Abundance of Ixodes ricinus Ticks (Acari: Ixodidae) and the Diversity of Borrelia Species in Northeastern Poland
Abstract
:1. Introduction
2. Materials and Methods
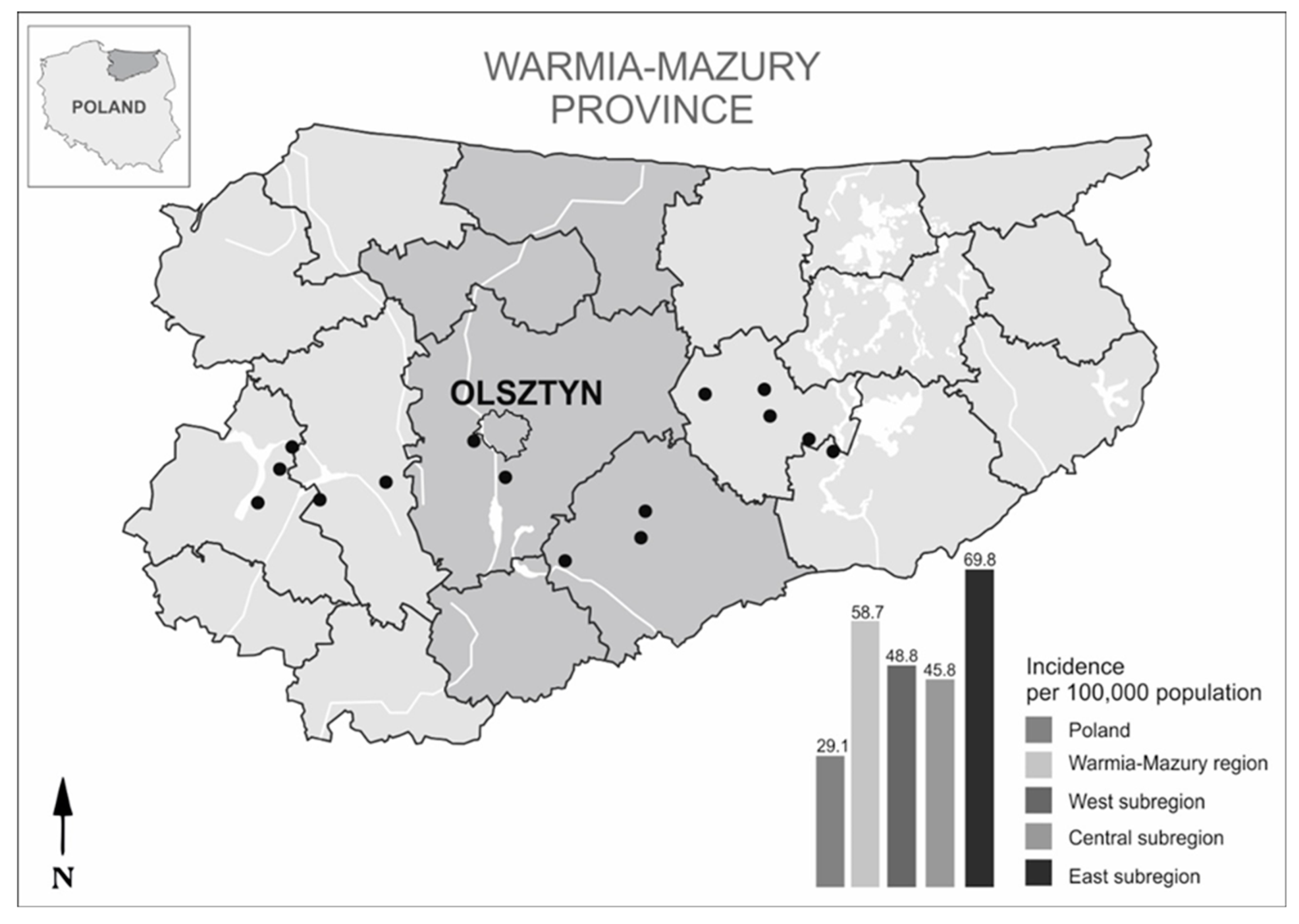
2.1. Study Area and Tick Collection
2.2. DNA Extraction
2.3. Borrelia Spirochaete DNA Detection
2.4. Borrelia Species Identification by the PCR-RFLP Method
2.5. Borrelia Species Identification by DNA Sequencing
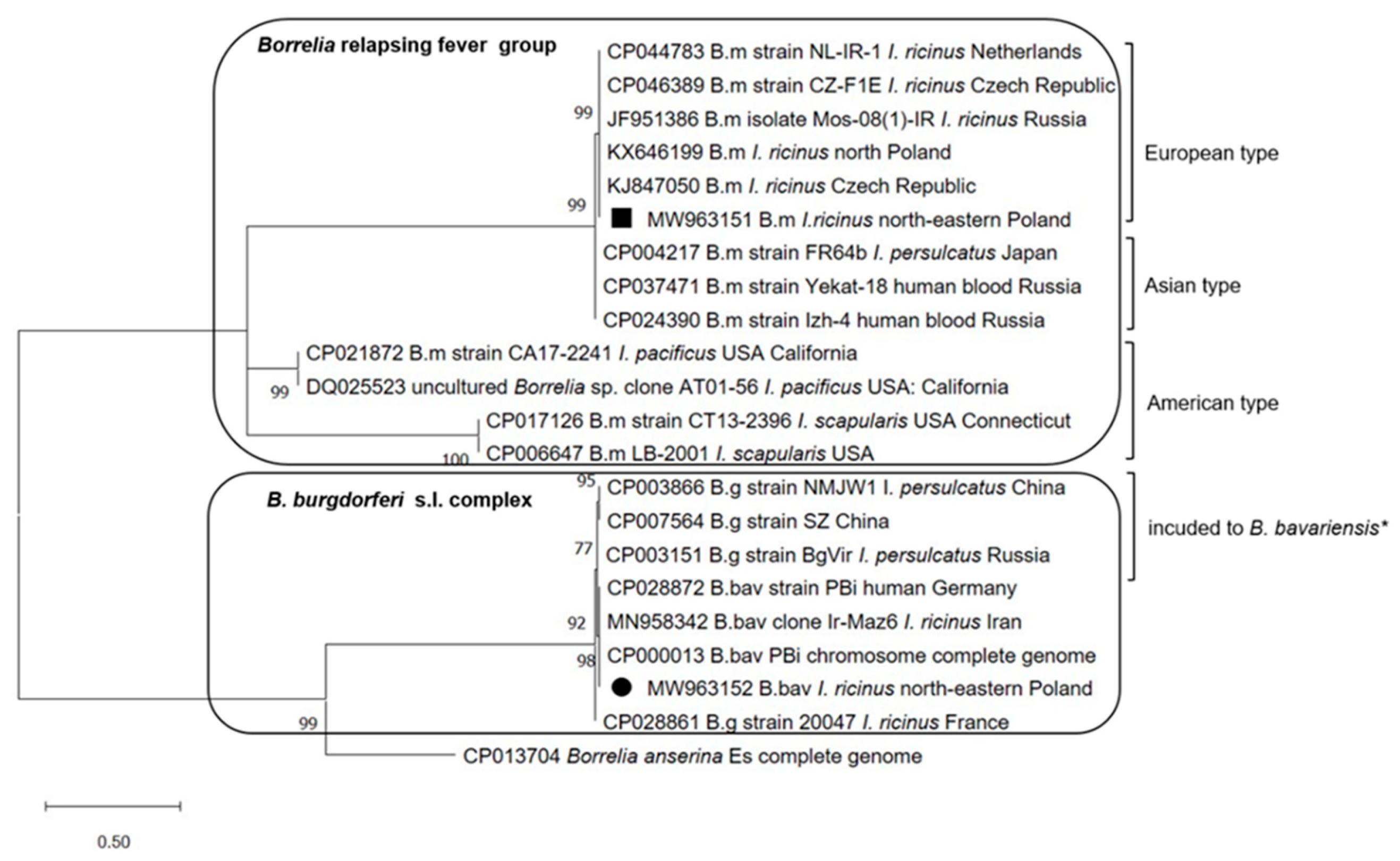
2.6. Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
3.1. Tick Density
3.2. Prevalence of Borrelia Spirochaetes
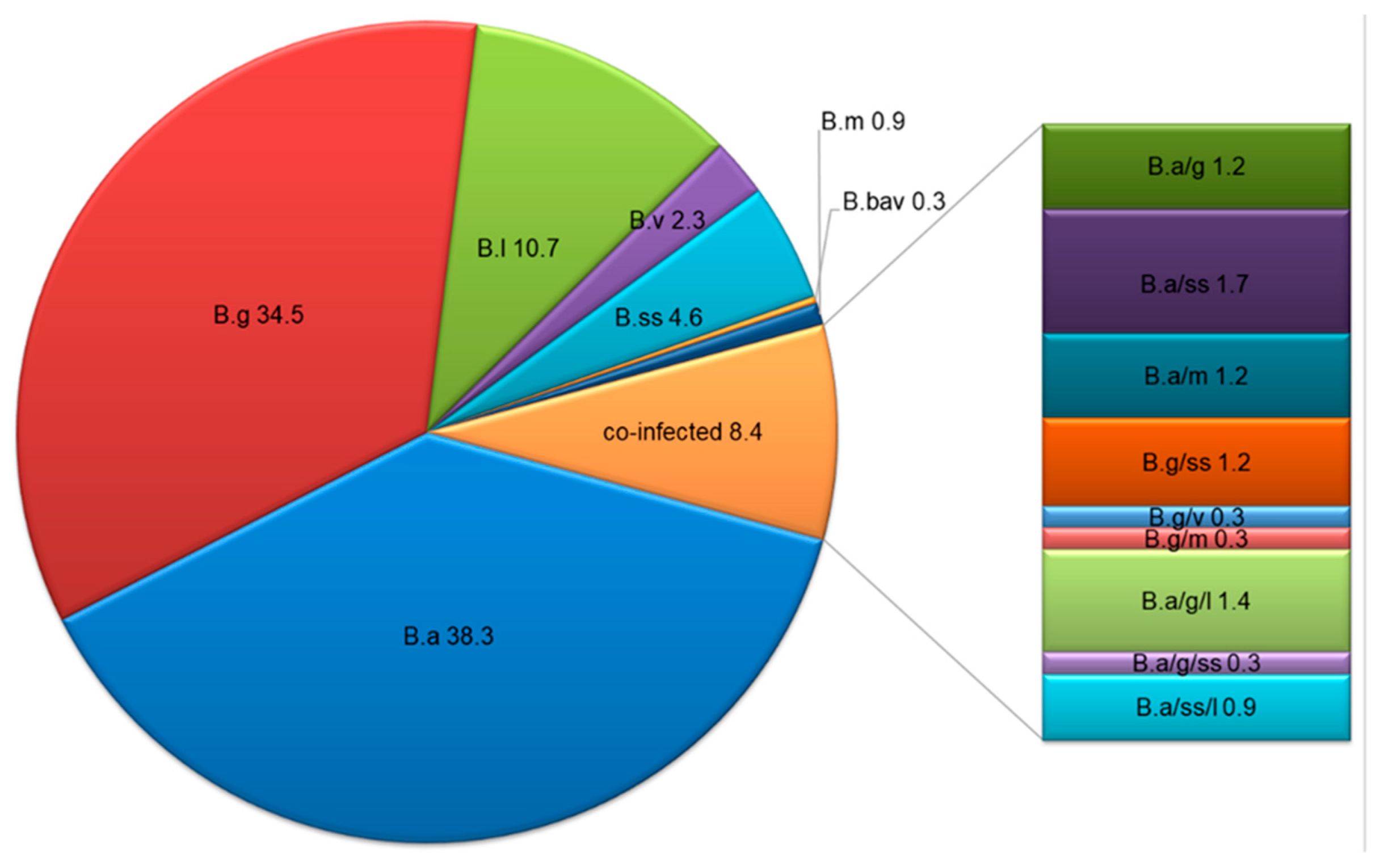
3.3. Borrelia Species Distribution
3.4. Genetic Diversity in flaB Gene in Borrelia Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clow, K.M.; Leighton, P.A.; Pearl, D.L.; Jardine, C.M. A Framework for Adaptive Surveillance of Emerging Tick-Borne Zoonoses. One Health 2019, 7, 100083. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Brief Guide to Emerging Infectious Diseases and Zoonoses; WHO Regional Office for South-East Asia: New Delhi, India, 2014.
- McArthur, D.B. Emerging Infectious Diseases. Nurs. Clin. N. Am. 2019, 54, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk Factors for Human Disease Emergence. Philos. Trans. R. Soc. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme Borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Radolf, J.D.; Strle, K.; Lemieux, J.E.; Strle, F. Lyme Disease in Humans. Curr. Issues Mol. Biol. 2020, 42, 333–384. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme Borreliosis. Nat. Rev. Dis. Prim. 2016, 2, 16090. [Google Scholar] [CrossRef] [Green Version]
- Stone, B.L.; Tourand, Y.; Brissette, C.A. Brave New Worlds: The Expanding Universe of Lyme Disease. Vector-Borne Zoonotic Dis. 2017, 17, 619–629. [Google Scholar] [CrossRef]
- Pritt, B.S.; Mead, P.S.; Johnson, D.K.H.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a Novel Pathogenic Borrelia Species Causing Lyme Borreliosis with Unusually High Spirochaetaemia: A Descriptive Study. Lancet Infect. Dis. 2016, 16, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-Wide Meta-Analysis of Borrelia Burgdorferi Sensu Lato Prevalence in Questing Ixodes Ricinus Ticks. Appl. Environ. Microbiol. 2017, 83, e0069-17. [Google Scholar] [CrossRef] [Green Version]
- Eisen, L. Vector Competence Studies with Hard Ticks and Borrelia Burgdorferi Sensu Lato Spirochetes: A Review. Ticks Tick. Borne Dis. 2020, 11, 101359. [Google Scholar] [CrossRef] [PubMed]
- Diza, E.; Papa, A.; Vezyri, E.; Tsounis, S.; Milonas, I.; Antoniadis, A. Borrelia Valaisiana in Cerebrospinal Fluid. Emerg. Infect. Dis. 2004, 10, 1692–1693. [Google Scholar] [CrossRef] [PubMed]
- Derdáková, M.; Lenčáková, D. Association of Genetic Variability within the Borrelia Burgdorferi Sensu Lato with the Ecology, Epidemiology of Lyme Borreliosis in Europe. Ann. Agric. Environ. Med. 2005, 12, 165–172. [Google Scholar] [CrossRef]
- Jungnick, S.; Margos, G.; Rieger, M.; Dzaferovic, E.; Bent, S.J.; Overzier, E.; Silaghi, C.; Walder, G.; Wex, F.; Koloczek, J.; et al. Borrelia Burgdorferi Sensu Stricto and Borrelia Afzelii: Population Structure and Differential Pathogenicity. Int. J. Med. Microbiol. 2015, 305, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Margos, G.; Sing, A.; Fingerle, V. Published Data Do Not Support the Notion That Borrelia Valaisiana Is Human Pathogenic. Infection 2017, 45, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Reiter, M. The Expanding Lyme Borrelia Complex—Clinical Significance of Genomic Species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, M.; Takahashi, Y.; Tsuruta, Y.; Matsushita, O.; Ralph, D.; McClelland, M.; Nakao, M. Genetic and Phenotypic Analysis of Borrelia Miyamotoi Sp. Nov., Isolated from the Ixodid Tick Ixodes Persulcatus, the Vector for Lyme Disease in Japan. Int. J. Syst. Bacteriol. 1995, 45, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Cutler, S.J.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. A New Borrelia on the Block: Borrelia Miyamotoi—A Human Health Risk? Eurosurveillance 2019, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, K.; Szczotko, M.; Dmitryjuk, M. Borrelia Miyamotoi—An Emerging Human Tick-Borne Pathogen in Europe. Microorganisms 2021, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Siński, E.; Welc-Falęciak, R.; Zajkowska, J. Borrelia Miyamotoi: A Human Tick-Borne Relapsing Fever Spirochete in Europe and Its Potential Impact on Public Health. Adv. Med. Sci. 2016, 61, 255–260. [Google Scholar] [CrossRef]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans Infected with Relapsing Fever Spirochete Borrelia Miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Rollend, L.; Fikrig, E.; Lepore, T.; Barbour, A.; Fish, D. Human Borrelia Miyamotoi Infection in the United States. N. Engl. J. Med. 2013, 368, 291–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molloy, P.J.; Telford, S.R.; Chowdri, H.R.; Lepore, T.J.; Gugliotta, J.L.; Weeks, K.E.; Hewins, M.E.; Goethert, H.K.; Berardi, V.P. Borrelia Miyamotoi Disease in the Northeastern United States a Case Series. Ann. Intern. Med. 2015, 163, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Franck, M.; Ghozzi, R.; Pajaud, J.; Lawson-Hogban, N.E.; Mas, M.; Lacout, A.; Perronne, C. Borrelia Miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients With Persistent Polymorphic Signs and Symptoms. Front. Med. 2020, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiecek, B.; Lewandowska, G.; Roguska, U.; Rozej-Bielicka, W.; Tylewska-Wierzbanowska, S.; Chmielewski, T. Borrelia Miyamotoi DNA in a Patient Suspected of Lyme Borreliosis. Available online: https://assets.researchsquare.com/files/rs-5981/v2/manuscript.pdf (accessed on 11 January 2021). [CrossRef] [Green Version]
- Hovius, J.W.R.; De Wever, B.; Sohne, M.; Brouwer, M.C.; Coumou, J.; Wagemakers, A.; Oei, A.; Knol, H.; Narasimhan, S.; Hodiamont, C.J.; et al. A Case of Meningoencephalitis by the Relapsing Fever Spirochaete Borrelia Miyamotoi in Europe. Lancet 2013, 382, 658. [Google Scholar] [CrossRef] [Green Version]
- Tobudic, S.; Burgmann, H.; Stanek, G.; Winkler, S.; Schtta, A.M.; Obermller, M.; Markowicz, M.; Lagler, H. Human Borrelia Miyamotoi Infection, Austria. Emerg. Infect. Dis. 2020, 26, 2201–2204. [Google Scholar] [CrossRef]
- Sato, K.; Takano, A.; Konnai, S.; Nakao, M.; Ito, T.; Koyama, K.; Kaneko, M.; Ohnishi, M.; Kawabata, H. Human Infections with Borrelia Miyamotoi, Japan. Emerg. Infect. Dis. 2014, 20, 1391–1393. [Google Scholar] [CrossRef]
- Jiang, B.G.; Jia, N.; Jiang, J.F.; Zheng, Y.C.; Chu, Y.L.; Jiang, R.R.; Wang, Y.W.; Liu, H.B.; Wei, R.; Zhang, W.H.; et al. Borrelia Miyamotoi Infections in Humans and Ticks, Northeastern China. Emerg. Infect. Dis. 2018, 24, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Lv, X.L.; Han, S.Z.; Wang, W.; Liu, Q.; Song, M. First Detection of Borrelia Miyamotoi Infections in Ticks and Humans from the Northeast of Inner Mongolia, China. Acta Trop. 2021, 217, 105857. [Google Scholar] [CrossRef]
- Strnad, M.; Grubhoffer, L.; Rego, R.O.M. Novel Targets and Strategies to Combat Borreliosis. Appl. Microbiol. Biotechnol. 2020, 104, 1915–1925. [Google Scholar] [CrossRef]
- Blazejak, K.; Raulf, M.K.; Janecek, E.; Jordan, D.; Fingerle, V.; Strube, C. Shifts in Borrelia Burgdorferi (s.l.) Geno-Species Infections in Ixodes Ricinus over a 10-Year Surveillance Period in the City of Hanover (Germany) and Borrelia Miyamotoi-Specific Reverse Line Blot Detection. Parasites Vectors 2018, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Peña, A.; De La Fuente, J. The Ecology of Ticks and Epidemiology of Tick-Borne Viral Diseases. Antiviral Res. 2014, 108, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Vu Hai, V.; Almeras, L.; Socolovschi, C.; Raoult, D.; Parola, P.; Pages, F. Monitoring Human Tick-Borne Disease Risk and Tick Bite Exposure in Europe: Available Tools and Promising Future Methods. Ticks Tick. Borne. Dis. 2014, 5, 607–619. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Public Health–National Institute of Hygiene–Department of Epidemiology Infectious Diseases and Poisonings in Poland. Available online: http://www.pzh.gov.pl/oldpage/epimeld/index_p.htm (accessed on 11 January 2021).
- Wegner, Z.; Stańczak, J.; Racewicz, M.; Kruminis-Lozowska, W.; Kubica-Biernat, B. Occurrence of Borrelia Spirochaetes in Ticks (Acari, Ixodidae) Collected in the Forest Areas in Olsztyn Province (North Central Poland). Bull. Inst. Marit. Trop. Med. Gdynia 1993, 44–45, 51–59. [Google Scholar]
- Stańczak, J.; Kubica-Biernat, B. Prevalence of Borrelia Burgdorferi in Ixodes Ricinus Ticks from Different Areas of Poland. Proc. Zent. Bakteriol. 1999, 289, 704–705. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Siński, E. Prevalence of Ixodes Ricinus Infection with Borrelia Burgdorferi s.l.: Seasonal and Annual Variations. Wiad Parazytol. 2004, 50, 253–258. [Google Scholar]
- Kowalec, M.; Szewczyk, T.; Welc-Falęciak, R.; Siński, E.; Karbowiak, G.; Bajer, A. Ticks and the City—Are There Any Differences between City Parks and Natural Forests in Terms of Tick Abundance and Prevalence of Spirochaetes? Parasites Vectors 2017, 10, 573. [Google Scholar] [CrossRef]
- Kubiak, K.; Dziekońska-Rynko, J.; Szymańska, H.; Kubiak, D.; Dmitryjuk, M.; Dzika, E. Questing Ixodes Ricinus Ticks (Acari, Ixodidae) as a Vector of Borrelia Burgdorferi Sensu Lato and Borrelia Miyamotoi in an Urban Area of North-Eastern Poland. Exp. Appl. Acarol. 2019, 78, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Nowak-Chmura, M. Fauna Kleszczy (Ixodida) Europy Środkowej; Wydawnictwo Naukowe Uniwersytetu Pedagogicznego: Krakow, Poland, 2013. [Google Scholar]
- Marconi, R.T.; Garon, C.F. Erratum: Development of Polymerase Chain Reaction Primer Sets for Diagnosis of Lyme Disease and for Species-Specific Identification of Lyme Disease Isolates by 16S RRNA Signature Nucleotide Analysis (Journal of Clinical Microbiology 30:11 (2831)). J. Clin. Microbiol. 1993, 31, 1026. [Google Scholar]
- Demaerschalck, I.; Ben Messaoud, A.; De Kesel, M.; Hoyois, B.; Lobet, Y.; Hoet, P.; Bigaignon, G.; Bollen, A.; Godfroid, E. Simultaneous Presence of Different Borrelia Burgdorferi Genospecies in Biological Fluids of Lyme Disease Patients. J. Clin. Microbiol. 1995, 33, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Stańczak, J.; Kubica-Biernat, B.; Burkiewicz, A.; Racewicz, M.; Kruminis-Łozowska, W.; Kur, J. Preliminary Studies of the Use of Polymerase Chain Reaction (PCR) for the Detection of Borrelia Burgdorferi Sensu in Ticks Ixodes Ricinus (Acari, Ixodidae). Probl. Hig. I Epidemiol. 1997, 54, 122–126. [Google Scholar]
- Strzelczyk, J.K.; Gaździcka, J.; Cuber, P.; Asman, M.; Trapp, G.; Gołąbek, K.; Zalewska-Ziob, M.; Nowak-Chmura, M.; Siuda, K.; Wiczkowski, A.; et al. Prevalence of Borrelia Burgdorferi Sensu Lato in Ixodes Ricinus Ticks Collected from Southern Poland. Acta Parasitol. 2015, 60, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Leońska, A.; Skotarczak, B. A Comparative Analysis of Molecular Markers for the Detection and Identification of Borrelia Spirochaetes in Ixodes Ricinus. J. Med. Microbiol. 2010, 59, 309–314. [Google Scholar] [CrossRef]
- Wagemakers, A.; Jahfari, S.; de Wever, B.; Spanjaard, L.; Starink, M.V.; de Vries, H.J.C.; Sprong, H.; Hovius, J.W. Borrelia Miyamotoi in Vectors and Hosts in The Netherlands. Ticks Tick. Borne Dis. 2017, 8, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B. FlaB Gene as a Molecular Marker for Distinct Identification of Borrelia Species in Environmental Samples by the PCR-Restriction Fragment Length Polymorphism Method. Appl. Environ. Microbiol. 2011, 77, 7088–7092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Becker, N.S.; Rollins, R.E.; Nosenko, K.; Paulus, A.; Martin, S.; Krebs, S.; Takano, A.; Sato, K.; Kovalev, S.Y.; Kawabata, H.; et al. High Conservation Combined with High Plasticity: Genomics and Evolution of Borrelia Bavariensis. BMC Genom. 2020, 21, 702. [Google Scholar] [CrossRef]
- Kubiak, K.; Sielawa, H.; Dziekońska-Rynko, J.; Kubiak, D.; Rydzewska, M.; Dzika, E. Dermacentor Reticulatus Ticks (Acari: Ixodidae) Distribution in North-Eastern Poland: An Endemic Area of Tick-Borne Diseases. Exp. Appl. Acarol. 2018, 75, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Pawełczyk, A.; Bednarska, M.; Hamera, A.; Religa, E.; Poryszewska, M.; Mierzejewska, E.J.; Welc-Falęciak, R. Long-Term Study of Borrelia and Babesia Prevalence and Co-Infection in Ixodes Ricinus and Dermacentor Recticulatus Ticks Removed from Humans in Poland, 2016–2019. Parasites Vectors 2021, 14, 348. [Google Scholar] [CrossRef]
- Springer, A.; Raulf, M.K.; Fingerle, V.; Strube, C. Borrelia Prevalence and Species Distribution in Ticks Removed from Humans in Germany, 2013–2017. Ticks Tick. Borne Dis. 2020, 11, 101363. [Google Scholar] [CrossRef] [PubMed]
- Lernout, T.; De Regge, N.; Tersago, K.; Fonville, M.; Suin, V.; Sprong, H. Prevalence of Pathogens in Ticks Collected from Humans through Citizen Science in Belgium. Parasites Vectors 2019, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Kalmár, Z.; Dumitrache, M.; D’Amico, G.; Matei, I.A.; Ionică, A.M.; Gherman, C.M.; Lupșe, M.; Mihalca, A.D. Multiple Tick-borne Pathogens in Ixodes Ricinus Ticks Collected from Humans in Romania. Pathogens 2020, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, K.; Dziekońska-Rynko, J. Seasonal Activity of the Common European Tick, Ixodes Ricinus (Linnaeus, 1758), in the Forested Areas of the City of Olsztyn and Its Surroundings. Wiadomości. Parazytol. 2006, 52, 59–64. [Google Scholar]
- Hamšíková, Z.; Coipan, C.; Mahríková, L.; Minichová, L.; Sprong, H.; Kazimírová, M. Borrelia Miyamotoi and Co-Infection with Borrelia Afzelii in Ixodes Ricinus Ticks and Rodents from Slovakia. Microb. Ecol. 2017, 73, 1000–1008. [Google Scholar] [CrossRef]
- Dobson, A.D.M.; Taylor, J.L.; Randolph, S.E. Tick (Ixodes Ricinus) Abundance and Seasonality at Recreational Sites in the UK: Hazards in Relation to Fine-Scale Habitat Types Revealed by Complementary Sampling Methods. Ticks Tick. Borne. Dis. 2011, 2, 67–74. [Google Scholar] [CrossRef]
- Estrada-Peña, A. Understanding the Relationships between Landscape Connectivity and Abundance of Ixodes Ricinus Ticks. Exp. App. Acarol. 2002, 28, 239–248. [Google Scholar] [CrossRef]
- Zarząd Województwa Warmińsko-Mazurskie. Program Ochrony Środowiska Województwa Warmińsko-Mazurskiego Do Roku 2020. Available online: https://bip.warmia.mazury.pl (accessed on 15 April 2021).
- Siński, E.; Pawełczyk, A.; Bajer, A.; Behnke, J.M. Abundance of Wild Rodents, Ticks and Environmental Risk of Lyme Borreliosis: A Longitudinal Study in an Area of Mazury Lakes District of Poland. Ann. Agric. Environ. Med. 2006, 13, 295–300. [Google Scholar]
- Michalski, M.M.; Kubiak, K.; Szczotko, M.; Dmitryjuk, M. Tick-Borne Pathogens in Ticks Collected from Wild Ungulates in North-Eastern Poland. Pathogens 2021, 10, 587. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Špitalská, E.; et al. Ixodes Ricinus and Its Transmitted Pathogens in Urban and Peri-Urban Areas in Europe: New Hazards and Relevance for Public Health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Dobson, A.D.M.; Levi, T.; Salkeld, D.J.; Swei, A.; Ginsberg, H.S.; Kjemtrup, A.; Padgett, K.A.; Jensen, P.M.; Fish, D.; et al. Lyme Disease Ecology in a Changing World: Consensus, Uncertainty and Critical Gaps for Improving Control. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160117. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E. EDEN-TBD sub-project team Human Activities Predominate in Determining Changing Incidence of Tick-Borne Encephalitis in Europe. Euro Surveill. 2010, 15, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karbowiak, G.; Biernat, B.; Stańczak, J.; Werszko, J.; Szewczyk, T.; Sytykiewicz, H. The Role of Particular Ticks Developmental Stages in the Circulation of Tick-Borne Pathogens in Central Europe. 5. Borreliaceae. Ann. Parasitol. 2018, 64, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Asman, M.; Witecka, J.; Korbecki, J.; Solarz, K. The Potential Risk of Exposure to Borrelia Garinii, Anaplasma Phagocytophilum and Babesia Microti in the Wolinski National Park (North-Western Poland). Sci. Rep. 2021, 11, 4860. [Google Scholar] [CrossRef]
- Stańczak, J.; Kubica-Biernat, B.; Racewicz, M.; Kruminis-Łozowska, W.; Kur, J. Detection of Three Genospecies of Borrelia Burgdorferi Sensu Lato in Ixodes Ricinus Ticks Collected in Different Regions of Poland. Int. J. Med. Microbiol. 2000, 290, 559–566. [Google Scholar] [CrossRef]
- Stańczak, J.; Racewicz, M.; Kubica-Biernat, B.; Kruminis-Łozowska, W.; Dabrowski, J.; Adamczyk, A.; Markowska, M. Prevalence of Borrelia Burgdorferi Sensu Lato in Ixodes Ricinus Ticks (Acari, Ixodidae) in Different Polish Woodlands. Ann. Agric. Environ. Med. 1999, 6, 127–132. [Google Scholar]
- Pawelczyk, A.; Sinski, E. Co-Infection of Borrelia Garinii and B. Afzelii in a Population of Wild Rodents from Woodland. Wiad Parazytol. 2001, 47, 741–746. [Google Scholar]
- Rauter, C.; Hartung, T. Prevalence of Borrelia Burgdorferi Sensu Lato Genospecies in Ixodes Ricinus Ticks in Europe: A Metaanalysis. Appl. Environ. Microbiol. 2005, 71, 7203–7216. [Google Scholar] [CrossRef] [Green Version]
- Tappe, J.; Jordan, D.; Janecek, E.; Fingerle, V.; Strube, C. Revisited: Borrelia Burgdorferi Sensu Lato Infections in Hard Ticks (Ixodes Ricinus) in the City of Hanover (Germany). Parasit. Vectors 2014, 7, 441. [Google Scholar] [CrossRef] [Green Version]
- Zintl, A.; Zaid, T.; McKiernan, F.; Naranjo-Lucena, A.; Gray, J.; Brosnan, S.; Browne, J.; O’Connor, J.; Mee, J.; Good, B.; et al. Update on the Presence of Ixodes Ricinus at the Western Limit of Its Range and the Prevalence of Borrelia Burgdorferi Sensu Lato. Ticks Tick. Borne. Dis. 2020, 11, 101518. [Google Scholar] [CrossRef]
- Michalski, M.M.; Kubiak, K.; Szczotko, M.; Chajęcka, M.; Dmitryjuk, M. Molecular Detection of Borrelia Burgdorferi Sensu Lato and Anaplasma Phagocytophilum in Ticks Collected from Dogs in Urban Areas of North-Eastern Poland. Pathogens 2020, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, S.; Ruyts, S.C.; Scherer-Lorenzen, M.; Bauhus, J.; Brunet, J.; Cousins, S.A.O.; Deconchat, M.; Decocq, G.; De Frenne, P.; De Smedt, P.; et al. Habitat Properties Are Key Drivers of Borrelia Burgdorferi (s.l.) Prevalence in Ixodes Ricinus Populations of Deciduous Forest Fragments. Parasites Vectors 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Rollins, R.E.; Yeyin, Z.; Wyczanska, M.; Alig, N.; Hepner, S.; Fingerle, V.; Margos, G.; Becker, N.S. Spatial Variability in Prevalence and Genospecies Distributions of Borrelia Burgdorferi Sensu Lato from Ixodid Ticks Collected in Southern Germany. Ticks Tick. Borne Dis. 2021, 12, 101589. [Google Scholar] [CrossRef] [PubMed]
- Margos, G.; Vollmer, S.A.; Cornet, M.; Garnier, M.; Fingerle, V.; Wilske, B.; Bormane, A.; Vitorino, L.; Collares-Pereira, M.; Drancourt, M.; et al. A New Borrelia Species Defined by Multilocus Sequence Analysis of Housekeeping Genes. Appl. Environ. Microbiol. 2009, 75, 5410–5416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokarska-Rodak, M.; Plewik, D.; Kozioł-Montewka, M.; Szepeluk, A.; Paszkiewicz, J. Ryzyko Zakażeń Zawodowych Borrelia Burgdorferi u Pracowników Leśnictwa i Rolników. Med. Pr. 2014, 65, 109–117. [Google Scholar] [CrossRef]
- Szekeres, S.; Lügner, J.; Fingerle, V.; Margos, G.; Földvári, G. Prevalence of Borrelia Miyamotoi and Borrelia Burgdorferi Sensu Lato in Questing Ticks from a Recreational Coniferous Forest of East Saxony, Germany. Ticks Tick. Borne. Dis. 2017, 8, 922–927. [Google Scholar] [CrossRef]
- Ćakić, S.; Veinović, G.; Cerar, T.; Mihaljica, D.; Sukara, R.; Ružić-Sabljić, E.; Tomanović, S. Diversity of Lyme Borreliosis Spirochetes Isolated from Ticks in Serbia. Med. Vet. Entomol. 2019, 33, 512–520. [Google Scholar] [CrossRef]
- Gern, L.; Douet, V.; López, Z.; Rais, O.; Cadenas, F.M. Diversity of Borrelia Genospecies in Ixodes Ricinus Ticks in a Lyme Borreliosis Endemic Area in Switzerland Identified by Using New Probes for Reverse Line Blotting. Ticks Tick. Borne. Dis. 2010, 1, 23–29. [Google Scholar] [CrossRef]
- Glatz, M.; Muellegger, R.R.; Hizo-Teufel, C.; Fingerle, V. Low Prevalence of Borrelia Bavariensis in Ixodes Ricinus Ticks in Southeastern Austria. Ticks Tick. Borne Dis. 2014, 5, 649–650. [Google Scholar] [CrossRef]
- Kiewra, D.; Stańczak, J.; Richter, M. Ixodes Ricinus Ticks (Acari, Ixodidae) as a Vector of Borrelia Burgdorferi Sensu Lato and Borrelia Miyamotoi in Lower Silesia, Poland—Preliminary Study. Ticks Tick. Borne Dis. 2014, 5, 892–897. [Google Scholar] [CrossRef]
- Fiecek, B.; Chmielewski, T. Borrelia Miyamotoi—New Etiologic Agent Of Neuroborreliosis? Przegl. Epidemiol. 2017, 71, 531–538. [Google Scholar] [PubMed]
- O’bier, N.S.; Hatke, A.L.; Camire, A.C.; Marconi, R.T. Human and Veterinary Vaccines for Lyme Disease. Curr. Issues Mol. Biol. 2021, 42, 191–222. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, A.; Milewski, R.; Pancewicz, S.; Dunaj, J.; Czupryna, P.; Milewska, A.J.; Róg-Makal, M.; Grygorczuk, S.; Moniuszko-Malinowska, A. Comparison of Tick-Borne Pathogen Prevalence in Ixodes Ricinus Ticks Collected in Urban Areas of Europe. Sci. Rep. 2020, 10, 6975. [Google Scholar] [CrossRef] [PubMed]
- Sykes, R.A.; Makiello, P. An Estimate of Lyme Borreliosis Incidence InWestern Europe. J. Public Health (Oxf.) 2017, 39, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Remesar, S.; Díaz, P.; Venzal, J.M.; Prieto, A.; Estrada-Peña, A.; López, C.M.; Panadero, R.; Fernández, G.; Díez-Baños, P.; Morrondo, P. Longitudinal Study of Infection with Borrelia Spp. in Questing Ticks from North-Western Spain. Vector-Borne Zoonotic Dis. 2019, 19, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Mathews-Martin, L.; Namèche, M.; Vourc’h, G.; Gasser, S.; Lebert, I.; Poux, V.; Barry, S.; Bord, S.; Jachacz, J.; Chalvet-Monfray, K.; et al. Questing Tick Abundance in Urban and Peri-Urban Parks in the French City of Lyon. Parasites Vectors 2020, 13, 576. [Google Scholar] [CrossRef]
- Hansford, K.M.; Fonville, M.; Gillingham, E.L.; Coipan, E.C.; Pietzsch, M.E.; Krawczyk, A.I.; Vaux, A.G.C.; Cull, B.; Sprong, H.; Medlock, J.M. Ticks and Borrelia in Urban and Peri-Urban Green Space Habitats in a City in Southern England. Ticks Tick. Borne Dis. 2017, 8, 353–361. [Google Scholar] [CrossRef]
| Locus | Primer Name | Primer Sequence 5′-3′ | Product Size [bp] | Reference | Annealing Step in PCR |
|---|---|---|---|---|---|
| Borrelia spp. | |||||
| 16S rRNA | LDF | ATGCACACTTGGTGTTAACTA | 357 | [43] | 53 °C/30 s |
| LDR | GACTTATCACCGGCAGTCTTA | ||||
| ospA | SL1 | AATAGGTCTAATAATAGCCTTAATAGC | 307 | [44] | 65 °C/90 s |
| SL2 | CTAGTGTTTTGCCATCTTCTTTGAAAA | ||||
| flaB | BFL1 | GCTCAATATAACCAAATGCACATG | 422 | [45,46] | 58 °C/45 s |
| BFL2 | CAAGTCTATTTTGGAAAGCACCTAA | ||||
| 132f | TGGTATGGGAGTTCTGG | 774 | [47] | 52 °C/30 s | |
| 905r | TCTGTCATTGTAGCATCTTT | ||||
| 220f | CAGACAACAGAGGGAAAT | 604 | 54 °C/20 s | ||
| 823r | TCAAGTCTATTTTGGAAAGCACC | ||||
| B. miyamotoi | |||||
| flaB | BmF | AACTTGCTGTTCAGTCTGGT | 424 | [40] | 54 °C/20 s |
| BmR | TTAACTCCACCTTGAACTGG | ||||
| glpQ | forward | ATGGGTTCAAACAAAAAGTCACC | 700 | [48] | 53 °C/30 s |
| reverse | CCAGGGTCCAATTTCATCAGAATATTGTGCAAC |
| Year | Subregion | Mean ± SD | |||
|---|---|---|---|---|---|
| West | Central | East | |||
| Nymphs | 2016 | 10.6 ± 3.25 a | 7.6 ± 2.37 b | 8.3 ± 2.91 a,b | 8.8 ± 3.08 a |
| 2017 | 7.7 ± 2.84 a | 7.6 ± 2.84 a | 4.9 ± 2.10 b | 6.8 ± 2.87 b | |
| Mean | 9.2 ± 3.33 a | 7.6 ± 2.56 b | 6.6 ± 3.03 b | 7.8 ± 3.14 | |
| Females | 2016 | 0.5 ± 0.45 a | 0.6 ± 0.78 a | 1.1 ± 1.31 a | 0.8 ± 0.94 a |
| 2017 | 0.7 ± 0.65 a | 1.1 ± 0.77 a | 1.3 ± 0.86 a | 1.0 ± 0.79 a | |
| Mean | 0.6 ± 0.56 a | 0.8 ± 0.79 a,b | 1.2 ± 1.10 b | 0.9 ± 0.87 | |
| Males | 2016 | 0.6 ± 0.53 a | 0.7 ± 0.84 a | 1.0 ± 1.18 a | 0.8 ± 0.89 a |
| 2017 | 0.9 ± 1.07 a | 1.5 ± 1.21 a | 1.5 ± 1.12 a | 1.3 ± 1.14 b | |
| Mean | 0.8 ± 0.85 a | 1.1 ± 1.09 a | 1.3 ± 1.15 a | 1.0 ± 1.05 | |
| Total | 2016 | 11.7 ± 3.25 a | 8.9 ± 2.49 b | 10.5 ± 3.39 a,b | 10.4 ± 3.22 a |
| 2017 | 9.4 ± 3.22 a | 10.2 ± 2.89 a | 7.7 ± 2.64 a | 9.1 ± 3.04 a | |
| Mean | 10.9 ± 4.40 a | 9.5 ± 2.73 a | 9.1 ± 3.30 a | 9.7 ± 3.2 | |
| No. of Tested Ticks | Borrelia-Positive n/% * (95% CI) | p-Value ** | ||
|---|---|---|---|---|
| Stage | Nymphs | 3420 | 189/5.5 a (4.8–6.3) | <0.001 |
| Females | 399 | 90/22.6 b (18.4–26.7) | ||
| Males | 462 | 66/14.3 c (11.1–17.5) | ||
| Year | 2016 | 2313 | 158/6.8 a (5.8–7.9) | <0.001 |
| 2017 | 1968 | 187/9.5 b (8.2–10.8) | ||
| Subregion | West | 1559 | 89/5.7 a (4.6–6.9) | <0.001 |
| Central | 1351 | 118/8.7 b (7.2–10.2) | ||
| East | 1371 | 138/10.1 b (8.5–11.7) | ||
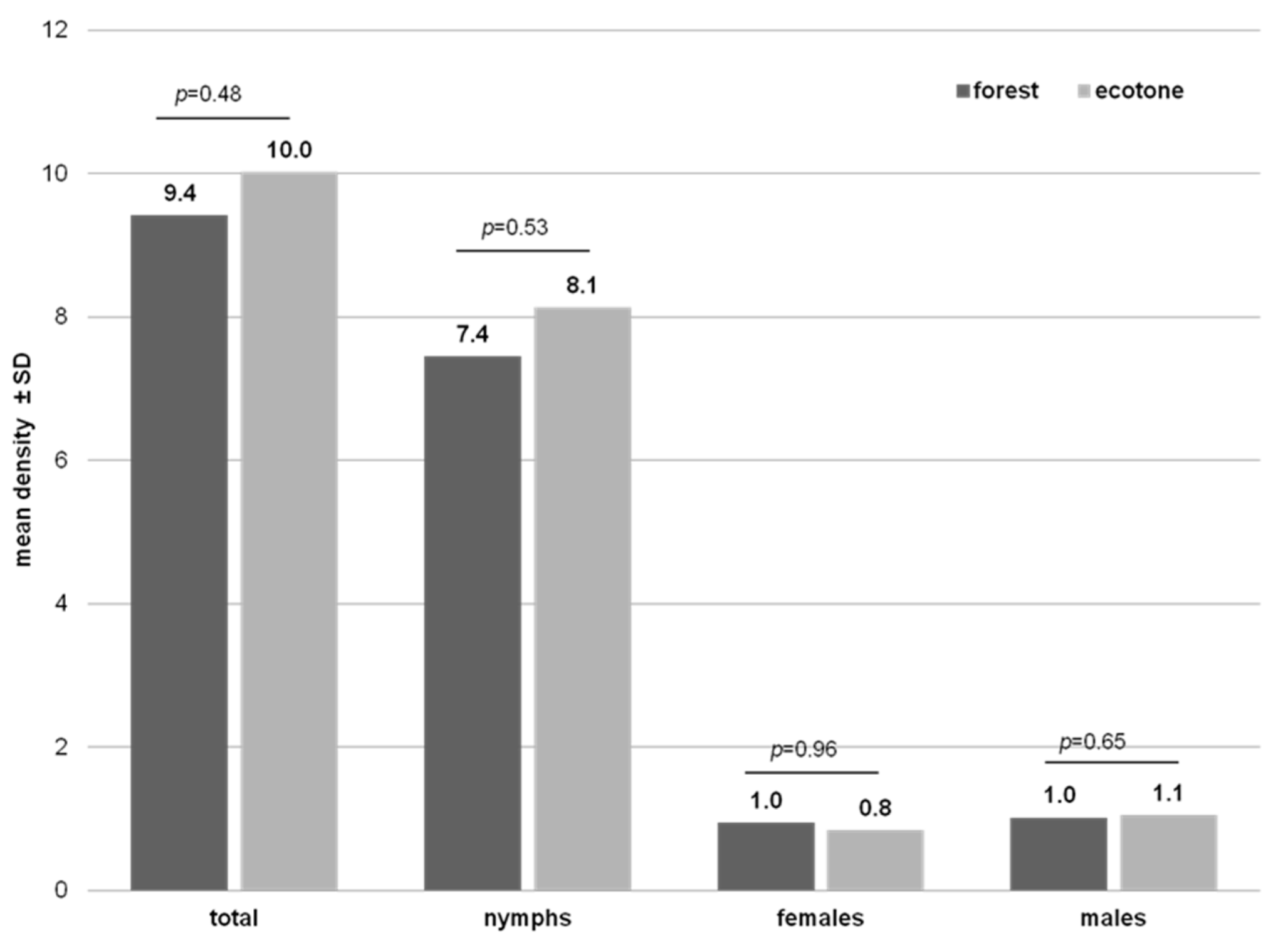
| Habitat | Forest | 1945 | 172/8.8 a (7.6–10.1) | 0.085 |
| Ecotone | 2336 | 173/7.4 a (6.3–8.5) | ||
| Total | 4281 | 345/8.1 (7.2–8.9) | ||
| Stage (n/%) | Year (n/%) | Subregion (n/%) | Habitat (n/%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | N * | 2016 | 2017 | West | Central | East | Forest | Ecotone | ||
| mono-infection | B.a | 38 a/42.2 | 27 a/40.9 | 67 a/35.4 | 65 a/41.1 | 67 a/35.8 | 27b/30.3 | 60 a/50.8 | 45b/32.6 | 65 a/37.8 | 67 a/38.7 |
| B.g | 27a.b/30.0 | 14b/21.2 | 78 a/41.3 | 51 a/32.3 | 68 a/36.4 | 38 a/42.7 | 34 a/28.8 | 47 a/34.1 | 60 a/34.9 | 59 a/34.1 | |
| B.l | 14 a/15.6 | 13 a/19.7 | 10 b/5.3 | 17 a/10.8 | 20 a/10.7 | 8 a,b/9.0 | 4 a/3.4 | 25b/18.1 | 14 a/8.1 | 23 a/13.3 | |
| B.v | 0 a/0.0 | 1 a/1.5 | 7 a/3.7 | 3 a/1.9 | 5 a/2.7 | 2 a/2.2 | 4 a/3.4 | 2 a/1.4 | 2 a/1.2 | 6 a/3.5 | |
| B.ss | 4 a/4.4 | 5 a/7.6 | 7 a/3.7 | 8 a/5.1 | 8 a/4.3 | 3 a/3.4 | 7 a/5.9 | 6 a/4.3 | 13 a/7.6 | 3 b/1.7 | |
| B.bav | 0 a/0.0 | 1 a/1.5 | 0 a/0.0 | 0 a/0.0 | 1 a/0.5 | 0 a/0.0 | 1 a/0.8 | 0 a/0.0 | 0 a/0.0 | 1 a/0.6 | |
| B.m | 0 a/0.0 | 1 a/1.5 | 2 a/1.1 | 1 a/0.6 | 2 a/1.1 | 1 a/1.1 | 1 a/0.8 | 1 a/0.7 | 1 a/0.6 | 2 a/1.2 | |
| Subtotal | 83 a/92.2 | 62 a/93.9 | 171 a/90.5 | 145 a/91.8 | 171 a/91.4 | 79 a/88.8 | 111 a/94.1 | 126 a/91.3 | 155 a/90.1 | 161 a/93.1 | |
| co-infection | B.a/B.g | 1 a/1.1 | 0 a/0.0 | 3 a/1.6 | 1 a/0.6 | 3 a/1.6 | 0 a/0.0 | 1 a/0.8 | 3 a/2.2 | 2 a/1.2 | 2 a/1.2 |
| B.a/B.ss | 4 a/4.4 | 2 a/3.0 | 0 b/0.0 | 0 a/0.0 | 6 b/3.2 | 1 a/1.1 | 2 a/1.7 | 3 a/2.2 | 4 a/2.3 | 2 a/1.2 | |
| B.a/B.m | 0 a/0.0 | 0 a/0.0 | 4 a/2.1 | 3 a/1.9 | 1 a/0.5 | 3 a/3.4 | 0 a/0.0 | 1 a/0.7 | 2 a/1.2 | 2 a/1.2 | |
| B.g/B.ss | 1 a/1.1 | 0 a/0.0 | 3 a/1.6 | 2 a/1.3 | 2 a/1.1 | 3 a/3.4 | 1 a/0.8 | 0 a/0.0 | 3 a/1.7 | 1 a/0.6 | |
| B.g/B.v | 1 a/1.1 | 0 a/0.0 | 0 a/0.0 | 1 a/0.6 | 0 a/0.0 | 0 a/0.0 | 0 a/0.0 | 1 a/0.7 | 1 a/0.6 | 0 a/0.0 | |
| B.g/B.m | 0 a/0.0 | 0 a/0.0 | 1 a/0.5 | 0 a/0.0 | 1 a/0.5 | 0 a/0.0 | 0 a/0.0 | 1 a/0.7 | 1 a/0.6 | 0 a/0.0 | |
| B.a/B.g/B.l | 0 a/0.0 | 0a/0.0 | 5 a/2.6 | 3 a/1.9 | 2 a/1.1 | 1 a/1.1 | 2 a/1.7 | 2 a/1.4 | 3 a/1.7 | 2 a/1.2 | |
| B.a/B.g/B.ss | 0 a/0.0 | 1 a/1.5 | 0 a/0.0 | 0 a/0.0 | 1 a/0.5 | 1 a/1.1 | 0 a/0.0 | 0 a/0.0 | 0 a/0.0 | 1 a/0.6 | |
| B.a/B.ss/B.l | 0 a/0.0 | 1 a/1.5 | 2 a/1.1 | 3 a/1.9 | 0 a/0.0 | 1 a/1.1 | 1 a/0.8 | 1 a/0.7 | 1 a/0.6 | 2 a/1.2 | |
| Subtotal | 7 a/7.8 | 4 a/6.1 | 18 a/9.5 | 13 a/8.2 | 16 a/8.6 | 10 a/11.2 | 7 a/5.9 | 12 a/8.7 | 17 a/9.9 | 12 a/6.9 | |
| p-value ** | 0.002 | 0.355 | 0.025 | 0.318 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, K.; Szymańska, H.; Dmitryjuk, M.; Dzika, E. Abundance of Ixodes ricinus Ticks (Acari: Ixodidae) and the Diversity of Borrelia Species in Northeastern Poland. Int. J. Environ. Res. Public Health 2022, 19, 7378. https://doi.org/10.3390/ijerph19127378
Kubiak K, Szymańska H, Dmitryjuk M, Dzika E. Abundance of Ixodes ricinus Ticks (Acari: Ixodidae) and the Diversity of Borrelia Species in Northeastern Poland. International Journal of Environmental Research and Public Health. 2022; 19(12):7378. https://doi.org/10.3390/ijerph19127378
Chicago/Turabian StyleKubiak, Katarzyna, Hanna Szymańska, Małgorzata Dmitryjuk, and Ewa Dzika. 2022. "Abundance of Ixodes ricinus Ticks (Acari: Ixodidae) and the Diversity of Borrelia Species in Northeastern Poland" International Journal of Environmental Research and Public Health 19, no. 12: 7378. https://doi.org/10.3390/ijerph19127378
APA StyleKubiak, K., Szymańska, H., Dmitryjuk, M., & Dzika, E. (2022). Abundance of Ixodes ricinus Ticks (Acari: Ixodidae) and the Diversity of Borrelia Species in Northeastern Poland. International Journal of Environmental Research and Public Health, 19(12), 7378. https://doi.org/10.3390/ijerph19127378







