Management for Caries Prevention in ADHD Children
1. Introduction
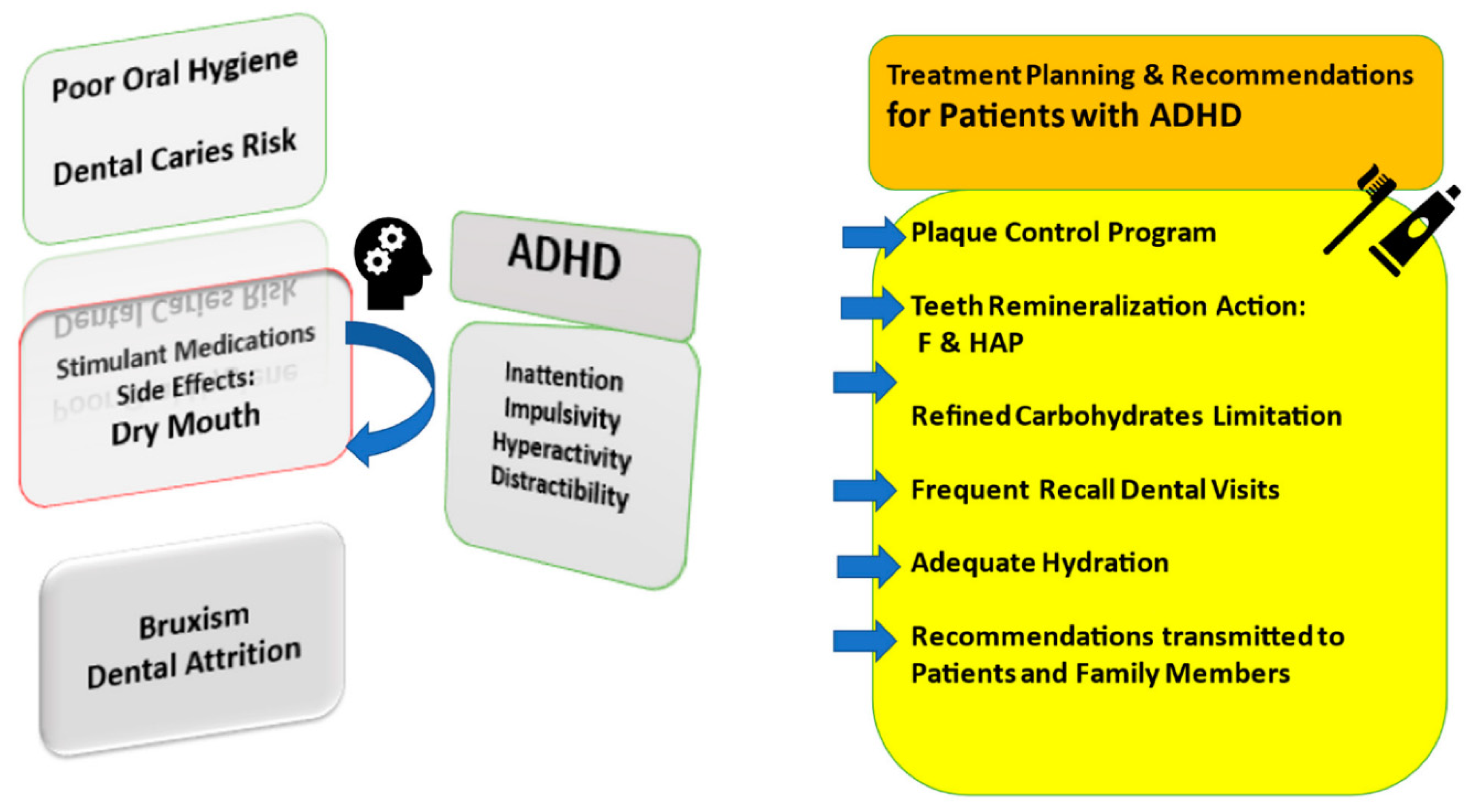
1.1. The Incidence of Carious Lesions among ADHD Children
1.2. Evaluation of Caries Prevention among ADHD Children
2. Conclusions
- Clinical data of ADHD trials suggest that effective prevention may depend on level of caries risk, patient’s cooperation and therapeutic management;
- Early strategies for individual patient cases and choices of remineralization products are needed to defend the oral health of children with ADHD against dental caries;
- All abovementioned preventive directions may be applied, and longer follow-up periods are suggested to evaluate the clinical approach in oral health among ADHD patients.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Polanczyk, G.V.; Willcutt, E.G.; Salum, G.A.; Kieling, C.; Rohde, L.A. ADHD Prevalence Estimates across Three Decades: An Updated Systematic Review and Meta-Regression Analysis. Int. J. Epidemiol. 2014, 43, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Enax, J.; Amaechi, B.T.; Limeback, H.; Fabritius, H.-O.; Ganss, B.; Pawinska, M.; Paszynska, E. Hydroxyapatite as Remineralization Agent for Children’s Dental Care. Front. Dent. Med. 2022, 3. [Google Scholar] [CrossRef]
- Broadbent, J.; Ayers, K.; Thomson, W. Is Attention-Deficit Hyperactivity Disorder a Risk Factor for Dental Caries? Caries Res. 2004, 38, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, M.; Holmberg, K.; Fernell, E.; Ek, U.; Dahllöf, G. Oral Health, Dental Anxiety, and Behavior Management Problems in Children with Attention Deficit Hyperactivity Disorder. Eur. J. Oral Sci. 2006, 114, 385–390. [Google Scholar] [CrossRef]
- Blomqvist, M.; Ahadi, S.; Fernell, E.; Ek, U.; Dahllöf, G. Dental Caries in Adolescents with Attention Deficit Hyperactivity Disorder: A Population-based Follow-up Study. Eur. J. Oral Sci. 2011, 119, 381–385. [Google Scholar] [CrossRef]
- Paszynska, E.; Dmitrzak-Weglarz, M.; Perczak, A.; Gawriolek, M.; Hanć, T.; Bryl, E.; Mamrot, P.; Dutkiewicz, A.; Roszak, M.; Tyszkiewicz-Nwafor, M.; et al. Excessive weight gain and dental caries experience among children affected by ADHD. Int. J. Environ. Res. Public Health 2020, 17, 5870. [Google Scholar] [CrossRef]
- Blomqvist, M.; Holmberg, K.; Lindblad, F.; Fernell, E.; Ek, U.; Dahllöf, G. Salivary cortisol levels and dental anxiety in children with attention deficit hyperactivity disorder. Eur. J. Oral Sci. 2007, 115, 1–6. [Google Scholar] [CrossRef]
- Begnini, G.J.; Brancher, J.A.; Guimarães, A.T.; de Araujo, M.R.; Pizzatto, E. Oral Health of Children and Adolescents with Attention Deficit Hyperactivity Disorder. Int. J. Clin. Pediatr. Dent. 2019, 12, 543–547. [Google Scholar] [CrossRef]
- Ehlers, V.; Callaway, A.; Wantzen, S.; Patyna, M.; Deschner, J.; Azrak, B. Oral health of children and adolescents with or without attention deficit hyperactivity disorder (ADHD) living in residential care in rural Rhineland-Palatinate, Germany. BMC Oral Health 2019, 19, 258. [Google Scholar] [CrossRef]
- Bimstein, E.; Wilson, J.; Guelmann, M.; Primosch, R. Oral characteristics of children with attention-deficit hyperactivity disorder. Spec. Care Dent. 2008, 28, 107–110. [Google Scholar] [CrossRef]
- Chandra, P.; Anandakrishna, L.; Ray, P. Caries Experience and Oral Hygiene Status of Children Suffering from Attention Deficit Hyperactivity Disorder. J. Clin. Pediatric Dent. 2009, 34, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Hidas, A.; Noy, A.F.; Birman, N.; Shapira, J.; Matot, I.; Steinberg, D.; Moskovitz, M. Oral Health Status, Salivary Flow Rate and Salivary Quality in Children, Adolescents and Young Adults with ADHD. Arch. Oral Biol. 2011, 56, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Hidas, A.; Birman, N.; Noy, A.F.; Shapira, J.; Matot, I.; Steinberg, D.; Moskovitz, M. Salivary bacteria and oral health status in medicated and non-medicated children and adolescents with attention deficit hyperactivity disorder (ADHD). Clin. Oral. Investig. 2013, 17, 1863–1867. [Google Scholar] [CrossRef]
- Chau, Y.C.; Peng, S.-M.; McGrath, C.P.; Yiu, C.K. Oral Health of Children with Attention Deficit Hyperactivity Disorder: Systematic Review and Meta-Analysis. J. Atten. Disord. 2020, 24, 947–962. [Google Scholar] [CrossRef]
- Rosenberg, S.S.; Kumar, S.; Williams, N.J. Attention Deficit/Hyperactivity Disorder Medication and Dental Caries in Children. Am. Dent. Hyg. Assoc. 2014, 88, 342–347. [Google Scholar]
- Blumer, S.; Khoury, R.S.; Peretz, B. The Prevalence of ADHD Patients among Pediatric Dentists in Israel and Knowledge of Dental and Behavioral Aspects of Treating Them. J. Clin. Pediatric Dent. 2018, 42, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, A.; Vafaei, I.; Noorazar, G.; Akbarzadeh, R.; Erfanparast, L.; Shirazi, S. Comparison of the Effect of Pharmacotherapy and Neuro-Feedback Therapy on Oral Health of Children with Attention Deficit Hyperactivity Disorder. J. Clin. Exp. Dent. 2018, 10, e306. [Google Scholar] [CrossRef][Green Version]
- Friedlander, A.H.; Friedlander, I. Dental Management Considerations in Children with Attention-Deficit Hyperactivity Disorder. ASDC J. Dent. Child. 1992, 59, 196–201. [Google Scholar]
- Walker, J.E.; Kozlowski, G.P. Neurofeedback Treatment of Epilepsy. Child Adolesc. Psychiatr. Clin. 2005, 14, 163–176. [Google Scholar] [CrossRef]
- Choudhry, Z.; Sengupta, S.M.; Grizenko, N.; Harvey, W.J.; Fortier, M.-E.; Schmitz, N.; Joober, R. Body Weight and ADHD: Examining the Role of Self-Regulation. PLoS ONE 2013, 8, e55351. [Google Scholar] [CrossRef]
- Hanć, T.; Słopień, A.; Wolańczyk, T.; Dmitrzak-Węglarz, M.; Szwed, A.; Czapla, Z.; Durda, M.; Ratajczak, J.; Cieślik, J. ADHD and Overweight in Boys: Cross-Sectional Study with Birth Weight as a Controlled Factor. Eur. Child Adolesc. Psychiatry 2015, 24, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Kuja-Halkola, R.; Martin, J.; Lu, Y.; Lichtenstein, P.; Norring, C.; Birgegård, A.; Yilmaz, Z.; Hübel, C.; Watson, H. Associations between Attention-Deficit/Hyperactivity Disorder and Various Eating Disorders: A Swedish Nationwide Population Study Using Multiple Genetically Informative Approaches. Biol. Psychiatry 2019, 86, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Giel, K.E.; Teufel, M.; Junne, F.; Zipfel, S.; Schag, K. Food-Related Impulsivity in Obesity and Binge Eating Disorder-A Systematic Update of the Evidence. Nutrients 2017, 9, 1170. [Google Scholar] [CrossRef]
- Carvalho, T.S.; Colon, P.; Ganss, C.; Huysmans, M.C.; Lussi, A.; Schlueter, N.; Schmalz, G.; Shellis, R.P.; Tveit, A.B.; Wiegand, A. Consensus Report of the European Federation of Conservative Dentistry: Erosive Tooth Wear—Diagnosis and Management. Clin. Oral Investig. 2015, 19, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Grohe, B.; Mittler, S. Advanced Non-Fluoride Approaches to Dental Enamel Remineralization: The next Level in Enamel Repair Management. Biomater. Biosyst. 2021, 4, 100029. [Google Scholar] [CrossRef]
- Ehlers, V.; Reuter, A.; Kehl, E.-B.; Enax, J.; Meyer, F.; Schlecht, J.; Schmidtmann, I.; Deschner, J. Efficacy of a Toothpaste Based on Microcrystalline Hydroxyapatite on Children with Hypersensitivity Caused by MIH: A Randomised Controlled Trial. Oral Health Prev. Dent. 2021, 19, 647–658. [Google Scholar] [CrossRef]
- Limeback, H.; Enax, J.; Meyer, F. Biomimetic Hydroxyapatite and Caries Prevention: A Systematic Review and Meta-Analysis. Can. J. Dent. Hyg. 2021, 55, 148–159. [Google Scholar]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U.; et al. Impact of a Toothpaste with Microcrystalline Hydroxyapatite on the Occurrence of Early Childhood Caries: A 1-Year Randomized Clinical Trial. Sci. Rep. 2021, 11, 2650. [Google Scholar] [CrossRef]
| Authors of Clinical Studies Country | Age Range Min-Max or Mean [years] | Number of ADHD Subjects | Control Group +Yes −No | Oral Examination Methodology | Significant Results for ADHD Group |
|---|---|---|---|---|---|
| Significant Conclusions | |||||
| Broadbent et al., 2004 [3] New Zeland | 11–14 | 64 | + | analysis of dental service records | higher caries experience (odds of 12 times) |
| ADHD condition may affect children’s dental caries experience | |||||
| Bimstein et al., 2008 [10] USA | 7.4 | 25 | + | analysis of dental service records | higher prevalence of toothache, bruxism, bleeding gums and oral trauma histories recorded; no differences in plaque accumulation, gingival inflammation, calculus, oral hygiene level, dental caries treatment |
| ADHD condition may affect children’s oral health | |||||
| Blomqvist et al., 2006 [4] Sweden | 11 | 25 | + | clinical dental examination, bitewing radiographs, parents’ questionnaire interview | higher caries prevalence, not significant degree of dental anxiety, but differences in behavioral management |
| ADHD children desire an intensive oral health control | |||||
| Blomqvist et al., 2007 [7] Sweden | 13 | 21 | + | clinical dental examination, parents’ questionnaire interview | no significant caries experience, poorer oral health behaviors |
| ADHD condition indicates for shorter intervals between dental examinations | |||||
| Blomqvist et al., 2011 [5] Sweden | 17 | 32 | + | clinical and radiographic dental examinations | higher caries prevalence and gingival inflammation |
| ADHD adolescents desire an intensive oral health control | |||||
| Chandra et al., 2009 [11] India | 8.9 | 40 | + | clinical dental examinations, parents’ questionnaire interview | significant caries in primary dentition, poorer oral hygiene and sweetened consumption control |
| ADHD children desire an intensive oral health control | |||||
| Hidas et al., 2011, 2013 [12,13] Israel | ADHD non-medicated 10.3 ADHD medicated 11.8 | 31 non-medicated 30 medicated ADHD patients | + | clinical dental examination, plaque index, oral mucosa pH and unstimulated whole salivary flow (USF), parents’ questionnaire interview | in both ADHD groups, no differences in caries incidence, diet/hygiene habits, significant lower USF and higher dental plaque |
| ADHD condition may be a factor contributing to caries in older age | |||||
| Chau et al., 2016 [14] Honk Kong China | 12–18 | 31 | + | intraoral dental/periodontal, salivary function, tooth wear examination, parents’ questionnaire interview | no significant differences between children, with or without ADHD, in dental caries, trauma prevalence, periodontal disease, plaque, tooth wear or USF significant difference in gingival bleeding, oral hygiene habits, higher attendance at dental clinic |
| poorer oral hygiene, more adverse oral-health attitudes | |||||
| Begnini et al., 2019 [8] Italy | 7–14 | 51 | + | intraoral dental/gingival examination, parents’ questionnaire interview | no differences in dental caries, although visible plaque, gingival bleeding were detected |
| ADHD children need supervision on oral health | |||||
| Ehlers et al., 2019 [9] Germany | 9–15 | 34 | + | intraoral dental/gingival, parents questionnaire interview | no differences in oral health; however, higher indices in secondary dentition |
| parents/guardians need instructions for better supervision of oral hygiene and dietary habits | |||||
| Paszynska et al., 2020 [6] Poland | 8.2 | 39 | + | physical measurements, clinical dental examination, parents’ questionnaire interview | significant prevalence of abnormal body weight, hip circumference, BMI, caries differences for primary/permanent teeth, primary tooth decay was corelated to sweet consumption |
| limiting sugar consumption might be one of preventive point against dental caries and overweight/obesity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszynska, E.; Krahel, A.; Pawinska, M.; Dmitrzak-Węglarz, M.; Perczak, A.; Słopień, A.; Gawriolek, M. Management for Caries Prevention in ADHD Children. Int. J. Environ. Res. Public Health 2022, 19, 7455. https://doi.org/10.3390/ijerph19127455
Paszynska E, Krahel A, Pawinska M, Dmitrzak-Węglarz M, Perczak A, Słopień A, Gawriolek M. Management for Caries Prevention in ADHD Children. International Journal of Environmental Research and Public Health. 2022; 19(12):7455. https://doi.org/10.3390/ijerph19127455
Chicago/Turabian StylePaszynska, Elzbieta, Anna Krahel, Malgorzata Pawinska, Monika Dmitrzak-Węglarz, Aleksandra Perczak, Agnieszka Słopień, and Maria Gawriolek. 2022. "Management for Caries Prevention in ADHD Children" International Journal of Environmental Research and Public Health 19, no. 12: 7455. https://doi.org/10.3390/ijerph19127455
APA StylePaszynska, E., Krahel, A., Pawinska, M., Dmitrzak-Węglarz, M., Perczak, A., Słopień, A., & Gawriolek, M. (2022). Management for Caries Prevention in ADHD Children. International Journal of Environmental Research and Public Health, 19(12), 7455. https://doi.org/10.3390/ijerph19127455






