A Transparency Checklist for Carbon Footprint Calculations Applied within a Systematic Review of Virtual Care Interventions
Abstract
:1. Introduction
2. Methods
2.1. Development of a Transparency Catalogue
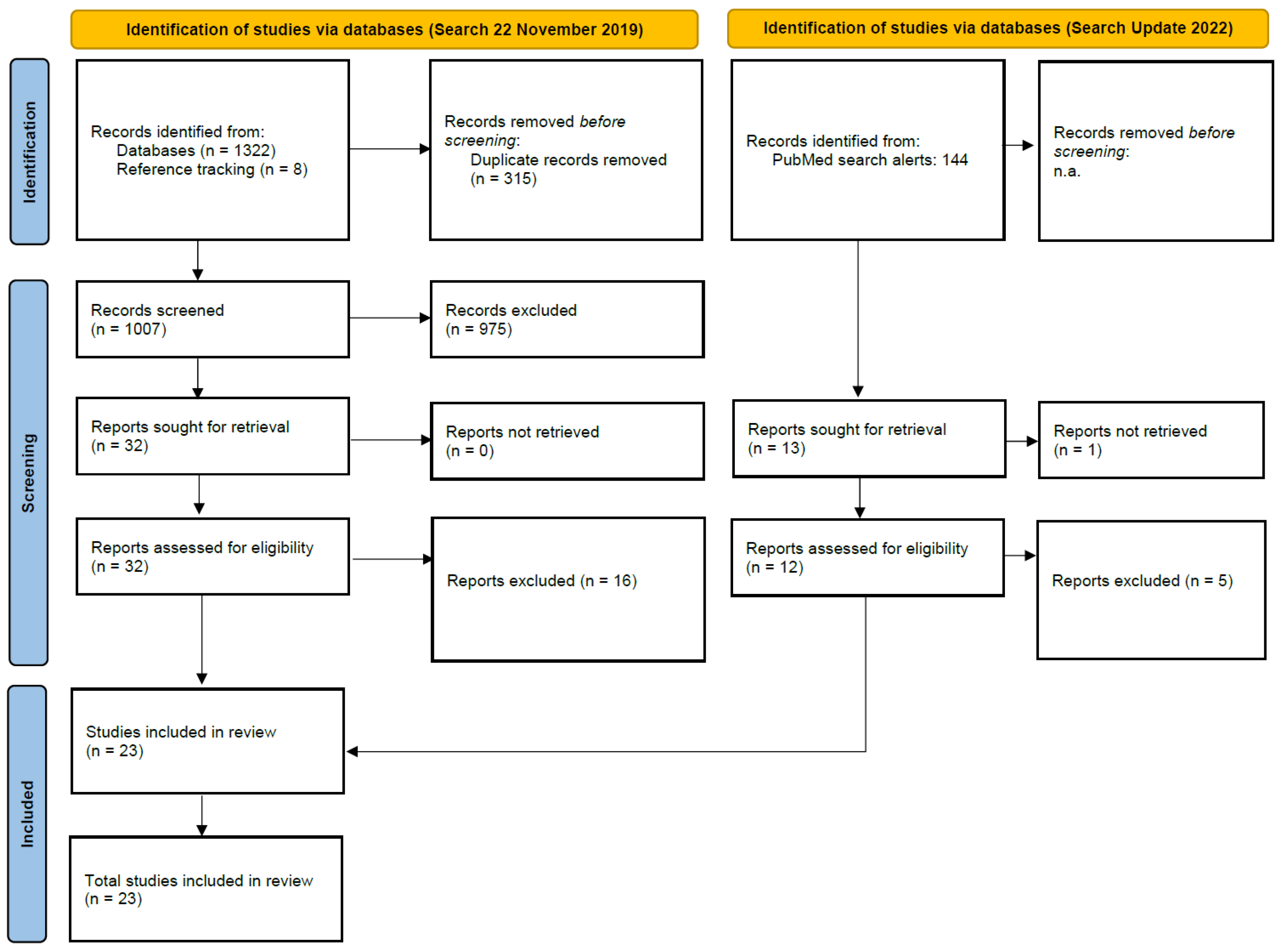
2.2. Search Strategy and Selection Criteria
2.3. Data Extraction and Analysis
3. Results
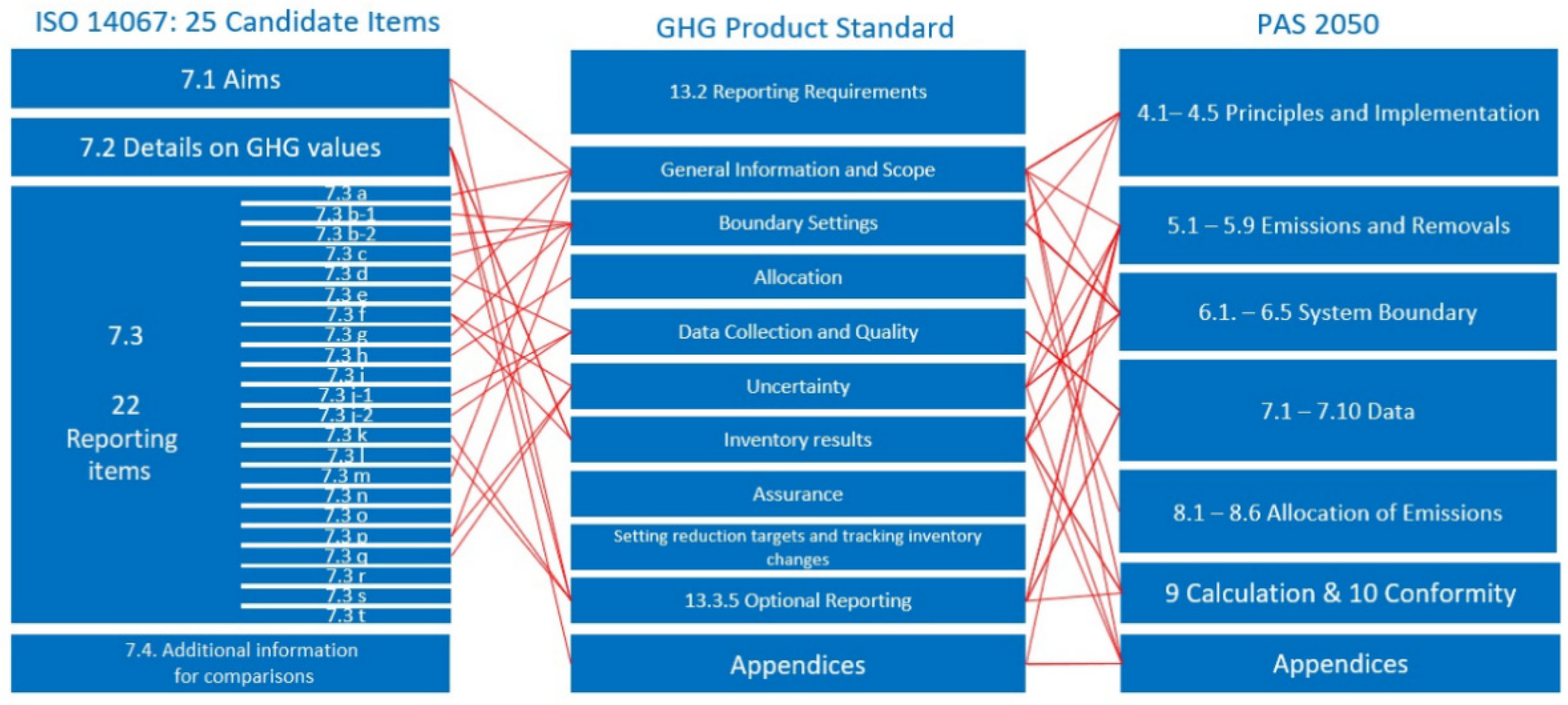
3.1. Development of Transparency Catalogue
3.2. Results of the Systematic Review
4. Discussion
4.1. Statement of Principal Findings
4.2. Limitations
4.3. Implications for Practice and Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harmer, A.; Eder, B.; Gepp, S.; Leetz, A.; van de Pas, R. WHO Should Declare Climate Change a Public Health Emergency. BMJ 2020, 368, m797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, J.D.; MacNeill, A.; Thiel, C. Reducing Pollution from the Health Care Industry. JAMA 2019, 322, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Haines, A.; Scheelbeek, P. The Health Case for Urgent Action on Climate Change. BMJ 2020, 368, m1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, J.; Hamilton, I.; Woodcock, J.; Williams, M.; Davies, M.; Wilkinson, P.; Haines, A. Health Benefits of Policies to Reduce Carbon Emissions. BMJ 2020, 368, l6758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straus, S.E.; Glasziou, P.; Richardson, W.S.; Haynes, R.B. Evidence-Based Medicine E-Book: How to Practice and Teach EBM; Elsevier Health Sciences: London, UK, 2018. [Google Scholar]
- EQUATOR Network. Enhancing the Quality and Transparency of Health Research. Available online: https://www.equator-network.org/ (accessed on 1 April 2022).
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. Value Health 2013, 16, e1–e5. [Google Scholar] [CrossRef] [Green Version]
- European Committee for Standardization. Greenhouse Gases-Carbon Footprint of Products-Requirements and Guidelines for Quantification (Iso 14067:2019); German and English Version EN ISO 14067; European Committee for Standardization: Brussels, Belgium, 2019. [Google Scholar]
- Gao, T.; Liu, Q.; Wang, J.P. A Comparative Study of Carbon Footprint and Assessment Standards. Int. J. Low Carbon Technol. 2014, 9, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Soode, E.; Weber-Blaschke, G.; Richter, K. Comparison of Product Carbon Footprint Standards with a Case Study on Poinsettia (Euphorbia Pulcherrima). Int. J. Life Cycle Assess. 2013, 18, 1280–1290. [Google Scholar] [CrossRef]
- Bathia, P.C.; Draucker, L.; Rich, D.; Lahd, H.; Brown, A. Greenhouse Gas Protocol. Product Life Cycle Accounting and Reporting Standard; World Resources Institute: Washington, DC, USA, 2011. [Google Scholar]
- Pas 2050:2011; Specification for the Assessment of the Life Cycle Greenhouse Gas Emissions of Goods and Services. BSI: Hong Kong, China, 2011.
- Rizan, C.; Steinbach, I.; Nicholson, R.; Lillywhite, R.; Reed, M.; Bhutta, M.F. The Carbon Footprint of Surgical Operations: A Systematic Review. Ann. Surg. 2020, 272, 986–995. [Google Scholar] [CrossRef]
- Drew, J.; Christie, S.D.; Tyedmers, P.; Smith-Forrester, J.; Rainham, D. Operating in a Climate Crisis: A State-of-the-Science Review of Life Cycle Assessment within Surgical and Anesthetic Care. Environ. Health Perspect. 2021, 129, 076001. [Google Scholar] [CrossRef]
- Weidema, B. Guidelines for Critical Review of Product LCA; SPOLD: Brussels, Belgium, 1997. [Google Scholar]
- Topol, E. The Topol Review: Preparing the Healthcare Workforce to Deliver the Digital Future; Health Education England: London, UK, 2019.
- Fatehi, F.; Samadbeik, M.; Kazemi, A. What Is Digital Health? Review of Definitions. Stud. Health Technol. Inform. 2020, 275, 67–71. [Google Scholar]
- Lowery, C. What Is Digital Health and What Do I Need to Know About It? Obstet. Gynecol. Clin. N. Am. 2020, 47, 215–225. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. White Paper Digital Transformation of Industries: Healthcare; World Economic Forum: Cologny, Switzerland, 2016. [Google Scholar]
- Galvin, R. The Ict/Electronics Question: Structural Change and the Rebound Effect. Ecol. Econ. 2015, 120, 23–31. [Google Scholar] [CrossRef]
- Court, V.; Sorrell, S. Digitalisation of Goods: A Systematic Review of the Determinants and Magnitude of the Impacts on Energy Consumption. Environ. Res. Lett. 2020, 15, 043001. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhou, D.Q.; Wang, Q.W.; Su, B. How Information and Communication Technology Drives Carbon Emissions: A Sector-Level Analysis for China. Energy Econ. 2019, 81, 380–392. [Google Scholar] [CrossRef]
- Purohit, A.; Smith, J.; Hibble, A. Does Telemedicine Reduce the Carbon Footprint of Healthcare? A Systematic Review. Future Healthc. J. 2021, 8, e85–e91. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Miah, S.; Dunford, C.; Edison, M.; Eldred-Evans, D.; Gan, C.; Shah, T.T.; Lunn, P.; Winkler, M.; Ahmed, H.U.; Gibbons, N.; et al. A Prospective Clinical, Cost and Environmental Analysis of a Clinician-Led Virtual Urology Clinic. Ann. R. Coll. Surg. Engl. 2019, 101, 30–34. [Google Scholar] [CrossRef]
- Whetten, J.; Montoya, J.; Yonas, H. Access to Better Health and Clear Skies: Telemedicine and Greenhouse Gas Reduction. Telemed. J. E Health 2019, 25, 960–965. [Google Scholar] [CrossRef]
- Holmner, A.; Ebi, K.L.; Lazuardi, L.; Nilsson, M. Carbon Footprint of Telemedicine Solutions—Unexplored Opportunity for Reducing Carbon Emissions in the Health Sector. PLoS ONE 2014, 9, e105040. [Google Scholar] [CrossRef] [Green Version]
- Connor, M.J.; Miah, S.; Edison, M.A.; Brittain, J.; Smith, M.K.; Hanna, M.; El-Husseiny, T.; Dasgupta, R. Clinical, Fiscal and Environmental Benefits of a Specialist-Led Virtual Ureteric Colic Clinic: A Prospective Study. BJU Int. 2019, 124, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, C.; Ferguson, J.; Ah-See, K.; Barr, C.; Lalla, K.; van der Pol, M.; McKenzie, L.; Wootton, R. Head and Neck Cancer Assessment by Flexible Endoscopy and Telemedicine. J. Telemed. Telecare 2009, 15, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Alaball, J.; Franch-Parella, J.; Lopez Segui, F.; Garcia Cuyas, F.; Mendioroz Pena, J. Impact of a Telemedicine Program on the Reduction in the Emission of Atmospheric Pollutants and Journeys by Road. Int. J. Environ. Res. Public Health 2019, 16, 4366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dullet, N.W.; Geraghty, E.M.; Kaufman, T.; Kissee, J.L.; King, J.; Dharmar, M.; Smith, A.C.; Marcin, J.P. Impact of a University-Based Outpatient Telemedicine Program on Time Savings, Travel Costs, and Environmental Pollutants. Value Health 2017, 20, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Paquette, S.; Lin, J.C. Outpatient Telemedicine Program in Vascular Surgery Reduces Patient Travel Time, Cost, and Environmental Pollutant Emissions. Ann. Vasc. Surg. 2019, 59, 167–172. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Barlow, J.; Goncalves, L.; Bayer, S. Teleconsultations Reduce Greenhouse Gas Emissions. J. Health Serv. Res. Policy 2013, 18, 209–214. [Google Scholar] [CrossRef]
- Andrew, N.; Barraclough, K.A.; Long, K.; Fazio, T.N.; Holt, S.; Kanhutu, K.; Hughes, P.D. Telehealth Model of Care for Routine Follow up of Renal Transplant Recipients in a Tertiary Centre: A Case Study. J. Telemed. Telecare 2020, 26, 232–238. [Google Scholar] [CrossRef]
- Smith, A.J.; Tennison, I.; Roberts, I.; Cairns, J.; Free, C. The Carbon Footprint of Behavioural Support Services for Smoking Cessation. Tob. Control 2013, 22, 302–307. [Google Scholar] [CrossRef]
- Masino, C.; Rubinstein, E.; Lem, L.; Purdy, B.; Rossos, P.G. The Impact of Telemedicine on Greenhouse Gas Emissions at an Academic Health Science Center in Canada. Telemed. J. E Health 2010, 16, 973–976. [Google Scholar] [CrossRef]
- Turley, M.; Porter, C.; Garrido, T.; Gerwig, K.; Young, S.; Radler, L.; Shaber, R. Use of Electronic Health Records Can Improve the Health Care Industry’s Environmental Footprint. Health Aff. 2011, 30, 938–946. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.; Tranter, G.; Axford, A.T. Use of Videoconferencing in Wales to Reduce Carbon Dioxide Emissions, Travel Costs and Time. J. Telemed. Telecare 2009, 15, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.; Mortimer, F.; Higgins, R. The Follow-up of Renal Transplant Recipients by Telephone Consultation: Three Years Experience from a Single UK Renal Unit. Clin Med. 2011, 11, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Wiljer, D.; Bender, J.L.; Masino, C.; Brierley, J. Alternative Methods for Sustainable Survivorship Care. In Proceedings of the Iadis International Conference E-Health, Lisbon, Portugal, 17–19 July 2012; pp. 237–241. [Google Scholar]
- Blenkinsop, S.; Foley, A.; Schneider, N.; Willis, J.; Fowler, H.J.; Sisodiya, S.M. Carbon Emission Savings and Short-Term Health Care Impacts from Telemedicine: An Evaluation in Epilepsy. Epilepsia 2021, 62, 2732–2740. [Google Scholar] [CrossRef]
- Bonsall, A. Unleashing Carbon Emissions Savings with Regular Teledermatology Clinics. Clin. Exp. Dermatol. 2021, 46, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Filfilan, A.; Anract, J.; Chartier-Kastler, E.; Parra, J.; Vaessen, C.; de La Taille, A.; Roupret, M.; Pinar, U. Positive Environmental Impact of Remote Teleconsultation in Urology During the Covid-19 Pandemic in a Highly Populated Area. Prog. Urol. 2021, 31, 1133–1138. [Google Scholar] [CrossRef]
- O’Connell, G.; O’Connor, C.; Murphy, M. Every Cloud Has a Silver Lining: The Environmental Benefit of Teledermatology During the Covid-19 Pandemic. Clin. Exp. Dermatol. 2021, 46, 1589–1590. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.R.; Kanhutu, K.; Sasadeusz, J.; Watkinson, S.; Biggs, B.A. Using Telehealth to Improve Access to Hepatitis C Treatment in the Direct-Acting Antiviral Therapy Era. J. Telemed. Telecare 2020, 26, 180–185. [Google Scholar] [CrossRef]
- Sellars, H.; Ramsay, G.; Sunny, A.; Gunner, C.K.; Oliphant, R.; Watson, A.J.M. Video Consultation for New Colorectal Patients. Colorectal. Dis. 2020, 22, 1015–1021. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Strohbehn, G.W.; Dedinsky, R.M.; Raupp, S.M.; Pannecouk, B.M.; Yentz, S.E.; Ramnath, N. Teleoncology for Veterans: High Patient Satisfaction Coupled with Positive Financial and Environmental Impacts. JCO Oncol. Pract. 2021, 17, e1362–e1374. [Google Scholar] [CrossRef]
- Rockstrom, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S., 3rd; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A Safe Operating Space for Humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef]

| Item | Assessment Question | How to Extract | |
|---|---|---|---|
| Aim | 1 | Does the study specify its aim, e.g., in terms of the product or service for which CF is assessed? | Assess whether the healthcare goods or services subject to the study are described; if yes, extract a brief description. In particular, extract the name of the product subject to the assessment; its comparator and the aim of comparison if applicable; and its predecessor and the aim of performance tracking if applicable. |
| 2a | Does the study specify the functional unit? | Assess whether a functional unit is specified; if yes, extract the functional unit. | |
| 2b | Assess whether no final use of the product is known and/or whether the study explicitly justifies the limitation of a partial carbon footprint; if yes, report ‘Partial CF’. | ||
| 3 | Does the study specify the reference flow? | Assess whether the reference flow is specified; if yes, extract information about the reference flow. | |
| 4 | Does the study provide a description of the life cycle stages? | Assess whether the life cycle phases of the product or service under investigation are explicitly addressed and described; if yes, extract stated life cycle phases. | |
| 5 | Does the study provide a list of important unit processes? | Assess whether a list of unit processes is provided; if yes, extract the list of unit processes. | |
| 6 | Does the study specify exclusions and reasons for exclusions? | Assess whether exclusions of unit processes or single energy or material flows are reported; if yes, extract a list of data exclusions. | |
| 7 | Does the study specify the system boundary? | Assess whether, in the methods section, the system boundary is specified and justified. If yes, extract information on the system boundary. | |
| Data | 8 | Does the study provide sources for all data used in the analysis? | Assess whether all data sources are provided (these may include primary and secondary data); if yes, extract all data sources. |
| 9 | Does the study assess the temporal representativeness of the data? | Assess whether the data year or other details about the period for which the data are relevant are reported; if yes, extract exemplary information on temporal representativeness. | |
| 10 | Does the study assess the geographical representativeness of the data? | Assess whether information about the geographical region to which the data apply is reported; if yes, extract exemplary information on geographical representativeness. | |
| 11 | Does the study assess the technological representativeness of the data? | Assess whether information about the technology for which the data are relevant is reported; if yes, extract exemplary information on technology coverage. | |
| 12 | Does the study assess the completeness of the data? | Assess whether information about the completeness is provided; if yes, extract this information. | |
| Analysis | 13 | Does the study estimate CF in terms of CO2e? | Assess whether an outcome is specified in terms of CO2e [Ref. ISO 14067:2018, 7.2]; if yes, state ‘yes’. |
| 14a | Does the study provide a list of GHGs taken into account? | Assess whether a list of GHGs taken into account is provided [Ref. ISO 14067:2018, 7.3 e)]; if yes, extract included GHGs. | |
| 14b | If CO2 is analysed only, has it been justified as to why this is the only relevant GHG? If yes, extract the justification on which it was based (see also item 5) | ||
| 15a | Does the study specify the selected characterisation factors? | Assess whether characterisation factors are reported; if yes, extract the source of the values. | |
| 15b | If CO2 is analysed only, has it been justified why this is the only relevant GHG? If yes, extract justification it was based on (see also items 5 and 12b). | ||
| 16a | Does the study report the selected allocation procedures? | Assess whether allocation procedures are addressed. If yes, extract shared processes and allocation procedures. | |
| 16b | If no allocation is addressed, was it why allocation is not relevant to the study justified? If yes, extract justification (see also item 5). | ||
| Results | 17 | Does the study report the outcomes per unit of analysis? | Assess whether data on CF per unit of analysis is provided; if yes, extract the figure and the unit of analysis. |
| 18 | Does the study report CF separately per specific component? | Assess whether CF is reported separately per component; if yes, extract component and CFP per component (which may include that some components of emissions or removals amount to zero). | |
| 19 | Does the study report CF according to life cycle phases? | Assess whether total GHG emissions are differentiated by life cycle phases; if yes, extract data. | |
| 20 | Does the study report a qualitative statement on the influence of key uncertainties or methodological choices on the result? | Assess whether the impact of at least one uncertainty or methodological assumption on results is reported; if yes, extract the most influential ones. | |
| 21 | Does the study perform a quantitative sensitivity analysis? | Assess whether quantitative sensitivity analyses are reported; if yes, extract type of sensitivity analysis (e.g., one-way or two-way sensitivity analysis, tornado diagram, probabilistic analysis). | |
| 22 | Does the study critically discuss limitations, e.g., appropriateness of system boundary, data quality, or methods of analysis? | Assess whether limitations of the CF study are critically discussed; if yes, extract exemplary reported limitations. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, O.; Plath, J.; Dziggel, T.F.; Karpa, D.F.; Keil, M.; Becker, T.; Rogowski, W.H. A Transparency Checklist for Carbon Footprint Calculations Applied within a Systematic Review of Virtual Care Interventions. Int. J. Environ. Res. Public Health 2022, 19, 7474. https://doi.org/10.3390/ijerph19127474
Lange O, Plath J, Dziggel TF, Karpa DF, Keil M, Becker T, Rogowski WH. A Transparency Checklist for Carbon Footprint Calculations Applied within a Systematic Review of Virtual Care Interventions. International Journal of Environmental Research and Public Health. 2022; 19(12):7474. https://doi.org/10.3390/ijerph19127474
Chicago/Turabian StyleLange, Oliver, Julian Plath, Timo F. Dziggel, David F. Karpa, Mattis Keil, Tom Becker, and Wolf H. Rogowski. 2022. "A Transparency Checklist for Carbon Footprint Calculations Applied within a Systematic Review of Virtual Care Interventions" International Journal of Environmental Research and Public Health 19, no. 12: 7474. https://doi.org/10.3390/ijerph19127474
APA StyleLange, O., Plath, J., Dziggel, T. F., Karpa, D. F., Keil, M., Becker, T., & Rogowski, W. H. (2022). A Transparency Checklist for Carbon Footprint Calculations Applied within a Systematic Review of Virtual Care Interventions. International Journal of Environmental Research and Public Health, 19(12), 7474. https://doi.org/10.3390/ijerph19127474






