Dust Storms Increase the Risk of Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
2.2. Methods
3. Results
3.1. Descriptive Statistics
3.1.1. Outpatient Care
3.1.2. Inpatient Care
3.2. Regression Results
3.2.1. Outpatient Care
3.2.2. Inpatient Care
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Serener, A.; Serte, S. Dry and Wet Age-Related Macular Degeneration Classification Using Oct Images and Deep Learning. In Proceedings of the 2019 Scientific Meeting on Electrical-Electronics & Biomedical Engineering and Computer Science (EBBT), Istanbul, Turkey, 24–26 April 2019; pp. 1–4. [Google Scholar]
- Kumar-Singh, R. The role of complement membrane attack complex in dry and wet AMD—From hypothesis to clinical trials. Exp. Eye Res. 2019, 184, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.C.; Wu, M.M.; Wu, C.M.; Sung, F.C.; Muo, C.H.; Te, A.; Su, F.H. Increased Risk of Age-Related Macular Degeneration with Chronic Hepatitis C Virus Infection: A Nationwide Population-Based Propensity Score-Matched Cohort Study in Taiwan. Viruses 2021, 13, 790. [Google Scholar] [CrossRef]
- He, M.S.; Chang, F.L.; Lin, H.Z.; Wu, J.L.; Hsieh, T.C.; Lee, Y.C. The association between diabetes and age-related macular degeneration among the elderly in Taiwan. Diabetes Care 2018, 41, 2202–2211. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.D.; Lee, C.Z.; Kao, L.T.; Lin, H.C.; Tsai, M.C.; Sheu, J.J. Association between neovascular age-related macular degeneration and dementia: A population-based case-control study in Taiwan. PLoS ONE 2015, 10, e0120003. [Google Scholar] [CrossRef]
- Chang, K.H.; Hsu, P.Y.; Lin, C.J.; Lin, C.L.; Juo, S.H.H.; Liang, C.L. Traffic-related air pollutants increase the risk for age-related macular degeneration. J. Investig. Med. 2019, 67, 1076–1081. [Google Scholar] [CrossRef]
- Chua, S.Y.; Warwick, A.; Peto, T.; Balaskas, K.; Moore, A.T.; Reisman, C.; Patel, P.J. Association of ambient air pollution with age-related macular degeneration and retinal thickness in UK Biobank. Br. J. Ophthalmol. 2021, 106, 705–711. [Google Scholar] [CrossRef]
- Ju, M.J.; Kim, J.; Park, S.K.; Kim, D.H.; Choi, Y.H. Long-term exposure to ambient air pollutants and age-related macular degeneration in middle-aged and older adults. Environ. Res. 2022, 204, 111953. [Google Scholar] [CrossRef]
- Liu, T.C.; Tang, H.H.; Lei, S.Y.; Peng, Y.I. Asian dust storms result in a higher risk of the silicosis hospital admissions. J. Environ. Health Sci. Eng. 2022, 20, 305–314. [Google Scholar] [CrossRef]
- Teng, J.C.Y.; Chan, Y.S.; Peng, Y.I.; Liu, T.C. Influence of Asian dust storms on daily acute myocardial infarction hospital admissions. Public Health Nurs. 2016, 33, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chen, C.S.; Lin, C.L. The threat of Asian dust storms on asthma patients: A population-based study in Taiwan. Glob. Public Health 2014, 9, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.M.; Pouran, H.; Majbouri, M.; Moradi-Lakeh, M.; Hakimian, H. Saharan sand and dust storms and neonatal mortality: Evidence from Burkina Faso. Sci. Total Environ. 2020, 729, 139053. [Google Scholar] [CrossRef]
- Cai, Q.L.; Dai, X.R.; Li, J.R.; Tong, L.; Hui, Y.; Cao, M.Y.; Xiao, H. The characteristics and mixing states of PM2. 5 during a winter dust storm in Ningbo of the Yangtze River Delta, China. Sci. Total Environ. 2020, 709, 136146. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Naddafi, K.; Hassanvand, M.S.; Nabizadeh, R.; Yunesian, M.; Ghanbarian, M.; Atafar, Z.; Faraji, M.; Nazmara, S.; Mahmoudi, B.; et al. Physiochemical characteristics and oxidative potential of ambient air particulate matter (PM10) during dust and non-dust storm events: A case study in Tehran, Iran. J. Environ. Health Sci. Eng. 2018, 16, 147–158. [Google Scholar] [CrossRef]
- Faraji, M.; Pourpak, Z.; Naddafi, K.; Nodehi, R.N.; Nicknam, M.H.; Shamsipour, M.; Mesdaghinia, A. Chemical composition of PM10 and its effect on in vitro hemolysis of human red blood cells (RBCs): A comparison study during dust storm and inversion. J. Environ. Health Sci. Eng. 2019, 17, 493–502. [Google Scholar] [CrossRef]
- Kang, J.H.; Keller, J.J.; Chen, C.S.; Lin, H.C. Asian dust storm events are associated with an acute increase in pneumonia hospitalization. Ann. Epidemiol. 2012, 22, 257–263. [Google Scholar] [CrossRef]
- Chen, C.S.; Chan, Y.S.; Liu, T.C. Tracheitis hospital admissions are associated with Asia dust storm. Int. J. Environ. Health Res. 2022, 32, 1337–1343. [Google Scholar] [CrossRef]
- Liu, S.T.; Liao, C.Y.; Kuo, C.Y.; Kuo, H.W. The effects of PM2. 5 from Asian dust storms on emergency room visits for cardiovascular and respiratory diseases. Int. J. Environ. Res. Public Health 2017, 14, 428. [Google Scholar] [CrossRef] [Green Version]
- Chien, L.C.; Lien, Y.J.; Yang, C.H.; Yu, H.L. Acute increase of children’s conjunctivitis clinic visits by Asian dust storms exposure-A spatiotemporal study in Taipei, Taiwan. PLoS ONE 2014, 9, e109175. [Google Scholar]
- Mu, H.; Battsetseg, B.; Ito, T.Y.; Otani, S.; Onishi, K.; Kurozawa, Y. International perspectives: Health effects of dust storms: Subjective eye and respiratory system symptoms in inhabitants in Mongolia. J. Environ. Health 2011, 73, 18–21. [Google Scholar] [PubMed]
- Lee, P.P.; Feldman, Z.W.; Ostermann, J.; Brown, D.S.; Sloan, F.A. Longitudinal prevalence of major eye diseases. Arch. Ophthalmol. 2003, 121, 1303–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. 2021, 105, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Brannas, K. Explanatory variables in the AR (1) count data model. Umea Econ. Stud. 1995, 381, 1–22. [Google Scholar]
- Brännäs, K.; Hellström, J. Generalized integer-valued autoregression. Econom. Rev. 2001, 20, 425–443. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Zwiener, I. Prevalence of age-related macular degeneration in Europe: The past and the future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Jonasson, F.; Fisher, D.E.; Eiriksdottir, G.; Sigurdsson, S.; Klein, R.; Launer, L.J.; Cotch, M.F. Five-year incidence, progression, and risk factors for age-related macular degeneration: The age, gene/environment susceptibility study. Ophthalmology 2014, 121, 1766–1772. [Google Scholar] [CrossRef] [Green Version]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Kaufman, J.D. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Lee, H.; Kim, H. Association between exposure to ambient air pollution and age-related cataract: A nationwide population-based retrospective cohort study. Int. J. Environ. Res. Public Health 2020, 17, 9231. [Google Scholar] [CrossRef]
- Rudnicka, A.R.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.; Owen, C.G. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: A meta-analysis. Ophthalmology 2012, 119, 571–580. [Google Scholar] [CrossRef]
- Huang, E.J.; Wu, S.H.; Lai, C.H.; Kuo, C.N.; Wu, P.L.; Chen, C.L.; Wu, P.C. Prevalence and risk factors for age-related macular degeneration in the elderly Chinese population in south-western Taiwan: The Puzih eye study. Eye 2014, 28, 705–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, L.K.; Eaton, A. Age-related macular degeneration. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
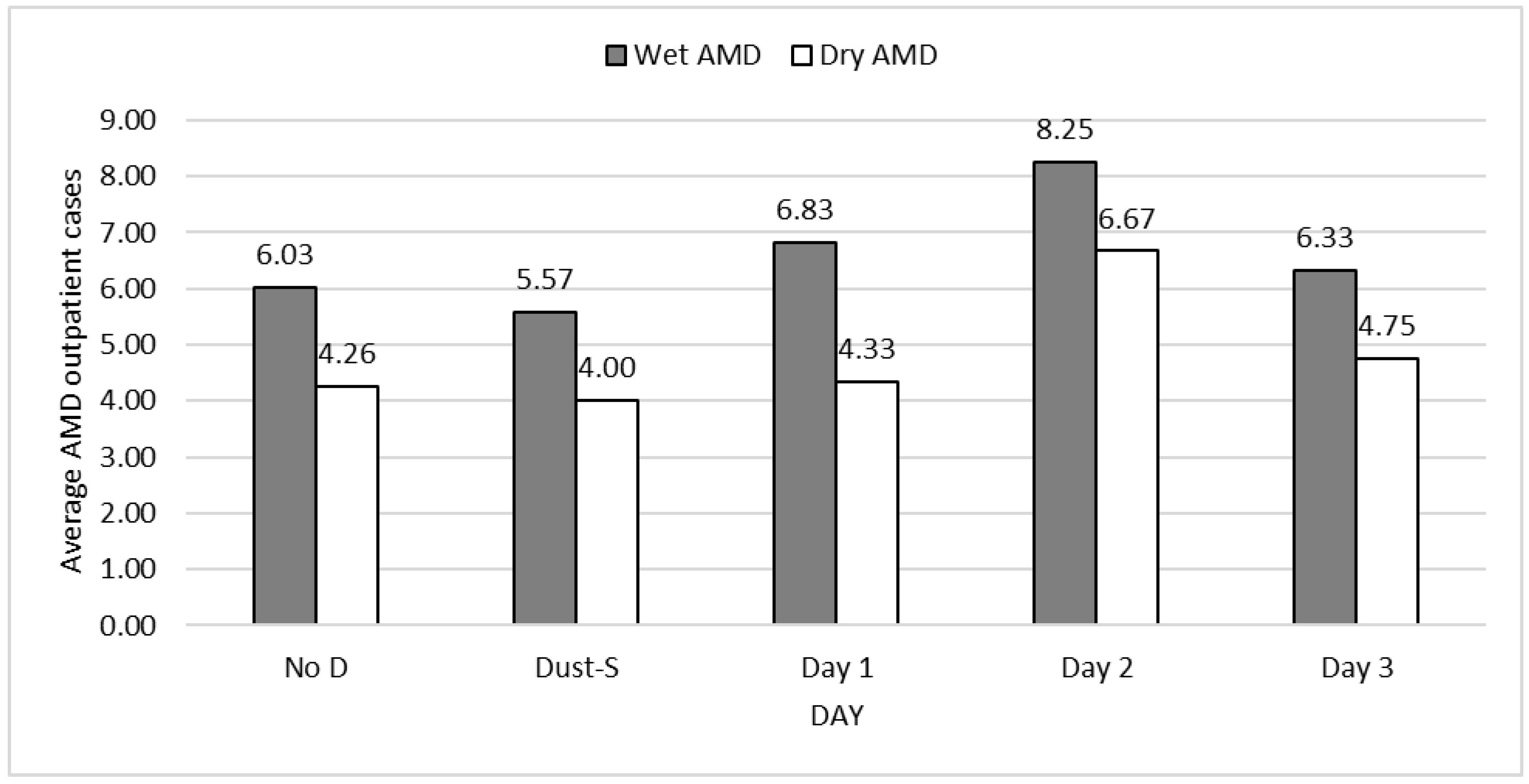

| No | Day 0 | Day 1 | Day 2 | Day 3 | Obs. | |
|---|---|---|---|---|---|---|
| Wet AMD | ||||||
| Total | 6.03 | 5.57 | 6.83 | 8.25 | 6.33 | 11,043 |
| Male | 3.59 | 3.35 | 3.50 | 5.08 | 3.50 | 6567 |
| Female | 2.44 | 2.22 | 3.33 | 3.17 | 2.83 | 4476 |
| ≤50 | 0.57 | 0.61 | 0.33 | 0.67 | 0.50 | 1046 |
| 51–60 | 1.04 | 0.96 | 1.33 | 1.42 | 1.58 | 1921 |
| 61–70 | 1.49 | 1.22 | 1.25 | 1.92 | 1.33 | 2714 |
| 71–80 | 1.59 | 1.57 | 1.75 | 2.50 | 2.25 | 2933 |
| ≥81 | 1.33 | 1.22 | 2.17 | 1.75 | 0.67 | 2429 |
| Dry AMD | ||||||
| Total | 4.26 | 4.00 | 4.33 | 6.67 | 4.75 | 7812 |
| Male | 2.50 | 2.43 | 2.33 | 4.00 | 3.17 | 4587 |
| Female | 1.76 | 1.57 | 2.00 | 2.67 | 1.58 | 3225 |
| ≤50 | 0.15 | 0.13 | 0.25 | 0.42 | 0.17 | 287 |
| 51–60 | 0.36 | 0.30 | 0.33 | 0.75 | 0.17 | 662 |
| 61–70 | 0.79 | 0.52 | 0.67 | 0.83 | 1.50 | 1447 |
| 71–80 | 1.42 | 1.70 | 1.25 | 2.50 | 1.25 | 2618 |
| ≥81 | 1.53 | 1.35 | 1.83 | 2.17 | 1.67 | 2798 |
| No | Day 0 | Day 1 | Day 2 | Day 3 | Obs. | |
|---|---|---|---|---|---|---|
| Wet AMD | ||||||
| Total | 0.26 | 0.22 | 0.58 | 0.42 | 0.25 | 471 |
| Male | 0.15 | 0.04 | 0.33 | 0.08 | 0.25 | 279 |
| Female | 0.10 | 0.17 | 0.25 | 0.33 | 0.00 | 192 |
| ≤50 | 0.03 | 0.00 | 0.00 | 0.08 | 0.00 | 50 |
| 51–60 | 0.05 | 0.00 | 0.25 | 0.08 | 0.08 | 92 |
| 61–70 | 0.08 | 0.17 | 0.08 | 0.17 | 0.00 | 152 |
| 71–80 | 0.06 | 0.04 | 0.17 | 0.08 | 0.08 | 107 |
| ≥81 | 0.04 | 0.00 | 0.08 | 0.00 | 0.08 | 70 |
| Dry AMD | ||||||
| Total | 0.33 | 0.30 | 0.75 | 0.33 | 0.17 | 609 |
| Male | 0.22 | 0.22 | 0.50 | 0.25 | 0.08 | 399 |
| Female | 0.11 | 0.09 | 0.25 | 0.08 | 0.08 | 210 |
| ≤50 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 7 |
| 51–60 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 22 |
| 61–70 | 0.05 | 0.04 | 0.08 | 0.00 | 0.08 | 83 |
| 71–80 | 0.12 | 0.09 | 0.25 | 0.17 | 0.00 | 226 |
| ≥81 | 0.15 | 0.13 | 0.42 | 0.17 | 0.08 | 271 |
| Total | Male | Female | ≤50 | 51–60 | 61–70 | 71–80 | ≥81 | |
|---|---|---|---|---|---|---|---|---|
| Intercept | 0.0980 | −0.0982 | −1.2900 *** | −0.3380 | −2.0440 *** | −1.5910 *** | −1.0490 ** | −1.8150 *** |
| (0.1720) | (0.2140) | (0.2560) | (0.5130) | (0.3760) | (0.3050) | (0.3270) | (0.3460) | |
| Day of DS | 0.0650 | 0.0968 | 0.0132 | 0.1220 | 0.0515 | −0.0408 | 0.2130 | −0.0027 |
| (0.0971) | (0.1200) | (0.1460) | (0.2760) | (0.2140) | (0.1820) | (0.1810) | (0.1940) | |
| 1 day post DS | 0.1820 | 0.0144 | 0.3770 * | −0.4520 | 0.2790 | −0.2330 | 0.2470 | 0.5290 * |
| (0.1260) | (0.1690) | (0.1720) | (0.5340) | (0.2600) | (0.2550) | (0.2500) | (0.2120) | |
| 2 days post DS | 0.1600 | 0.1730 | 0.1120 | 0.2450 | 0.1260 | 0.0592 | 0.3260 | 0.0821 |
| (0.1140) | (0.1410) | (0.1730) | (0.378) | (0.2520) | (0.2060) | (0.2040) | (0.2280) | |
| 3 days post DS | −0.0607 | −0.1350 | 0.0256 | −0.2210 | 0.3360 | −0.2600 | 0.2750 | −0.6990 |
| (0.1300) | (0.1700) | (0.1830) | (0.4280) | (0.2400) | (0.2460) | (0.2160) | (0.3680) | |
| Temperature | 0.0096 * | 0.0136 ** | 0.0036 | 0.0020 | 0.0070 | 0.0075 | 0.0087 | 0.0162 * |
| (0.0038) | (0.0048) | (0.0057) | (0.0115) | (0.0083) | (0.0067) | (0.0073) | (0.0076) | |
| NO2 | 0.0113 *** | 0.0076 | 0.0155 ** | 0.0007 | 0.0140 | 0.0145 * | 0.0135 * | 0.0081 |
| (0.0033) | (0.0041) | (0.0049) | (0.0100) | (0.0071) | (0.0058) | (0.0063) | (0.0066) | |
| O3 | −0.0004 | −0.0006 | 0.0008 | −0.0050 | −0.0023 | 0.0012 | 0.0043 | −0.0037 |
| (0.0013) | (0.0016) | (0.0020) | (0.0041) | (0.0029) | (0.0023) | (0.0025) | (0.0026) | |
| Trend | 0.0104 *** | 0.0081 *** | 0.0137 *** | 0.0017 | 0.0134 *** | 0.0116 *** | 0.0081 *** | 0.0132 *** |
| (0.0006) | (0.0008) | (0.0009) | (0.0019) | (0.0014) | (0.0011) | (0.0012) | (0.0012) | |
| Spring | −0.0602 | −0.0549 | −0.0633 | 0.0299 | 0.0009 | −0.0756 | −0.2730 *** | 0.0843 |
| (0.0414) | (0.0514) | (0.0624) | (0.1260) | (0.0911) | (0.0721) | (0.0816) | (0.0810) | |
| Autumn | 0.0035 | 0.0006 | −0.0019 | 0.0898 | 0.0551 | −0.1260 * | −0.0074 | 0.0528 |
| (0.0341) | (0.0421) | (0.0515) | (0.1030) | (0.0754) | (0.0604) | (0.0649) | (0.0681) | |
| Winter | −0.0808 | −0.0611 | −0.1010 | −0.0352 | −0.0476 | −0.2060 * | −0.1060 | 0.0154 |
| (0.0530) | (0.0660) | (0.0792) | (0.1610) | (0.1160) | (0.0932) | (0.1020) | (0.1060) | |
| AR1 | 0.0086 | 0.0060 | −0.0016 | 0.0003 | 0.0060 | 0.0365 | 0.0079 * | −0.0007 |
| AR2 | 0.0043 | 0.0039 | −0.0035 | −0.0021 | −0.0072 | −0.0137 | 0.0024 | 0.0010 |
| AR3 | 0.0035 | 0.0052 | −0.0025 | 0.0155 * | −0.0036 | 0.0104 | −0.0001 | 0.0003 |
| AR4 | 0.0049 | 0.0034 | −0.0011 | 0.0036 | −0.0111 | 0.0073 | −0.0022 | −0.0024 |
| AR5 | 0.0019 | 0.0006 | 0.0050 | −0.0048 | −0.0010 | −0.0016 | 0.0055 | 0.0004 |
| AR6 | −0.0056 | −0.0056 | −0.0066 | 0.0022 | −0.0080 | −0.0242 | −0.0089 | 0.0091 |
| AR7 | −0.0026 | 0.0009 | 0.0748 ** | 0.0700 ** | 0.0712 ** | 0.0125 | 0.0250 | −0.0218 |
| N | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 |
| Total | Male | Female | ≤50 | 51–60 | 61–70 | 71–80 | ≥81 | |
|---|---|---|---|---|---|---|---|---|
| Intercept | 0.6720 *** | 0.4490 | −0.6580 * | 0.0724 | −3.2920 *** | −1.3640 ** | 0.5820 | −1.1040 *** |
| (0.1960) | (0.2530) | (0.2960) | (0.9480) | (0.6320) | (0.4300) | (0.3250) | (0.3190) | |
| Day of DS | −0.0208 | 0.0139 | −0.0646 | −0.1810 | −0.0334 | −0.3740 | 0.1590 | −0.0368 |
| (0.1110) | (0.1410) | (0.1710) | (0.5820) | (0.3760) | (0.2890) | (0.1650) | (0.1840) | |
| 1 day post DS | −0.0036 | −0.0807 | 0.0970 | 0.4870 | 0.0773 | −0.1490 | −0.1770 | 0.1250 |
| (0.1540) | (0.2090) | (0.2180) | (0.6230) | (0.5160) | (0.3680) | (0.2800) | (0.2260) | |
| 2 days post DS | 0.2730 * | 0.3040 | 0.2340 | 0.9640 * | 0.3950 | −0.1400 | 0.4140 * | 0.1880 |
| (0.1230) | (0.1580) | (0.1880) | (0.4870) | (0.3420) | (0.3250) | (0.1960) | (0.2070) | |
| 3 days post DS | −0.0405 | 0.0743 | −0.2520 | 0.1250 | −0.8230 | 0.4100 | −0.1910 | −0.0750 |
| (0.1450) | (0.1760) | (0.2440) | (0.7500) | (0.7320) | (0.2490) | (0.2730) | (0.2330) | |
| Temperature | 0.0161 *** | 0.0167 ** | 0.0154 * | 0.0023 | 0.0039 | 0.0183 | 0.0202 ** | 0.0153 * |
| (0.0044) | (0.0057) | (0.0066) | (0.0215) | (0.0139) | (0.0096) | (0.0073) | (0.0071) | |
| NO2 | 0.0153 *** | 0.0092 | 0.0217 *** | 0.0244 | 0.0337 ** | 0.0188 * | 0.0171 ** | 0.0077 |
| (0.0038) | (0.0049) | (0.0056) | (0.0183) | (0.0120) | (0.0082) | (0.0063) | (0.0062) | |
| O3 | 0.0013 | 0.0035 | −0.0016 | −0.0040 | 0.0113 * | −0.0001 | 0.0005 | 0.0009 |
| (0.0015) | (0.0019) | (0.0023) | (0.0076) | (0.0047) | (0.0033) | (0.0025) | (0.0024) | |
| Trend | 0.0029 *** | 0.0014 | 0.0052 *** | −0.0115 ** | 0.0085 *** | 0.0049 ** | −0.0047 *** | 0.0093 *** |
| (0.0007) | (0.0009) | (0.0011) | (0.0035) | (0.0023) | (0.0016) | (0.0012) | (0.0011) | |
| Spring | 0.0422 | 0.0179 | 0.0783 | −0.1580 | −0.0862 | 0.0666 | 0.0678 | 0.0225 |
| (0.0480) | (0.0616) | (0.0720) | (0.2370) | (0.1610) | (0.1050) | (0.0785) | (0.0760) | |
| Autumn | 0.0699 | 0.0153 | 0.1380 * | −0.1370 | 0.2720 * | 0.0245 | 0.0666 | 0.0450 |
| (0.0397) | (0.0509) | (0.0598) | (0.1980) | (0.1300) | (0.0882) | (0.0653) | (0.0629) | |
| Winter | −0.0174 | −0.0006 | −0.0404 | −0.2570 | −0.1300 | 0.0180 | −0.0209 | −0.0011 |
| (0.0614) | (0.0794) | (0.0919) | (0.3020) | (0.1990) | (0.1340) | (0.1020) | (0.0990) | |
| AR1 | 0.0081 | 0.0047 | 0.0052 | −0.0082 | −0.0001 | 0.0000 | 0.0062 * | −0.0018 |
| AR2 | 0.0128 * | 0.0148 *** | 0.0014 | −0.0069 | 0.0060 | 0.0043 | 0.0033 | 0.0010 |
| AR3 | 0.0057 | 0.0150 ** | 0.0024 | −0.0062 | −0.0077 | −0.0046 | −0.0015 | 0.0033 |
| AR4 | −0.0025 | 0.0035 | 0.0044 | −0.0059 | −0.0089 | 0.0115 | 0.0020 | 0.0008 |
| AR5 | 0.0102 | 0.0034 | 0.0048 | −0.0046 | −0.0086 | 0.0012 | 0.0050 | 0.0002 |
| AR6 | 0.0061 | −0.0054 | 0.0000 | −0.0098 | −0.0070 | −0.0104 | 0.0101 | −0.0041 |
| AR7 | −0.0035 | −0.0216 | 0.0176 | 0.0105 | 0.0201 | −0.0160 | 0.0018 | −0.0560 * |
| N | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 |
| Total | Male | Female | ≤50 | 51–60 | 61–70 | 71–80 | ≥81 | |
|---|---|---|---|---|---|---|---|---|
| Intercept | −1.5410 * | −2.9960 ** | −1.1430 | −3.6810 | −4.0970 * | −0.2970 | −1.1780 | −11.8800 *** |
| (0.7840) | (1.0290) | (1.1930) | (2.3310) | (1.7960) | (1.4270) | (1.5650) | (2.3090) | |
| Day of DS | −0.0969 | −1.3500 | 0.7020 | −14.6200 | −13.4300 | 0.8670 | −0.0693 | −14.3300 |
| (0.4610) | (1.0090) | (0.5200) | (1140.4000) | (484.7000) | (0.5500) | (1.0420) | (938.7000) | |
| 1 day post DS | 0.8020 | 0.9300 | 0.6280 | −15.0400 | 1.8500 ** | −0.1810 | 1.2130 | 0.8350 |
| (0.4110) | (0.5420) | (0.6170) | (1716.9000) | (0.6660) | (1.0830) | (0.8180) | (1.0340) | |
| 2 days post DS | 0.4480 | −0.5850 | 1.0930 * | 0.9840 | 0.5550 | 0.7260 | 0.2580 | −14.6200 |
| (0.4750) | (1.0410) | (0.5370) | (1.0400) | (1.0460) | (0.7800) | (1.1000) | (1393.8000) | |
| 3 days post DS | −0.1130 | 0.4850 | −14.2000 | −14.8200 | −0.1550 | −13.6600 | 0.7790 | 1.0410 |
| (0.6190) | (0.6250) | (685.2000) | (1993.4000) | (1.0840) | (658.9000) | (1.1420) | (1.0420) | |
| Temperature | 0.0373 * | 0.0561 * | 0.0142 | 0.0202 | 0.1090 * | −0.0033 | −0.0061 | 0.1680 ** |
| (0.0183) | (0.0241) | (0.0276) | (0.0521) | (0.0434) | (0.0323) | (0.0371) | (0.0537) | |
| NO2 | 0.0065 | 0.0298 | −0.0310 | −0.0545 | 0.0011 | −0.0260 | 0.0401 | 0.0707 |
| (0.0155) | (0.0195) | (0.0249) | (0.0481) | (0.0351) | (0.0299) | (0.0303) | (0.0385) | |
| O3 | 0.0036 | −0.0076 | 0.0188 * | −0.0102 | 0.0131 | 0.0032 | 0.0053 | −0.0014 |
| (0.0059) | (0.0078) | (0.0087) | (0.0180) | (0.0129) | (0.0104) | (0.0127) | (0.0154) | |
| Trend | −0.0004 | 0.0024 | −0.0047 | 0.0099 | −0.0091 | −0.0056 | −0.0079 | 0.0240 ** |
| (0.0028) | (0.0036) | (0.0043) | (0.0083) | (0.0063) | (0.0050) | (0.0059) | (0.0074) | |
| Spring | −0.1770 | −0.1130 | −0.2300 | 0.3930 | 0.1780 | −0.1590 | −0.7880 | 0.0707 |
| (0.1840) | (0.2370) | (0.2870) | (0.5250) | (0.4140) | (0.3300) | (0.4270) | (0.4370) | |
| Autumn | −0.1810 | −0.1310 | −0.2370 | −0.0077 | 0.0976 | −0.1030 | −0.4600 | −0.2790 |
| (0.1520) | (0.1960) | (0.2340) | (0.4620) | (0.3330) | (0.2660) | (0.3420) | (0.3950) | |
| Winter | −0.0237 | 0.0691 | −0.0906 | 0.4730 | 0.8670 | −0.5710 | −0.4190 | 0.6410 |
| (0.2480) | (0.3200) | (0.3870) | (0.7300) | (0.5710) | (0.4540) | (0.5190) | (0.6440) | |
| AR1 | 0.0100 | −0.0139 | 0.0014 | −0.0091 | −0.0103 | −0.0036 | 0.0008 | −0.0175 |
| AR2 | 0.0211 | 0.0361 * | 0.0110 | 0.0010 | 0.0046 | −0.0072 | −0.0052 | −0.0052 |
| AR3 | 0.0053 | 0.0142 | −0.0044 | −0.0002 | −0.0053 | −0.0055 | −0.0064 | −0.0005 |
| AR4 | −0.0022 | 0.0048 | −0.0028 | −0.0105 | −0.0216 | 0.0075 | −0.0012 | −0.0107 |
| AR5 | 0.0043 | −0.0086 | −0.0247 | 0.0087 | −0.0173 | 0.0008 | 0.0003 | −0.0111 |
| AR6 | 0.0177 | 0.0138 | 0.0152 | 0.0000 | −0.0219 | 0.0190 | −0.0010 | −0.0007 |
| AR7 | 0.0020 | −0.0052 | 0.0157 | −0.031 | −0.0167 | 0.0095 | −0.0247 | 0.0522 * |
| N | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 |
| Total | Male | Female | 51–60 | 61–70 | 71–80 | ≥81 | |
|---|---|---|---|---|---|---|---|
| Intercept | 0.8220 | 0.5160 | −0.4700 | −2.4830 | −0.3430 | 0.4220 | −0.2650 |
| (0.6780) | (0.8360) | (1.1660) | (2.9490) | (1.6560) | (1.1250) | (1.0020) | |
| Day of DS | −0.1440 | −0.1430 | −0.2050 | −16.0500 | 0.1710 | −0.2590 | −0.4070 |
| (0.3900) | (0.4580) | (0.7380) | (2555.3000) | (0.9620) | (0.7230) | (0.5870) | |
| 1 day post DS | 0.8930 * | 0.8940 * | 0.9890 | −16.6800 | 1.0710 | 0.8790 | 0.9810 * |
| (0.3740) | (0.4490) | (0.6750) | (4133.4000) | (1.1240) | (0.6420) | (0.4940) | |
| 2 days post DS | −0.4020 | −0.2610 | −0.6250 | −17.1300 | −16.7900 | 0.3270 | −0.5910 |
| (0.5290) | (0.6120) | (1.0610) | (4192.0000) | (3202.2000) | (0.7520) | (0.7410) | |
| 3 days post DS | −0.7360 | −0.9950 | −0.3620 | −16.8400 | 0.925 | −15.6100 | −0.7110 |
| (0.7620) | (1.0600) | (1.1090) | (4784.5000) | (1.1160) | (1216.0000) | (1.0490) | |
| Temperature | 0.0276 | 0.0377 * | 0.0068 | −0.0316 | 0.0029 | 0.0271 | 0.0321 |
| (0.0154) | (0.0190) | (0.0265) | (0.0659) | (0.0376) | (0.0260) | (0.0225) | |
| NO2 | 0.0262 * | 0.0411 ** | −0.0005 | 0.0272 | 0.0140 | 0.0182 | 0.0349 |
| (0.0130) | (0.0158) | (0.0230) | (0.0591) | (0.0325) | (0.0218) | (0.0189) | |
| O3 | −0.0007 | −0.0089 | 0.0143 | 0.0469 * | 0.0001 | 0.0069 | −0.0127 |
| (0.0052) | (0.0065) | (0.0089) | (0.0198) | (0.0134) | (0.0085) | (0.0078) | |
| Trend | −0.0170 *** | −0.0188 *** | −0.0139 ** | −0.0135 | −0.0199 ** | −0.0207 *** | −0.0146 *** |
| (0.0025) | (0.0031) | (0.0043) | (0.0108) | (0.0063) | (0.0041) | (0.0037) | |
| Spring | 0.0716 | 0.1690 | −0.1570 | −0.7150 | −0.2580 | −0.2440 | 0.4240 |
| (0.1610) | (0.1940) | (0.2880) | (0.7000) | (0.4320) | (0.2670) | (0.2320) | |
| Autumn | 0.0002 | 0.0121 | −0.0570 | −0.5880 | 0.2300 | −0.1450 | −0.0068 |
| (0.1360) | (0.1670) | (0.2350) | (0.5770) | (0.3360) | (0.2160) | (0.2090) | |
| Winter | −0.1400 | −0.1600 | −0.1580 | −1.3010 | −0.0507 | −0.2920 | −0.0607 |
| (0.2140) | (0.2620) | (0.3720) | (0.9530) | (0.5320) | (0.3540) | (0.3140) | |
| AR1 | 0.0054 | 0.0228 | −0.0041 | −0.0152 | −0.0122 | 0.0003 | 0.0010 |
| AR2 | 0.0083 | −0.0031 | −0.0052 | −0.0132 | −0.0085 | −0.0071 | 0.0065 |
| AR3 | −0.0001 | 0.0000 | −0.0043 | −0.0211 | −0.0278 | −0.0086 | −0.0017 |
| AR4 | −0.0080 | −0.0035 | −0.0015 | −0.0196 | 0.0165 | −0.0002 | −0.0078 |
| AR5 | −0.0055 | −0.0053 | −0.0053 | −0.0118 | 0.0334 | −0.0048 | −0.0027 |
| AR6 | −0.0158 | −0.0135 | −0.0059 | −0.0127 | 0.0114 | −0.0065 | −0.0088 |
| AR7 | 0.0130 | 0.0055 | 0.0151 | 0.0225 | 0.0186 | 0.0057 | 0.0027 |
| N | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 | 1820 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-S.; Chen, C.; Liu, T.-C. Dust Storms Increase the Risk of Age-Related Macular Degeneration. Int. J. Environ. Res. Public Health 2022, 19, 7403. https://doi.org/10.3390/ijerph19127403
Chen C-S, Chen C, Liu T-C. Dust Storms Increase the Risk of Age-Related Macular Degeneration. International Journal of Environmental Research and Public Health. 2022; 19(12):7403. https://doi.org/10.3390/ijerph19127403
Chicago/Turabian StyleChen, Chin-Shyan, Conmin Chen, and Tsai-Ching Liu. 2022. "Dust Storms Increase the Risk of Age-Related Macular Degeneration" International Journal of Environmental Research and Public Health 19, no. 12: 7403. https://doi.org/10.3390/ijerph19127403
APA StyleChen, C.-S., Chen, C., & Liu, T.-C. (2022). Dust Storms Increase the Risk of Age-Related Macular Degeneration. International Journal of Environmental Research and Public Health, 19(12), 7403. https://doi.org/10.3390/ijerph19127403





