009/1223/2019-08-13 (accessed on 22 June 2021).
- Uter, W.; Johansen, J.D.; Havmose, M.S.; Kezic, S.; van der Molen, H.F.; Macan, J.; Babić, Ž.; Turk, R.; Symanzik, C.; John, S.M. Protocol for a systematic review on systemic and skin toxicity of important hazardous hair and nail cosmetic ingredients in hairdressers. BMJ Open 2021, 11, e050612. [Google Scholar] [CrossRef]
- Symanzik, C.; Johansen, J.D.; Weinert, P.; Babić, Ž.; Hallmann, S.; Havmose, M.S.; Kezic, S.; Macan, M.; Macan, J.; Strahwald, J.; et al. Differences between hairdressers and consumers in skin exposure to hair cosmetic products: A review. Contact Dermat. 2022, 86, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Symanzik, C.; Weinert, P.; Babić, Ž.; Hallmann, S.; Havmose, M.S.; Johansen, J.D.; Kezic, S.; Macan, M.; Macan, J.; Strahwald, J.; et al. Allergic contact dermatitis caused by 2-hydroxyethyl methacrylate and ethyl cyanoacrylate contained in cosmetic glues among hairdressers and beauticians who perform nail treatments and eyelash extension as well as hair extension applications: A systematic review. Contact Dermat. 2022, 86, 480–492. [Google Scholar] [CrossRef]
- Kezic, S.; Nunez, R.; Babić, Ž.; Hallmann, S.; Havmose, M.S.; Johansen, J.D.; John, S.M.; Macan, M.; Symanzik, C.; Uter, W.; et al. Occupational Exposure of Hairdressers to Airborne Hazardous Chemicals: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 4176. [Google Scholar] [CrossRef]
- Macan, J.; Babić, Ž.; Hallmann, S.; Havmose, M.S.; Johansen, J.D.; John, S.M.; Macan, M.; Symanzik, C.; Uter, W.; Weinert, P.; et al. Respiratory toxicity of persulphate salts and their adverse effects on airways in hairdressers: A systematic review. Int. Arch. Occup. Environ. Health 2022. [Google Scholar] [CrossRef]
- Uter, W.; Johansen, J.D.; Havmose, M.S.; Kezic, S.; van der Molen, H.; Macan, J.; Babić, Ž.; Turk, R.; Hallmann, S.; Strahwald, J.; et al. Protocol for a Systematic Review on Skin and Systemic Toxicity of Important Hazardous Substances in Hair Cosmetics and Hand Eczema in Hairdressers. PROSPERO. 2021. CRD42021238118. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021238118 (accessed on 22 June 2021).
- Tacconelli, E. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Lancet Infect. Dis. 2010, 10, 226. [Google Scholar] [CrossRef]
- Uter, W.; Gefeller, O.; John, S.M.; Schnuch, A.; Geier, J. Contact allergy to ingredients of hair cosmetics—A comparison of female hairdressers and clients based on IVDK 2007–2012 data. Contact Dermat. 2014, 71, 13–20. [Google Scholar] [CrossRef]
- Uter, W.; Lessmann, H.; Geier, J.; Schnuch, A. Contact allergy to hairdressing allergens in female hairdressers and client—Current data from the IVDK, 2003–2006. JDDG J. Dtsch. Dermatol. Ges. 2007, 5, 993–1001. [Google Scholar] [CrossRef]
- Uter, W.; Lessmann, H.; Geier, J.; Schnuch, A. Contact allergy to ingredients of hair cosmetics in female hairdressers and clients—An 8-year analysis of IVDK data. Contact Dermat. 2003, 49, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Smith, H.R.; Ross, J.S.; White, I.R. Sensitization to cocamidopropylbetaine: An 8-year review. Contact Dermat. 1999, 40, 335–336. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; van der Walle, H.B.; Weyland, J.W. Contact allergy to cocamidopropyl betaine. Contact Dermat. 1995, 33, 419–422. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Pub Lux N°L 97.5.4.2006: 1–528.
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foti, C.; Bonamonte, D.; Mascolo, G.; Corcelli, A.; Lobasso, S.; Rigano, L.; Angelini, G. The role of 3-dimethylaminopropylamine and amidoamine in contact allergy to cocamidopropylbetaine. Contact Dermat. 2003, 48, 194–198. [Google Scholar] [CrossRef]
- Angelini, G.; Rigano, L. The allergen cocamidopropyl betaine. Contact Dermat. 1998, 39, 210–211. [Google Scholar] [CrossRef]
- Pinola, A.; Estlander, T.; Jolanki, R.; Tarvainen, K.; Kanerva, L. Occupational allergic contact dermatitis due to coconut diethanolamide (cocamide DEA). Contact Dermat. 1993, 29, 262–265. [Google Scholar] [CrossRef]
- Kadivar, S.; Belsito, D.V. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis 2015, 26, 177–183. [Google Scholar] [CrossRef]
- De Groot, A.C. Cosmetic dermatitis. Clin. Dermatol. 1997, 15, 485–491. [Google Scholar] [CrossRef]
- Zirwas, M.; Moennich, J. Shampoos. Dermatitis 2009, 20, 106–110. [Google Scholar] [CrossRef]
- Mehling, A.; Chkarnat, C.; Degwert, J.; Ennen, J.; Fink, E.; Matthies, W.; Roethlisberger, R.; Rossow, U.; Schnitker, J.; Tronnier, H.; et al. Interlaboratory studies with a proposed patch test design to evaluate the irritation potential of surfactants. Contact Dermat. 2010, 62, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Cameli, N.; Tosti, G.; Venturo, N.; Tosti, A. Eyelid dermatitis due to cocamidopropyl betaine in a hard contact lens solution. Contact Dermat. 1991, 25, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Ojo, E.O.; Gowda, A.; Nedorost, S. Scalp Dermatitis in Patients Sensitized to Components of Hair Products. Dermatitis 2019, 30, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Boonchai, W.; Desomchoke, R.; Iamtharachai, P. Trend of contact allergy to cosmetic ingredients in Thais over a period of 10 years. Contact Dermat. 2011, 65, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.; Scalf, L.A.; Yiannias, J.A.; Cheng, J.F.; El-Azhary, R.A.; Rohlinger, A.L.; Farmer, S.A.; Fett, D.D.; Johnson, J.S.; Linehan, D.L.; et al. Changing trends and allergens in the patch test standard series: A mayo clinic 5-year retrospective review, january 1, 2001, through december 31, 2005. Arch. Dermatol. 2008, 144, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeKoven, J.G.; Warshaw, E.M.; Belsito, D.V.; Sasseville, D.; Maibach, H.I.; Taylor, J.S.; Marks, J.G.; Fowler, J.F., Jr.; Mathias, C.G.; DeLeo, V.A.; et al. North American Contact Dermatitis Group Patch Test Results 2013–2014. Dermatitis 2017, 28, 33–46. [Google Scholar] [CrossRef]
- DeKoven, J.G.; Warshaw, E.M.; Zug, K.A.; Maibach, H.I.; Belsito, D.V.; Sasseville, D.; Taylor, J.S.; Fowler, J.F., Jr.; Mathias, C.G.T.; Marks, J.G.; et al. North American Contact Dermatitis Group Patch Test Results: 2015–2016. Dermatitis 2018, 29, 297–309. [Google Scholar] [CrossRef]
- Fransway, A.F.; Zug, K.A.; Belsito, D.V.; Deleo, V.A.; Fowler, J.F., Jr.; Maibach, H.I.; Marks, J.G.; Mathias, C.G.; Pratt, M.D.; Rietschel, R.L.; et al. North American Contact Dermatitis Group patch test results for 2007–2008. Dermatitis 2013, 24, 10–21. [Google Scholar] [CrossRef]
- Garg, T.; Agarwal, S.; Chander, R.; Singh, A.; Yadav, P. Patch testing in patients with suspected cosmetic dermatitis: A retrospective study. J. Cosmet. Dermatol. 2018, 17, 95–100. [Google Scholar] [CrossRef]
- Gregoriou, S.; Mastraftsi, S.; Hatzidimitriou, E.; Tsimpidakis, A.; Nicolaidou, E.; Stratigos, A.; Katsarou, A.; Rigopoulos, D. Occupational and non-occupational allergic contact dermatitis to hair dyes in Greece. A 10-year retrospective study. Contact Dermat. 2020, 83, 277–285. [Google Scholar] [CrossRef]
- Hasan, T.; Rantanen, T.; Alanko, K.; Harvima, R.J.; Jolanki, R.; Kalimo, K.; Lahti, A.; Lammintausta, K.; Lauerma, A.I.; Laukkanen, A.; et al. Patch test reactions to cosmetic allergens in 1995–1997 and 2000–2002 in Finland—A multicentre study. Contact Dermat. 2005, 53, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hillen, U.; Grabbe, S.; Uter, W. Patch test results in patients with scalp dermatitis: Analysis of data of the Information Network of Departments of Dermatology. Contact Dermat. 2007, 56, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Krecisz, B.; Kiec-Swierczynska, M.; Chomiczewska, D. Dermatological screening and results of patch testing among Polish apprentice hairdressers. Contact Dermat. 2011, 64, 90–95. [Google Scholar] [CrossRef]
- Li, L.F. A study of the sensitization rate to cocamidopropyl betaine in patients patch tested in a university hospital of Beijing. Contact Dermat. 2008, 58, 24–27. [Google Scholar] [CrossRef]
- Lyons, G.; Roberts, H.; Palmer, A.; Matheson, M.; Nixon, R. Hairdressers presenting to an occupational dermatology clinic in Melbourne, Australia. Contact Dermat. 2013, 68, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Belsito, D.V. The detection of clinically relevant contact allergens with a standard screening tray of 28 allergens. Contact Dermat. 2012, 66, 154–158. [Google Scholar] [CrossRef]
- Pratt, M.D.; Belsito, D.V.; DeLeo, V.A.; Fowler, J.F., Jr.; Fransway, A.F.; Maibach, H.I.; Marks, J.G.; Mathias, C.G.; Rietschel, R.L.; Sasseville, D.; et al. North American Contact Dermatitis Group patch-test results, 2001–2002 study period. Dermatitis 2004, 15, 176–183. [Google Scholar]
- Salverda, J.G.; Bragt, P.J.; de Wit-Bos, L.; Rustemeyer, T.; Coenraads, P.J.; Tupker, R.A.; Kunkeler, L.C.; Laheij-de Boer, A.M.; Stenveld, H.J.; van Ginkel, C.J.; et al. Results of a cosmetovigilance survey in The Netherlands. Contact Dermat. 2013, 68, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Saripalli, Y.V.; Achen, F.; Belsito, D.V. The detection of clinically relevant contact allergens using a standard screening tray of twenty-three allergens. J. Am. Acad. Dermatol. 2003, 49, 65–69. [Google Scholar] [CrossRef]
- Schnuch, A.; Lessmann, H.; Geier, J.; Uter, W. Is cocamidopropyl betaine a contact allergen? Analysis of network data and short review of the literature. Contact Dermat. 2011, 64, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Schwensen, J.F.; Johansen, J.D.; Veien, N.K.; Funding, A.T.; Avnstorp, C.; Osterballe, M.; Andersen, K.E.; Paulsen, E.; Mortz, C.G.; Sommerlund, M.; et al. Occupational contact dermatitis in hairdressers: An analysis of patch test data from the Danish contact dermatitis group, 2002–2011. Contact Dermat. 2014, 70, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, B.K.; Yang, B.; Pasha, M.A. Experience in patch testing: A 6-year retrospective review from a single academic allergy practice. Ann. Allergy Asthma Immunol. 2019, 122, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Suuronen, K.; Pesonen, M.; Aalto-Korte, K. Occupational contact allergy to cocamidopropyl betaine and its impurities. Contact Dermat. 2012, 66, 286–292. [Google Scholar] [CrossRef]
- Tam, I.; Schalock, P.C.; González, E.; Yu, J. Patch Testing Results From the Massachusetts General Hospital Contact Dermatitis Clinic, 2007–2016. Dermatitis 2020, 31, 202–208. [Google Scholar] [CrossRef]
- Toholka, R.; Wang, Y.S.; Tate, B.; Tam, M.; Cahill, J.; Palmer, A.; Nixon, R. The first Australian Baseline Series: Recommendations for patch testing in suspected contact dermatitis. Australas. J. Dermatol. 2015, 56, 107–115. [Google Scholar] [CrossRef]
- Tomar, J.; Jain, V.K.; Aggarwal, K.; Dayal, S.; Gupta, S. Contact allergies to cosmetics: Testing with 52 cosmetic ingredients and personal products. J. Dermatol. 2005, 32, 951–955. [Google Scholar] [CrossRef]
- Van der Walle, H.B.; Brunsveld, V.M. Dermatitis in hairdressers. (I). The experience of the past 4 years. Contact Dermat. 1994, 30, 217–221. [Google Scholar] [CrossRef]
- Veverka, K.K.; Hall, M.R.; Yiannias, J.A.; Drage, L.A.; El-Azhary, R.A.; Killian, J.M.; Johnson, J.S.; Nordberg Linehan, D.L.; Singh, N.; Davis, M.D.P. Trends in Patch Testing With the Mayo Clinic Standard Series, 2011–2015. Dermatitis 2018, 29, 310–315. [Google Scholar] [CrossRef]
- Wang, M.Z.; Farmer, S.A.; Richardson, D.M.; Davis, M.D. Patch-testing with hairdressing chemicals. Dermatitis 2011, 22, 16–26. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Belsito, D.V.; DeLeo, V.A.; Fowler, J.F., Jr.; Maibach, H.I.; Marks, J.G.; Toby Mathias, C.G.; Pratt, M.D.; Rietschel, R.L.; Sasseville, D.; et al. North American Contact Dermatitis Group patch-test results, 2003–2004 study period. Dermatitis 2008, 19, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Belsito, D.V.; Taylor, J.S.; Sasseville, D.; DeKoven, J.G.; Zirwas, M.J.; Fransway, A.F.; Mathias, C.G.; Zug, K.A.; DeLeo, V.A.; et al. North American Contact Dermatitis Group patch test results: 2009 to 2010. Dermatitis 2013, 24, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Buchholz, H.J.; Belsito, D.V.; Maibach, H.I.; Fowler, J.F., Jr.; Rietschel, R.L.; Zug, K.A.; Mathias, C.G.; Pratt, M.D.; Sasseville, D.; et al. Allergic patch test reactions associated with cosmetics: Retrospective analysis of cross-sectional data from the North American Contact Dermatitis Group, 2001–2004. J. Am. Acad. Dermatol. 2009, 60, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Ruggiero, J.L.; DeKoven, J.G.; Maibach, H.I.; Atwater, A.R.; Taylor, J.S.; Zug, K.A.; Reeder, M.J.; Silverberg, J.I.; Sasseville, D.; et al. Contact Dermatitis Associated with Hair Care Products: A Retrospective Analysis of the North American Contact Dermatitis Group Data, 2001–2016. Dermatitis 2022, 33, 91–102. [Google Scholar] [CrossRef]
- Aalto-Korte, K.; Pesonen, M.; Kuuliala, O.; Suuronen, K. Occupational allergic contact dermatitis caused by coconut fatty acids diethanolamide. Contact Dermat. 2014, 70, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Grey, K.R.; Hanson, J.L.; Hagen, S.L.; Hylwa, S.A.; Warshaw, E.M. Epidemiology and Co-Reactivity of Novel Surfactant Allergens: A Double-Blind Randomized Controlled Study. Dermatitis 2016, 27, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Mertens, S.; Gilissen, L.; Goossens, A. Allergic contact dermatitis caused by cocamide diethanolamine. Contact Dermat. 2016, 75, 20–24. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Maibach, H.I.; Taylor, J.S.; Sasseville, D.; DeKoven, J.G.; Zirwas, M.J.; Fransway, A.F.; Mathias, C.G.; Zug, K.A.; DeLeo, V.A.; et al. North American contact dermatitis group patch test results: 2011–2012. Dermatitis 2015, 26, 49–59. [Google Scholar] [CrossRef]
- Ito, A.; Nishioka, K.; Kanto, H.; Yagami, A.; Yamada, S.; Sugiura, M.; Yasunaga, C.; Yoshii, K.; Kobayashi, H.; Adachi, A.; et al. A multi-institutional joint study of contact dermatitis related to hair colouring and perming agents in Japan. Contact Dermat. 2017, 77, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.; Delaney, T.A. Allergic contact dermatitis to cocamidopropylbetaine in hair shampoo. Australas. J. Dermatol. 1998, 39, 121–122. [Google Scholar] [CrossRef]
- Korting, H.C.; Parsch, E.M.; Enders, F.; Przybilla, B. Allergic contact dermatitis to cocamidopropyl betaine in shampoo. J. Am. Acad. Dermatol. 1992, 27, 1013–1015. [Google Scholar] [CrossRef]
- Su, L.H.; Sun, C.C. Positive patch test to cocamidopropyl betaine in a hairdresser. Contact Dermat. 1998, 38, 168–169. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.P.; Ross, J.S.; White, I.R.; Basketter, D.A. Clinical allergy to cocamidopropyl betaine: Reactivity to cocamidopropylamine and lack of reactivity to 3-dimethylaminopropylamine. Contact Dermat. 2001, 45, 72–74. [Google Scholar] [CrossRef]
- Moreau, L.; Sasseville, D. Allergic contact dermatitis from cocamidopropyl betaine, cocamidoamine, 3-(dimethylamino)propylamine, and oleamidopropyl dimethylamine: Co-reactions or cross-reactions? Dermat. Contact Atopic Occup. Drug 2004, 15, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Mowad, C.M. Cocamidopropyl betaine allergy. Am. J. Contact Dermat. 2001, 12, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; White, I.R. Eyelid dermatitis due to cocamidopropyl betaine in an eye make-up remover. Contact Dermat. 1991, 25, 64. [Google Scholar] [CrossRef]
- Taniguchi, S.; Katoh, J.; Hisa, T.; Tabata, M.; Hamada, T. Shampoo dermatitis due to cocamidopropyl betaine. Contact Dermat. 1992, 26, 139. [Google Scholar] [CrossRef]
- Badaoui, A.; Amsler, E.; Raison-Peyron, N.; Vigan, M.; Pecquet, C.; Frances, C.; Soria, A. An outbreak of contact allergy to cocamide diethanolamide? Contact Dermat. 2015, 72, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Dejobert, Y.; Delaporte, E.; Piette, F.; Thomas, P. Eyelid dermatitis with positive patch test to coconut diethanolamide. Contact Dermat. 2005, 52, 173. [Google Scholar] [CrossRef]
- Fowler, J.F., Jr. Allergy to cocamide DEA. Am. J. Contact Dermat. 1998, 9, 40–41. [Google Scholar]
- Isaksson, M.; van der Walle, H. Occupational contact allergy to cysteamine hydrochloride in permanent-wave solutions. Contact Dermat. 2007, 56, 295–296. [Google Scholar] [CrossRef]
- Landers, M.C.; Law, S.; Storrs, F.J. Permanent-wave dermatitis: Contact allergy to cysteamine hydrochloride. Am. J. Contact Dermat. Off. J. Am. Contact Dermat. Soc. 2003, 14, 157–160. [Google Scholar] [CrossRef]
- Nishioka, K.; Koizumi, A.; Takita, Y. Allergic contact dermatitis caused by cysteamine hydrochloride in permanent wave agent—A new allergen for hairdressers in Japan. Contact Dermat. 2019, 80, 174–175. [Google Scholar] [CrossRef]
- Buonomo, M.; Warshaw, E.M. Allergic contact dermatitis due to polyvinylpyrrolidone (PVP)/eicosene copolymer. Contact Dermat. 2021, 85, 458–460. [Google Scholar] [CrossRef]
- Pastor, N.; Silvestre, J.F.; Mataix, J.; Lucas, A.; Pérez, M. Contact cheilitis from bisabolol and polyvinylpyrrolidone/hexadecene copolymer in lipstick. Contact Dermat. 2008, 58, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Quartier, S.; Garmyn, M.; Becart, S.; Goossens, A. Allergic contact dermatitis to copolymers in cosmetic—Case report and review of the literature. Contact Dermat. 2006, 55, 257–267. [Google Scholar] [CrossRef]
- Scheman, A.; Cummins, R. Contact allergy to PVP/hexadecene copolymer. Contact Dermat. 1998, 39, 201. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.R.; Armstrong, K.; Wakelin, S.H.; White, I.R. Contact allergy to PVP/eicosene copolymer. Contact Dermat. 1999, 40, 283. [Google Scholar] [CrossRef] [PubMed]
- Waas, R.L.V.; Hill, G. Allergic contact dermatitis caused by vinylpyrrolidone/eicosene copolymer in a sunscreen. Contact Dermat. 2019, 80, 63. [Google Scholar] [CrossRef] [Green Version]
- Le Coz, C.J.; Lefèbvre, C.; Ludmann, F.; Grosshans, E. Polyvinylpyrrolidone (PVP)/eicosene copolymer: An emerging cosmetic allergen. Contact Dermat. 2000, 43, 61–62. [Google Scholar]
- Elder, R.L. Final Report on the Safety Assessment of Sodium Laureth Sulfate and Ammonium Laureth Sulfate. J. Am. Coll. Toxicol. 1983, 2, 1–34. [Google Scholar] [CrossRef]
- Charbonnier, V.; Morrison, B.M., Jr.; Paye, M.; Maibach, H.I. Subclinical, non-erythematous irritation with an open assay model (washing): Sodium lauryl sulfate (SLS) versus sodium laureth sulfate (SLES). Food Chem. Toxicol. 2001, 39, 279–286. [Google Scholar] [CrossRef]
- Löffler, H.; Happle, R. Profile of irritant patch testing with detergents: Sodium lauryl sulfate, sodium laureth sulfate and alkyl polyglucoside. Contact Dermat. 2003, 48, 26–32. [Google Scholar] [CrossRef]
- Symanzik, C.; Kezic, S.; Jakasa, I.; Skudlik, C.; John, S.M.; Brans, R.; Sonsmann, F.K. Effects of skin washing frequency on the epidermal barrier function and inflammatory processes of the epidermis: An experimental study. Contact Dermat. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bárány, E.; Lindberg, M.; Lodén, M. Biophysical characterization of skin damage and recovery after exposure to different surfactants. Contact Dermat. 1999, 40, 98–103. [Google Scholar] [CrossRef]
- Fowler, J.F.; Fowler, L.M.; Hunter, J.E. Allergy to cocamidopropyl betaine may be due to amidoamine: A patch test and product use test study. Contact Dermat. 1997, 37, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.F.J. Cocamidopropyl Betaine: Contact Allergen of the Year. Dermatitis 2004, 15, 3–4. [Google Scholar] [CrossRef]
- Angelini, G.; Rigano, L.; Foti, C.; Rossi, P.; Vena, G.A. Pure cocamidopropylbetaine is not the allergen in patients with positive reactions to commercial cocamidopropylbetaine. Contact Dermat. 1996, 35, 252–253. [Google Scholar] [CrossRef]
- European Commission. Ingredient: COCAMIDOPROPYL BETAINE. Available online: https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.details_v2&id=75231 (accessed on 25 February 2022).
- Geens, T.; Aerts, E.; Borguet, M.; Haufroid, V.; Godderis, L. Exposure of hairdressers to aromatic diamines: An interventional study confirming the protective effect of adequate glove use. Occup. Environ. Med. 2016, 73, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Oreskov, K.W.; Søsted, H.; Johansen, J.D. Glove use among hairdressers: Difficulties in the correct use of gloves among hairdressers and the effect of education. Contact Dermat. 2015, 72, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.-L.; Johnsson, S.; Lidén, C.; Meding, B.; Boman, A. The influence of hydrogen peroxide on the permeability of protective gloves to resorcinol in hairdressing. Contact Dermat. 2015, 72, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Antelmi, A.; Young, E.; Svedman, C.; Zimerson, E.; Engfeldt, M.; Foti, C.; Bruze, M. Are gloves sufficiently protective when hairdressers are exposed to permanent hair dyes? An in vivo study. Contact Dermat. 2015, 72, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.D.; Aalto-Korte, K.; Agner, T.; Andersen, K.E.; Bircher, A.; Bruze, M.; Cannavó, A.; Giménez-Arnau, A.; Gonçalo, M.; Goossens, A.; et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—Recommendations on best practice. Contact Dermat. 2015, 73, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Strahwald, J.; Hallmann, S.; Johansen, J.D.; Havmose, M.S.; Kezic, S.; van der Molen, H.; Macan, J.; Babić, Ž.; Franić, Z.; et al. Systematic review on skin adverse effects of important hazardous hair cosmetic ingredients with a focus on hairdressers. Contact Dermat. 2022; submitted for publication. [Google Scholar]
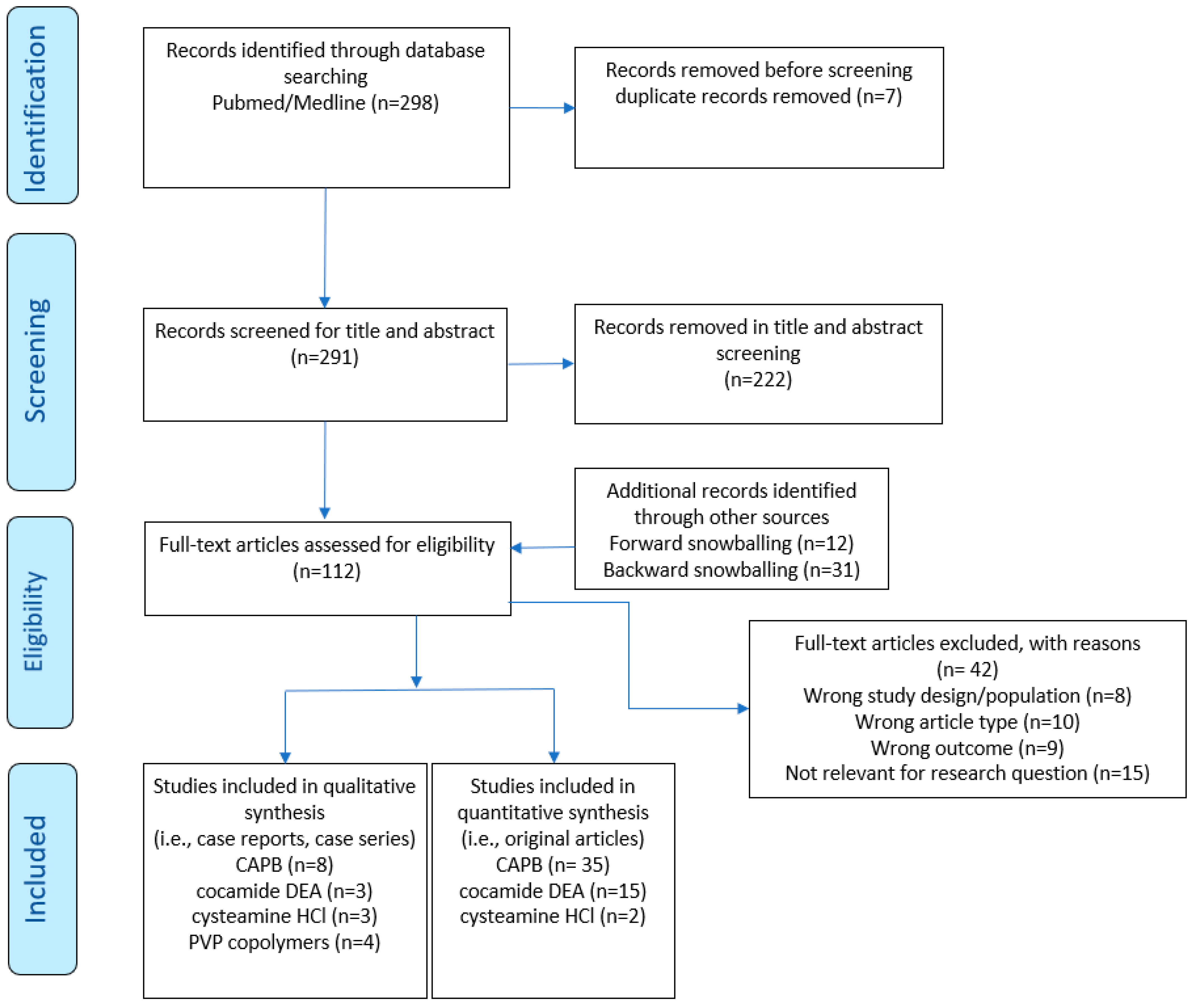
| Criterion | Inclusion | Exclusion |
|---|---|---|
| Participants | Hairdressers, Patients, Products | None |
| Exposure | Exposure to (an) eligible chemical(s) † | n/a |
| Comparator | Clients, Consumers, normal Population (no or less exposure) | n/a |
| Outcome | Skin toxicity event (contact allergy, irritancy) | n/a |
| Study design | Experimental studies | Other qualitative studies |
| Observational studies | ||
| Case reports | ||
| Case series |
| Study | Study Period | Country | Positive Results for Cocamide DEA |
|---|---|---|---|
| DeKoven et al. [41] | 2013–2014 | USA | 1 of 4859 (0.02%) |
| Toholka et al. [60] | 2001–2010 | Australia | 1 of 4297 (0.02%) |
| Warshaw et al. [67] | 2001–2004 | USA | 1 of 4304 (0.02%) |
| DeKoven et al. [42] | 2015–2016 | USA | 2 of 5594 (0.04%) |
| Warshaw et al. [72] | 2011–2012 | USA | 4 of 64,230 (0.1%) |
| Veverka et al. [63] | 2011–2015 | USA | 2 of 2573 (0.1%) |
| Fransway et al. [43] | 2007–2008 | USA | 4 of 5082 (0.1%) |
| Davis et al. [40] | 2001–2005 | USA | 1 of 410 (0.2%) |
| Sundquist, Yang, and Pasha [57] | 2010–2016 | Canada | 3 of 385 (0.8%) |
| Mertens, Gilissen, and Goossens [71] | 1990–2015 | Belgium | 18 of 1767 (1.0%) |
| Aalto-Korte et al. [69] | 1993–2011 | Finland | 25 of 2572 (1.0%) |
| Warshaw et al. [65] | 2003–2004 | USA | 56 of 5137 (1.1%) |
| Warshaw et al. [66] | 2009–2010 | USA | 28 of 609 (4.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symanzik, C.; Weinert, P.; Babić, Ž.; Hallmann, S.; Havmose, M.S.; Johansen, J.D.; Kezic, S.; Macan, M.; Macan, J.; Strahwald, J.; et al. Skin Toxicity of Selected Hair Cosmetic Ingredients: A Review Focusing on Hairdressers. Int. J. Environ. Res. Public Health 2022, 19, 7588. https://doi.org/10.3390/ijerph19137588
Symanzik C, Weinert P, Babić Ž, Hallmann S, Havmose MS, Johansen JD, Kezic S, Macan M, Macan J, Strahwald J, et al. Skin Toxicity of Selected Hair Cosmetic Ingredients: A Review Focusing on Hairdressers. International Journal of Environmental Research and Public Health. 2022; 19(13):7588. https://doi.org/10.3390/ijerph19137588
Chicago/Turabian StyleSymanzik, Cara, Patricia Weinert, Željka Babić, Sarah Hallmann, Martin Stibius Havmose, Jeanne Duus Johansen, Sanja Kezic, Marija Macan, Jelena Macan, Julia Strahwald, and et al. 2022. "Skin Toxicity of Selected Hair Cosmetic Ingredients: A Review Focusing on Hairdressers" International Journal of Environmental Research and Public Health 19, no. 13: 7588. https://doi.org/10.3390/ijerph19137588
APA StyleSymanzik, C., Weinert, P., Babić, Ž., Hallmann, S., Havmose, M. S., Johansen, J. D., Kezic, S., Macan, M., Macan, J., Strahwald, J., Turk, R., van der Molen, H. F., John, S. M., & Uter, W. (2022). Skin Toxicity of Selected Hair Cosmetic Ingredients: A Review Focusing on Hairdressers. International Journal of Environmental Research and Public Health, 19(13), 7588. https://doi.org/10.3390/ijerph19137588








