Tranexamic Acid for Postpartum Hemorrhage Treatment in Low-Resource Settings: A Rapid Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Literature Search Strategy
2.3. Eligibility Criteria
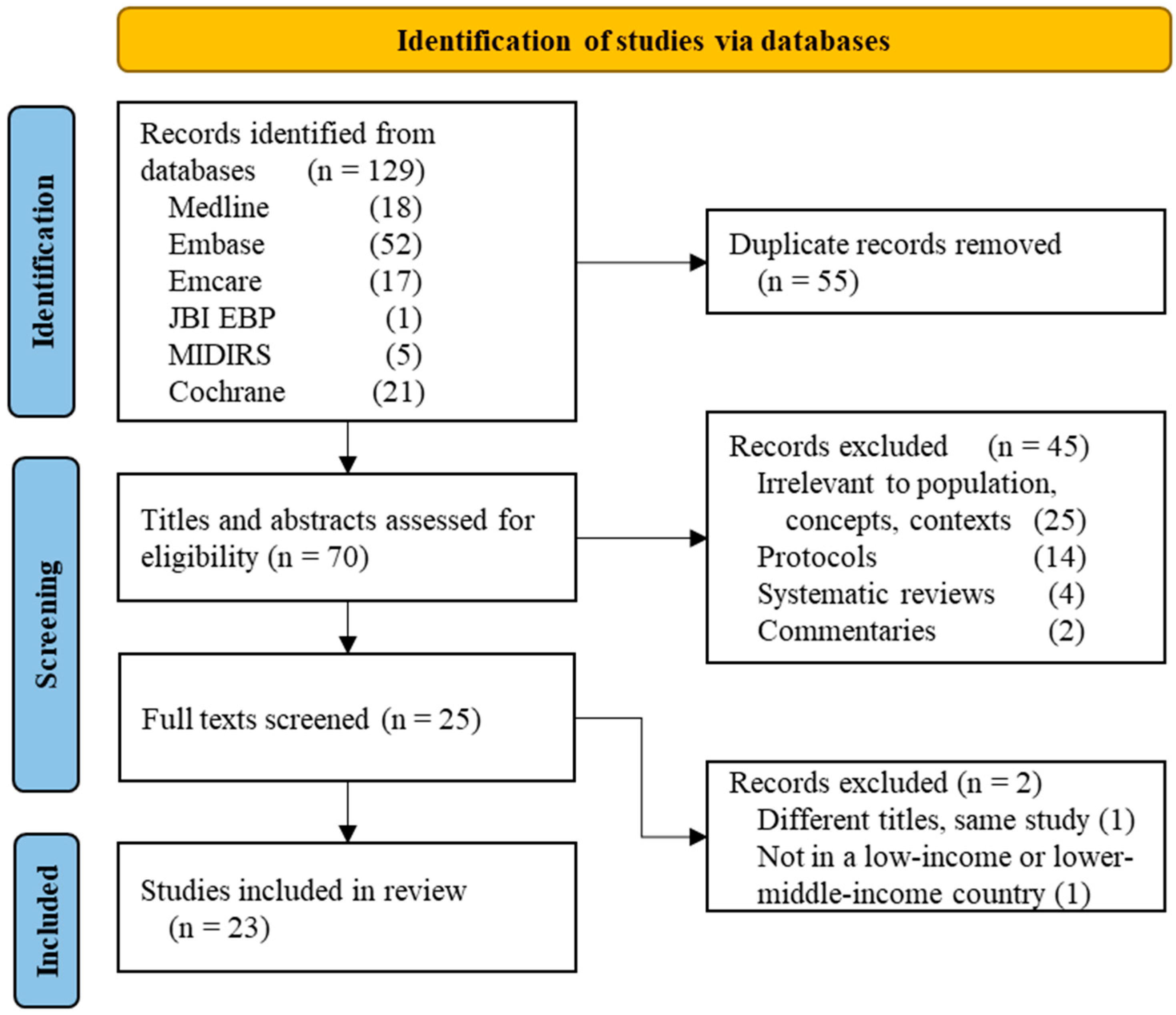
2.4. Study Selection and Data Abstraction
2.5. Methodological Quality Appraisal
2.6. Synthesis
3. Results
3.1. Quantity of Research Available
3.2. Study Design
| Study & Year | Location | Aim | Method/Design | Study Population and Sample Size | Intervention Type & Outcome Measures | Relevant Findings |
|---|---|---|---|---|---|---|
| Abdel-Aleem, 2013 [18] | Women’s Health Hospital, Assiut University, Assiut, Egypt | To assess the possible effect of TXA on blood loss during and after elective c-section | Two-arm non-blinded RCT | 740 pregnant women with singleton fetus at ≥37 weeks planned to have elective c-section | TXA 1 g IV, 10 min before c-section Primary outcome: Blood loss measured during and 2 h after operation | Pre-operative use of TXA is associated with reduced blood loss during and after elective c-section. This could benefit anemic women or those who refuse blood transfusion. Mean total blood loss was 241.6 (SE 6.77) mL in the TXA group versus 510 (SE 7.72) mL in the control group. The mean drop in hematocrit and hemoglobin levels were statistically significantly lower in the TXA group than in the control group. There were no statistically or clinically significant differences in other outcomes. The study was not powered to assess the efficacy of TXA in prevention of severe PPH or to assess its safety especially thromboembolic complications. |
| Agrawal, 2018 [19] (Conference abstract only) | BPKIHS medical university in eastern Nepal | To evaluate the effect of preoperative administration of IV TXA on blood loss during and after elective c-section | RCT, blinding not mentioned | 160 pregnant women at ≥37 weeks with elective c-section | TXA 1 g IV vs. normal saline Primary outcome: blood loss during and for 24 h after operation | The mean estimated blood loss was significantly lower in women treated with TXA compared with women in the placebo group (392.13 ± 10.06 mL versus 498.69 ± 15.87 mL) |
| Ajroudi, 2015 [32] (Conference abstract only) | Mongi Slim Hospital, La Marsa, Tunisia | To assess the efficacy of a new protocol including TXA in the management of PPH | Non-randomized trial | 40 women with PPH following vaginal or cesarean delivery | TXA loading dose 1 g/10 min, then infusion of 1 g/h over 3 h, in addition to the classic protocol including oxytocin and prostaglandins Primary outcome: protocol success rate | TXA reduces blood loss and maternal morbidity in PPH: the protocol succeeded in 81.1% of the cases, no adverse effects of TXA, 18% of patients required a blood transfusion. |
| Bose, 2017 [20] | Hospital-based Malabar Institute of Medical Sciences, Calicut, Kerala, India | To compare misoprostol vs. TXA in reducing blood loss during c-section | RCT, non-blinded | 163 pregnant women with emergency/elective c-section Gestational age not mentioned | TXA 500 mg IV vs. misoprostol 600 mcg SL Primary outcome: blood loss reduction, additional uterotonic use | TXA significantly reduced blood loss compared with misoprostol (416 vs. 505 mL, p = 0.023) in patients without high-risk factors for PPH, but not in patients with PPH risk factors. Misoprostol caused significantly higher minor side effects while TXA reduced operation time (by 5 min). Medication costs mentioned but cost analysis not done (misoprostol tablet manufactured by Cipla at INR 52 per dose vs. INR 57 per TXA dose, manufactured by Ozone). |
| Briki, 2018 [33] | Farhat Hached University Hospital, Sousse, Tunisia. | To evaluate the combination of TXA and fibrinogen concentrates in severe PPH | Retrospective observational study | 166 women with >24 weeks pregnancy and severe PPH (≥500 mL if vaginal delivery or ≥1000 mL if c-section) | Mean doses: TXA 1.98 g & fibrinogen 2.25 g Primary outcome: blood loss | Significant decrease in the fall of hemoglobin and blood transfusion in intervention group. Hemoglobin levels post-delivery: 6.23 ± 1.56 g/dl for control, 7.31 ± 2.09 for TXA & fibrinogen, p = 0.003 |
| Dimassi, 2018 [34] (Conference abstract only) | Moni Slim Hospital, Tunis, Tunisia | To evaluate the results of a therapeutic protocol with TXA and sulprostone for PPH care management | Prospective descriptive study | 70 women with PPH after vaginal or cesarean delivery. Gestational age not mentioned | TXA and sulprostone, unknown posology Primary outcome: success rate of protocol | The success rate of medical care management was 87.1%. The mean time for the diagnosis of the bleeding was 32 min. |
| Diop, 2020 [21] | 4 hospitals in Senegal and Vietnam | To evaluate the efficacy, safety, and acceptability of oral TXA when used as an adjunct to sublingual misoprostol to treat PPH following vaginal delivery. | Double-blind RCT | 258 women with PPH (defined as ≥700 mL) after vaginal birth. Gestational age not mentioned | TXA PO 1950 mg with misoprostol 800 mcg SL vs. placebo with misoprostol 800 mcg SL Primary outcome: blood loss | Adjunct use of oral TXA with misoprostol to treat PPH had similar clinical and acceptability outcomes when compared to treatment with misoprostol alone. Proportion of women with active bleeding controlled with trial drugs alone and no additional interventions was similar in both groups: 77 (60.2%) placebo; 74 (56.9%) TXA, p = 0.59). Use of other interventions to control bleeding, including uterotonics, did not differ significantly between groups. Median blood loss at PPH diagnosis was 700 mL in both groups. Reports of side effects and acceptability were similar in the two groups. |
| Dutta, 2017 [35] (Conference abstract only) | Tertiary care hospital in Nadia, West Bengal, India | To evaluate a new surgical technique (Dutta’s) to prevent PPH due to major degree placenta previa during c-section | Non-randomized trial | 94 pregnant women with major degree placenta previa undergoing c-section | Injection TXA 1 g IM + oxytocin 10 IU IV infusion Primary outcome: blood loss | Simple, safe, quick, effective procedure: intraoperative blood loss less than 300 cc in 89 (94.68%) cases. It reduces perfusion pressure, permits time for further steps. This technique is suitable for rural-based hospital in absence of adequate blood transfusion facility. |
| Joudeh, 2021 [36] | 22 District Hospitals in Bihar, India | To assess PPH diagnoses and management, hypertensive disorders of pregnancy, birth asphyxia, and low birth weight, as part of the CARE’s AMANAT program (Comprehensive Emergency Obstetric and Neonatal Readiness.) | Non-randomized trial | 11,259 pregnant women (diagnosis analysis) and 11,800 pregnant women (management analysis) | Physicians and nurse mentors conducted clinical instruction, simulations (PRONTO International curriculum & training kits) and teamwork and communication activities, infrastructure and management support, and data collection for 5 days weekly during 6 consecutive months. PPH management: IV fluids, uterotonics, TXA Primary outcome: level of PPH diagnosis and management | Lower level of PPH diagnosis than expected. But among PPH patients, 96% received fluids, 85% received uterotonics and 11% received TXA. There was a significant positive trend in the number of patients receiving TXA for PPH (6% to 13.8%, p trend = 0.03) |
| Khaing, 2021 [22] (Conference abstract only) | Central Women’s Hospital, Mandalay, Myanmar | To evaluate prophylactic TXA effectiveness | RCT Blinding not mentioned | 220 pregnant women at low risk of PPH, vaginal delivery | TXA 1 g IV & oxytocin 10 IU IV vs. oxytocin 10 IU IV (without TXA) Primary outcome: blood loss | Mean total blood loss was significantly lower in the intervention group (213.1 ± 85.9 mL) than the control group (365.6 ± 203.4 mL). The mean measured blood loss from fetal delivery to 2 h postpartum was significantly lower in the intervention group (173.1 ± 56.0 mL) than the control group (227.7 ± 83.3 mL). The need of additional uterotonic drugs was significantly lower in the intervention group. |
| Li, 2018 [38] | Hospitals in Nigeria, Pakistan | To assess the cost-effectiveness of early administration (within 3 h after birth) of TXA added to usual care to treat PPH | Cost-effectiveness analysis using decision tree model & health-care provider perspective | No detail regarding Nigeria and Pakistan trial population (the trial recruited in total 20,000 women from 21 countries) | Primary outcome: costs (calculated in 2016 US$), life-years, and quality-adjusted life-years (QALYs) with and without TXA, incremental cost-effectiveness ratios (ICERs) | Intervention highly cost-effective in Nigeria and Pakistan: 0.18 QALYs at an additional cost of $37.12 per patient in Nigeria and an average gain of 0.08 QALYs at an additional cost of $6.55 per patient in Pakistan. The base case ICER results were $208 per QALY in Nigeria and $83 per QALY in Pakistan. These ICERs were below the lower bound of the cost-effectiveness threshold range in both countries. |
| McClure, 2015 [41] | Sub-Saharan African countries, unspecified Homes, clinics, and hospitals level of care | To determine the impact of TXA on PPH-related maternal mortality in sub-Saharan Africa | Mathematical model populated with baseline birth rates and mortality estimates based on a review of current interventions for PPH in sub-Saharan Africa, assuming 30% efficacy of TXA to reduce PPH; the model assessed prophylactic and treatment TXA use for deliveries at homes, clinics, and hospitals. | Not applicable | Not applicable Primary outcome: reduced maternal mortality ratio | With TXA only in the hospitals, less than 2% of the PPH mortality would be reduced. However, if TXA were available in the home and clinic settings for PPH prophylaxis and treatment, a nearly 30% reduction (nearly 22,000 deaths per year) in PPH mortality is possible. Given its feasibility to be given in the home, TXA can save many lives. |
| Mirghafourvand, 2013 [23] | Alzahra hospital, Tabriz, Iran | To determine the effect of prophylactic TXA on calculated and measured blood loss | Double-blind RCT | 120 women with a term (38–42 weeks) singleton pregnancy at PPH low-risk, vaginal delivery | TXA 1 g IV & oxytocin 10 IU IV vs. placebo IV & oxytocin 10 IU IV Primary outcome: blood loss | Prophylactic TXA reduces blood loss after vaginal delivery in women with a low PPH risk. The mean (SD) calculated total blood loss (519 (320) vs. 659 (402) mL, p = 0.036) and measured blood loss from placental delivery to 2 h postpartum (69 (39) vs. 108 (53) mL, p < 0.001) was significantly lower in the intervention group. The frequency of calculated blood loss > 1000 mL was lower in the TXA group (7% vs. 18%, p = 0.048) |
| Naeiji, 2021 [24] | Shahid Beheshti University of Medical Science (SBUMS), Tehran, Iran | To evaluate the efficacy and safety of preoperative administration of IV TXA on blood loss during and after elective c-section. | Double-blind RCT | 200 pregnant women with elective c-section. Gestational age not mentioned | TXA 1 to 1.5 g IV vs. distilled water before incision Primary outcome: intra-operative and post-operative blood loss and hemoglobin | Prophylactic use of IV TXA decreases the blood loss safely in women undergoing elective c-section: TXA decreased the mean blood loss by 25.3%. Mean volume of intra-operative blood loss was 391.1 (±67.4) mL in TXA group and 523.8 (±153.4) mL in control group which was statistically significant lesser with a 132.7 mL difference. Rate of >1000 mL and >500 mL bleeding and need to blood transfusion were also statistically significant lower in TXA group. Mean hemoglobin level was statistically significant lower in placebo group (11.77 ± 0.50 versus 11.31 ± 0.56) 6 h after c-section. No adverse reaction was documented. |
| Nargis, 2020 [25] | IBN SINA Medical College Hospital, Dhaka, Bangladesh | To evaluate the effectiveness IV TXA on blood loss in elective c-section | Double-blind RCT | 120 pregnant women pregnant women with elective c-section after 35 weeks | TXA 1 IV vs. distilled water immediately after delivery of baby Primary outcome: intra-operative and post-operative blood loss and hemoglobin | Prophylactic use of IV TXA decreased blood losses from both placental deliveries to the end of c-section and from end of c-section to 2 h postpartum were significantly lower in the study group (p < 0.05). Total amount of oxytocin required was significantly less in TXA group (p < 0.05) also the number of women requiring other uterotonics (injectable methyl ergometrine, injectable carboprost and misoprostol per rectum) was significantly less in TXA group (p < 0.05). The amount of intra-operative fluid required were significantly less in TXA group (p < 0.005). |
| Nwabueze, 2021 [26] (Conference abstract only) | Federal Teaching Hospital Abakaliki (FETHA), Nigeria | To evaluate the efficacy of TXA at reducing blood loss following vaginal delivery | Double-blind RCT | Women undergoing vaginal births; sample size not mention. | TXA vs. placebo. Posology not mentioned Primary outcome: blood loss | IV TXA following vaginal delivery reduced blood loss. It reduced the need for additional uterotonics to control blood loss. However, blood loss greater than 500 was not significantly reduced: the mean estimated blood loss was significantly lower in the TXA group compared with the placebo group (174.87 ± 119.84 mL versus 341.07 ± 67.97 mL respectively; p < 0.0001). Additional uterotonics was required more in the control group compared to the treatment group 14 (16.67%) versus 3 (3.85%) of the treatment group, p-value of 0.007. There were no major complications noticed in the treatment group. |
| Oseni, 2021 [27] | Aminu Kano Teaching Hospital, Kano, Nigeria | To evaluate the effectiveness IV TXA on blood loss. | Double-blind RCT | 244 pregnant women 37–42 weeks with emergency c-section | Pre-incision: TXA 1 g IV vs. normal saline water. Oxytocin in both groups Primary outcome: intra-operative and post-operative blood loss and hemoglobin | Significant reduction in blood loss TXA group: the average intraoperative blood loss was 414.0 mL in the study group and 773.8 mL in the control group (t = −16.18, p ≤ 0.01). |
| Resch, 2020 [39] (Conference abstract only) | Different levels of care Uttar Pradesh, India | To develop a PPH cost-effectiveness model to estimate the potential health impact and cost-effectiveness of a quality improvement program for PPH management featuring a first response bundle and a set of refractory PPH interventions in health facilities | Decision tree model to compare the status quo delivery of PPH care in two scenarios | 1 million women delivering at home, subcenters, primary-health clinics, community-health centers, and district hospitals | Status quo PPH care: IV fluids, uterotonics, and uterine massage delivered with a setting-specific probability that increases with the level of health facility Strengthened PPH care: status quo interventions with TXA in all PPH cases, plus manual placenta removal and suturing when indicated. Enhanced scenario: further enhanced through implementation of non-surgical interventions for managing refractory PPH (including uterine balloon tamponade, aortic compression, and non-pneumatic anti-shock garment). | Implementation of an enhanced PPH care program, including the first response bundle and non-surgical refractory PPH interventions, is likely to be cost-effective and lifesaving in Uttar Pradesh, India (reduced PPH-related maternal mortality in intervention facilities by 98%, from 10.7 to 0.3 per 100,000 deliveries, averting 450 deaths per year). Moreover, enhanced PPH care is likely to generate more health impact and cost-savings compared with strengthened PPH care because of the greater reduction in number of surgeries needed. |
| Sahu, 2019 [37] | Referral hospital situated at the tribal terrain of Chhattisgarh, India | To evaluate the effectiveness IV TXA on blood loss. | Non-randomized trial | 100 singleton pregnant women 35–42 weeks with elective and emergency c-section | Pre-incision: TXA 1 g IV vs. no TXA. Both groups received oxytocin 10 IU post baby delivery. Primary outcome: blood loss | Significant reduction in blood loss in TXA group: the mean blood loss (intra as well as postoperative) was 436.5 ± 118.07 mL in the study group in comparison to 616.5 ± 153.34 mL in the control group (p ≤ 0.05) |
| Sujata, 2016 [28] | Max Hospital, New Delhi, India | To evaluate the effectiveness IV TXA on blood loss in c-section among women at high PPH risk | Single-blinded RCT | 60 singleton pregnant women with elective or emergency c-section: gestational age not mentioned. | Per-op: TXA 1 g IV vs. normal saline water. Primary outcome: need for additional uterotonics | Significant reduction in blood loss in TXA group: uterotonic drugs were required in 7 (23%) patients assigned to TXA and 25 (83%) patients in the control group (p < 0.001) |
| Tabatabaie, 2021 [29] | Dr. Ali Shariati and Persian Gulf Hospitals of Bandar Abbas, Iran | To compare the effect of TXA and misoprostol on blood loss during and after c-section | Triple-arm RCT, non-blinded | 300 singleton pregnant women, 37–42 weeks | Group A: TXA 10 mg/kg IV; Group B: misoprostol 600 mcg rectal; Group C: 200 mL normal saline. All groups received oxytocin. Primary outcome: blood loss | Both medicines are effective in reducing the amount of blood loss during c-section with misoprostol being more effective than TXA. Level of blood loss in ml: 444.70 ± 100.58 (TXA); 299.98 ± 162.79 (misoprostol); 568.84 ± 147.07 (placebo), p < 0.001 |
| Tali, 2016 [30] (Conference abstract only) | Jose R. Reyes Memorial Medical Center, Manila, Philippines | To compare the effect of TXA on blood loss in vaginal delivery | Double-blind RCT | Not mentioned | TXA 1 g IV & oxytocin 10 IU IV vs. placebo IV & oxytocin 10 IU IV Primary outcome: blood loss | The prophylactic use of TXA may reduce blood loss: the mean (SD) calculated total blood loss (167 (162) versus 463 (348) mL, p < 0.001), measured blood loss from fetus delivery to placental delivery (133 (47) versus 207 (66) mL, p < 0.001), from placental delivery to 2 h postpartum (82 (33) versus 136 (88) ml, p < 0.001). The frequency of calculated blood loss >1000 mL was lower in the TXA group (0% versus 12%, p < 0.001) |
| Zargar, 2018 [31] | Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran | To compare the effect of TXA and prostaglandin analog on reducing PPH in cesarean or vaginal delivery. | Triple-blind RCT | 248 singleton pregnant women, 38–40 weeks | TXA IV: 4 g for an hour and then 1 g over 6 h infusion vs. prostaglandin analog IM 0.25 mg up to 8 doses (Hemebate). Primary outcome: blood loss | TXA had comparable effects with prostaglandin analog on reducing PPH in women with uterine atony and in those undergoing C section or vaginal delivery: postoperative bleeding did not significantly differ between the two groups (68.2 ± 6.1 mL and 69.1 ± 175.73 mL, respectively, p = 0.6). Moreover, hemoglobin declines were 1 ± 0.4 g/dL and 1.2 ± 0.5 g/dL in TXA and prostaglandin group respectively, indicating that the difference was not statistically significant (p = 0.7) |
3.3. Patient Population
3.4. Context
3.5. Concepts
3.5.1. Effectiveness
PPH Treatment
PPH Prevention
Maternal Mortality
3.5.2. Feasibility
3.5.3. Acceptability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage; World Health Organization: Geneva, Switzerland, 2012; Available online: https://www.who.int/reproductivehealth/topics/maternal_perinatal/pph-woman-trial/en/ (accessed on 8 October 2021).
- World Health Organization. Maternal Mortality: Levels and Trends 2000–2017; Estimates from WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/reproductivehealth/publications/maternal-mortality-2000-2017/en/ (accessed on 8 October 2021).
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Oyelese, Y.; Ananth, C.V. Postpartum hemorrhage: Epidemiology, risk factors, and causes. Clin. Obstet. Gynecol. 2010, 53, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Carroli, G.; Cuesta, C.; Abalos, E.; Gulmezoglu, A.M. Epidemiology of postpartum haemorrhage: A systematic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations: Uterotonics for the Prevention of Postpartum Haemorrhage; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/277276/9789241550420-eng.pdf (accessed on 8 October 2021).
- Ker, K.; Roberts, I.; Shakur, H.; Coats, T.J. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst. Rev. 2015, CD004896. [Google Scholar] [CrossRef]
- Shakur, H.; Roberts, I.; Fawole, B.; Chaudhri, R.; El-Sheikh, M.; Akintan, A.; Qureshi, Z.; Kidanto, H.; Vwalika, B.; Abdulkadir, A. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 2105–2116. [Google Scholar] [CrossRef]
- Shakur, H.; Beaumont, D.; Ker, K.; Pavord, S.; Gayet-Ageron, A.; Mousa, H.A. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst. Rev. 2018, 2018, CD012964. [Google Scholar] [CrossRef]
- Aziz, S.; Rossiter, S.; Homer, C.S.; Wilson, A.N.; Comrie-Thomson, L.; Scott, N.; Vogel, J.P. The cost-effectiveness of tranexamic acid for treatment of postpartum hemorrhage: A systematic review. Int. J. Gynecol. Obstet. 2021, 155, 331–344. [Google Scholar] [CrossRef]
- Novikova, N.; Hofmeyr, G.J.; Cluver, C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst. Rev. 2015, CD007872. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendation on Tranexamic Acid for the Treatment of Postpartum Haemorrhage; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/reproductivehealth/publications/tranexamic-acid-pph-treatment/en/ (accessed on 8 October 2021).
- World Health Organization. WHO Model Lists of Essential Medicines, 21st List 2019; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists (accessed on 8 October 2021).
- Ganann, R.; Ciliska, D.; Thomas, H. Expediting systematic reviews: Methods and implications of rapid reviews. Implement. Sci. 2010, 5, 56. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual: 2015 Edition/Supplement; The Joanna Briggs Institute: Adelaide, Australia, 2015. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Kastner, M.; Levac, D.; Ng, C.; Sharpe, J.P.; Wilson, K. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aleem, H.; Alhusaini, T.K.; Abdel-Aleem, M.A.; Menoufy, M.; Gulmezoglu, A.M. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: Randomized clinical trial. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2013, 26, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. Tranexamic acid (TA) in prevention of postpartum haemorrhage in elective cesarean section. Int. J. Gynaecol. Obstet. 2018, 143, 294–295. [Google Scholar] [CrossRef]
- Bose, D.; Beegum, R. Sublingual misoprostol vs intravenous tranexamic acid in reducing blood loss during cesarean section: A prospective randomized study. J. SAFOG 2017, 9, 9–13. [Google Scholar] [CrossRef]
- Diop, A.; Abbas, D.; Martin, R.; Winikoff, B.; Ngoc, N.T.N.; Razafi, A.; Tuyet, H.T.D. A double-blind, randomized controlled trial to explore oral tranexamic acid as adjunct for the treatment for postpartum hemorrhage. Reprod. Health 2020, 17, 34. [Google Scholar] [CrossRef]
- Khaing, C.; Hlaing Thin, T.; Myat Thin, T. Prophylactic Intravenous Tranexamic Acid in Women at Low Risk of Postpartum Haemorrhage. J. Obstet. Gynaecol. Res. 2021, 47, 2928–2929. [Google Scholar] [CrossRef]
- Mirghafourvand, M.; Alizadeh Charandabi, S.M.; Abasalizadeh, F.; Shirdel, M. The effect of intravenous tranexamic acid on hemoglobin and hematocrit levels after vaginal delivery: A randomized controlled trial. Iran. J. Obstet. Gynecol. Infertil. 2013, 16, 1–8. [Google Scholar]
- Naeiji, Z.; Saleh, S.; Moridi, A.; Delshadiyan, N.; Rahmati, N.; Fathi, M. Prophylactic use of tranexamic acid for decreasing the blood loss in elective cesarean section: A placebo-controlled randomized clinical trial. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101973. [Google Scholar]
- Nargis, N.; Dewan, F. Prophylactic use of tranexamic acid during caesarean section in preventing postpartum haemorrhage-a prospective randomised double blind placebo controlled study. Bangladesh J. Obstet. Gynecol. 2020, 33, 125–130. [Google Scholar] [CrossRef]
- Nwabueze Igboke, F.; Osaheni Lawani, L.; Okwuchukwu Obi, V.; Benedict Dimejesi, I. Tranexamic acid for reducing PPH following vaginal delivery: A randomized controlled trial. BJOG 2021, 128, 147. [Google Scholar] [CrossRef]
- Oseni, R.O.; Zakari, M.; Adamou, N.; Umar, U.A. Effectiveness of preoperative tranexamic acid in reducing blood loss during caesarean section at Aminu Kano Teaching Hospital, Kano: A randomized controlled trial. Pan Afr. Med. J. 2021, 39, 34. [Google Scholar] [CrossRef] [PubMed]
- Sujata, N.; Tobin, R.; Kaur, R.; Aneja, A.; Khanna, M.; Hanjoora, V.M. Randomized controlled trial of tranexamic acid among parturients at increased risk for postpartum hemorrhage undergoing cesarean delivery. Int. J. Gynaecol. Obstet. 2016, 133, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaie, S.S.; Alavi, A.; Bazaz, M. Comparison of the effect of tranexamic acid and misoprostol on blood loss during and after cesarean section: A randomized clinical trial. Razavi Int. J. Med. 2021, 9, 7–13. [Google Scholar] [CrossRef]
- Tali, K.; Ignacio Alensuela, A. The effect of prophylactic intravenous tranexamic acid in reducing blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: A prospective, randomised, double-blind, placebocontrolled study. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 61. [Google Scholar]
- Zargar, M.; Nikbakht, R.; Ahmadi, M. The effect of tranexamic acid on preventing post-partum hemorrhage due to uterine atony: A triple-blind randomized clinical trial. Curr. Clin. Pharmacol. 2018, 13, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Ajroudi, M.; Ammous, T.; Robbana, M.; Zakhama, K.; Ben Romdhane, M.; Dimassi, K.; Kammoun, S.; Ben Amor, A.; Triki, A.; Mbazaa, M.S.; et al. Use of tranexamic acid in the management of post partum hemorrhage: Tunisian preliminary study. Int. J. Gynecol. Obstet. 2015, 131, E483. [Google Scholar]
- Briki, R.; Ferhi, F.; Cherif, O.; Saadi, M.A.; Derouich, M.; Khlifi, A.; Boughizane, S.; Tarmiz, K. Place of Tranexamic Acid and Fibrinogen Association in the Management of Severe Postpartum Haemorrhage. Open J. Obstet. Gynecol. 2018, 8, 1040–1051. [Google Scholar] [CrossRef][Green Version]
- Dimassi, K.; Ahmed, H.; Triki, A. Management of post-partum haemorrhage: Results of the systematic association of the acid tranexamic. Int. J. Gynecol. Obstet. 2018, 143, 680. [Google Scholar] [CrossRef]
- Dutta, D.K.; Dutta, I. Management of major degree placenta previa during LSCS operation—A new surgical technique (Dutta’s). J. Obstet. Gynaecol. Res. 2017, 43, 52. [Google Scholar] [CrossRef]
- Joudeh, A.; Ghosh, R.; Spindler, H.; Handu, S.; Sonthalia, S.; Das, A.; Gore, A.; Mahapatra, T.; Walker, D. Increases in diagnosis and management of obstetric and neonatal complications in district hospitals during a high intensity nurse-mentoring program in Bihar, India. PLoS ONE 2021, 16, e0247260. [Google Scholar] [CrossRef]
- Sahu, J.; Mishra, N. Role of intravenous tranexamic acid in reducing blood loss during caesarean section: Study at tribal-dominated area hospital in Chhattisgarh, India. J. Obstet. Gynaecol. Res. 2019, 45, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Miners, A.; Shakur, H.; Roberts, I. Tranexamic acid for treatment of women with post-partum haemorrhage in Nigeria and Pakistan: A cost-effectiveness analysis of data from the WOMAN trial. Lancet Glob. Health 2018, 6, e222–e228. [Google Scholar] [CrossRef]
- Resch, S.; Ward, Z.; Guha, M.; Suarez Zarate, S.; Borovac-Pinheiro, A.; Omotayo, M.; Garg, L.; Hansel, S.; Burke, T. Cost-effectiveness of postpartum haemorrhage first response bundle and non-surgical interventions for refractory postpartum haemorrhage in India: An ex-ante modelling study. Lancet Glob. Health 2020, 8, S42. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Rosas-Bermudez, A.; Castano, F.; Norton, M.H. Effects of birth spacing on maternal, perinatal, infant, and child health: A systematic review of causal mechanisms. Stud. Fam. Plan. 2012, 43, 93–114. [Google Scholar] [CrossRef] [PubMed]
- McClure, E.M.; Jones, B.; Rouse, D.J.; Griffin, J.B.; Kamath-Rayne, B.D.; Downs, A.; Goldenberg, R.L. Tranexamic acid to reduce postpartum hemorrhage: A MANDATE systematic review and analyses of impact on maternal mortality. Am. J. Perinatol. 2015, 32, 469–474. [Google Scholar] [CrossRef]
- Hernández-Vásquez, A.; Chacón-Torrico, H.; Bendezu-Quispe, G. Prevalence of home birth among 880,345 women in 67 low-and middle-income countries: A meta-analysis of Demographic and Health Surveys. SSM-Popul. Health 2021, 16, 100955. [Google Scholar] [CrossRef]
- Bayo, P.; Belaid, L.; Tahir, E.O.; Ochola, E.; Dimiti, A.; Greco, D.; Zarowsky, C. “Midwives do not appreciate pregnant women who come to the maternity with torn and dirty clothing”: Institutional delivery and postnatal care in Torit County, South Sudan: A mixed method study. BMC Pregnancy Childbirth 2020, 20, 250. [Google Scholar] [CrossRef]
- Picetti, R.; Miller, L.; Shakur-Still, H.; Pepple, T.; Beaumont, D.; Balogun, E.; Asonganyi, E.; Chaudhri, R.; El-Sheikh, M.; Vwalika, B.; et al. The WOMAN Trial: Clinical and Contextual Factors Surrounding the Deaths of 483 Women following Post-Partum Hemorrhage in Developing Countries. Obstet. Gynecol. Surv. 2020, 75, 723–725. [Google Scholar] [CrossRef]
- Ronsmans, C.; Graham, W.J.; Lancet Maternal Survival Series Steering Group. Maternal mortality: Who, when, where, and why. Lancet 2006, 368, 1189–1200. [Google Scholar] [CrossRef]
- Nair, M.; Choudhury, M.K.; Choudhury, S.S.; Kakoty, S.D.; Sarma, U.C.; Webster, P.; Knight, M. Association between maternal anaemia and pregnancy outcomes: A cohort study in Assam, India. BMJ Global Health 2016, 1, e000026. [Google Scholar] [CrossRef]
- Ker, K.; Roberts, I.; Chaudhri, R.; Fawole, B.; Beaumont, D.; Balogun, E.; Prowse, D.; Pepple, T.; Javaid, K.; Kayani, A. Tranexamic acid for the prevention of postpartum bleeding in women with anaemia: Study protocol for an international, randomised, double-blind, placebo-controlled trial. Trials 2018, 19, 712. [Google Scholar] [CrossRef] [PubMed]
| Study Design & Publication Type | Randomized Controlled Trials; Non-Randomized Trials; Peer-Reviewed (No Grey Literature) |
|---|---|
| Timeline | Published between 1 January 2011 and 15 September 2021 |
| P (population) | Women who had a vaginal or cesarean birth |
| C (concept) | Postpartum hemorrhage; feasibility; acceptability; health system considerations |
| C (context) | Low-income countries; lower-middle-income countries |
| Item (n = 23) | Count | % | Comment | ||
|---|---|---|---|---|---|
| Publication type | |||||
| Full-text articles | 15 | 65 | Abdel-Aleem, Bose, Briki, Diop, Joudeh, Li, McClure, Mirghafourvand, Naeiji, Nargis, Oseni, Sahu, Sujata, Tabatabaie, Zargar | ||
| Conference abstracts | 8 | 35 | Agrawal, Ajroudi, Dimassi, Dutta, Khaing, Nwabueze, Resch, Tali | ||
| Study country | |||||
| Low-income | 1 | 4 | Unspecified countries in sub-Saharan Africa (McClure) | ||
| Lower-middle-income | 23 | 100 | |||
| India | 6 | 26 | Bose, Dutta, Joudeh, Resch, Sahu, Jujata, | ||
| Iran | 4 | 17 | Mirghafourvand, Naeiji, Tabatabaie, Zargar | ||
| Tunisia | 3 | 13 | Ajroudi, Briki, Dimassi | ||
| Nigeria | 3 | 13 | Li, Nwabueze, Oseni | ||
| Others | 9 | 39 | Bangladesh (Nargis), Egypt (Abdel-Aleem) Myanmar (Khaing), Nepal (Agrawal), Pakistan (Li), Philippines (Tali), Senegal (Diop), Vietnam (Diop), unspecified countries in sub-Saharan Africa (McClure) | ||
| Study type | |||||
| Effectiveness for PPH | 20 | 87 | |||
| Prevention in cesarean birth | 11 | 55 | Abdel-Aleem, Agrawal, Bose, Dutta, Naeiji, Nargis, Oseni, Sahu, Sujata, Tabatabaie, Zargar | ||
| Prevention in vaginal birth | 5 | 25 | Khaing, Mirghafourvand, Nwabueze, Tali, Zargar | ||
| Treatment in cesarean birth | 4 | 20 | Ajroudi, Briki, Dimassi, Joudeh | ||
| Treatment in vaginal birth | 5 | 25 | Ajroudi, Briki, Dimassi, Diop, Joudeh | ||
| Economic evaluation | 2 | 9 | Li, Resh | ||
| Maternal mortality modeling | 1 | 4 | McClure | ||
| Outcome of interest | |||||
| Acceptability | 1 | 4 | Diop | ||
| Feasibility (operational) | 3 | 13 | Bose, Joudeh, Resch | ||
| Feasibility (financial) | 3 | 13 | Bose, Li, Resh | ||
| Study & Year | Countries | Levels of Care | Outcomes of Interest | Health System Environment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low-Income | Lower Middle-Income | BEmOC | Hospital CEmOC | Feasibility | Acceptability | Effectiveness | Governance & Policy Alignment | Procurement & Commodity Security | Health Staff Awareness, Motivation & Training | Service Delivery | Health Information System | Financing | |
| Abdel-Aleem, 2013 [18] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Agrawal, 2018 [19] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Ajroudi, 2015 [32] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Bose, 2017 [20] | - | ✓ | - | ✓ | ✓ | - | ✓ | - | ✓ | - | - | - | ✓ |
| Briki, 2018 [33] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Dimassi, 2018 [34] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Diop, 2020 [21] | - | ✓ | - | ✓ | - | ✓ a | ✓ | - | - | - | - | - | - |
| Dutta, 2017 [35] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Joudeh, 2021 [36] | - | ✓ | - | ✓ | ✓ | - | - | - | - | ✓ | - | - | - |
| Khaing, 2021 [22] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Li, 2018 [38] | - | ✓ | - | ✓ | ✓ | - | - | - | - | - | - | - | ✓ |
| McClure, 2015 [41] | ✓ | ✓ | ✓ b | ✓ | - | - | ✓ c | ✓ | - | - | - | - | - |
| Mirghafourvand, 2013 [23] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Naeiji, 2021 [24] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Nargis, 2020 [25] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Nwabueze, 2021 [26] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Oseni, 2021 [27] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Resch, 2020 [39] | - | ✓ | ✓ | ✓ | ✓ | - | ✓ | - | - | - | ✓ | - | ✓ |
| Sahu, 2019 [37] | - | ✓ | - | ✓ | - | - | ✓ | - | ✓ | - | - | - | - |
| Sujata, 2016 [28] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Tabatabaie, 2021 [29] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Tali, 2016 [30] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
| Zargar, 2018 [31] | - | ✓ | - | ✓ | - | - | ✓ | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.T.; Bar-Zeev, S.; Schulte-Hillen, C.; Zeck, W. Tranexamic Acid for Postpartum Hemorrhage Treatment in Low-Resource Settings: A Rapid Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 7385. https://doi.org/10.3390/ijerph19127385
Tran NT, Bar-Zeev S, Schulte-Hillen C, Zeck W. Tranexamic Acid for Postpartum Hemorrhage Treatment in Low-Resource Settings: A Rapid Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(12):7385. https://doi.org/10.3390/ijerph19127385
Chicago/Turabian StyleTran, Nguyen Toan, Sarah Bar-Zeev, Catrin Schulte-Hillen, and Willibald Zeck. 2022. "Tranexamic Acid for Postpartum Hemorrhage Treatment in Low-Resource Settings: A Rapid Scoping Review" International Journal of Environmental Research and Public Health 19, no. 12: 7385. https://doi.org/10.3390/ijerph19127385
APA StyleTran, N. T., Bar-Zeev, S., Schulte-Hillen, C., & Zeck, W. (2022). Tranexamic Acid for Postpartum Hemorrhage Treatment in Low-Resource Settings: A Rapid Scoping Review. International Journal of Environmental Research and Public Health, 19(12), 7385. https://doi.org/10.3390/ijerph19127385





