Potential Cost Savings for the Healthcare System by Physical Activity in Different Chronic Diseases: A Pilot Study in the Veneto Region of Italy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
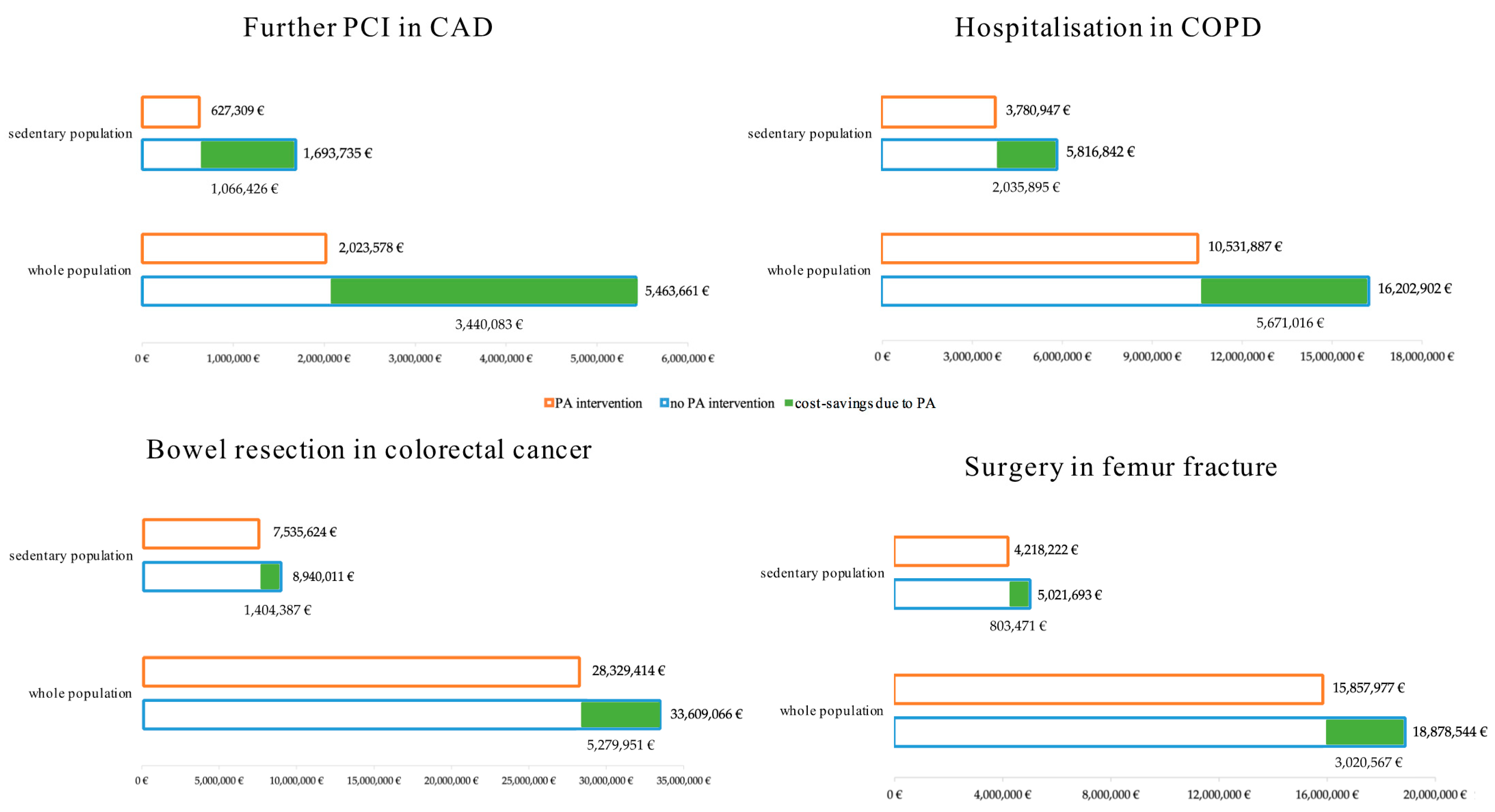
3.1. Coronary Artery Disease
3.2. Chronic Obstructive Pulmonary Disease
3.3. Colorectal Cancer
3.4. Femur Fracture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sedentary Behaviour Research Network. Standardized use of the terms ‘sedentary’ and ‘sedentary behaviours’. Appl. Physiol. Nutr. Metab. 2012, 37, 540–542. [Google Scholar] [CrossRef] [Green Version]
- Warburton, D.E.R.; Bredin, S.S.D. Reflections on Physical Activity and Health: What Should We Recommend? Can. J. Cardiol. 2016, 32, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.-M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults a systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef]
- Lee, I.-M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, E.; Gale, J.; Bauman, A.; Ekelund, U.; Hamer, M.; Ding, D. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J. Am. Coll. Cardiol. 2019, 73, 2062–2072. [Google Scholar] [CrossRef]
- Young, D.R.; Hivert, M.-F.; Alhassan, S.; Camhi, S.M.; Ferguson, J.F.; Katzmarzyk, P.; Lewis, C.E.; Owen, N.; Perry, C.; Siddique, J.; et al. Sedentary behavior and cardiovascular morbidity and mortality: A science advisory from the American Heart Association. Circulation 2016, 134, e262–e279. [Google Scholar] [CrossRef]
- Ramsey, K.A.; Rojer, A.G.; D’Andrea, L.; Otten, R.H.; Heymans, M.W.; Trappenburg, M.C.; Verlaan, S.; Whittaker, A.C.; Meskers, C.G.; Maier, A.B. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 67, 101266. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, J.L.; Mañas, A.; García-García, F.J.; Ara, I.; Carnicero, J.A.; Walter, S.; Rodríguez-Mañas, L. Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: Isotemporal substitution model. J. Cachexia Sarcopenia Muscle 2019, 10, 188–198. [Google Scholar] [CrossRef]
- Iolascon, G.; de Sire, A.; Calafiore, D.; Benedetti, M.G.; Cisari, C.; Mauro, G.L.; Migliaccio, S.; Nuti, R.; Resmini, G.; Gonnelli, S.; et al. Multifactorial Assessment of Risk of Falling in 753 Post-Menopausal Women: A Multicenter Cross- Sectional Study by the Italian Group for the Study of Metabolic Bone Diseases. Clin. Interv. Aging 2020, 7, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulens, M.; Vansant, G.; Lysens, R.; Claessens, A.L.; Muls, E. Exercise capacity in lean versus obese women. Scand. J. Med. Sci. 2001, 11, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U.; Lancet Physical Activity Series Working Group. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- Kohl, H.W., III; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S.; for the Lancet Physical Activity Series Working Group. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise Capacity and Mortality among Men Referred for Exercise Testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [Green Version]
- Foccardi, G.; Vecchiato, M.; Neunhaeuserer, D.; Mezzaro, M.; Quinto, G.; Battista, F.; Duregon, F.; Carlon, R.; Ermolao, A. Effectiveness of Text Messaging as an Incentive to Maintain Physical Activity after Cardiac Rehabilitation: A Randomized Controlled Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 6645. [Google Scholar] [CrossRef]
- Lee, D.-C.; Pate, R.R.; Lavie, C.J.; Sui, X.; Church, T.S.; Blair, S.N. Leisure-time running reduces all-cause and cardiovascular mortality risk. J. Am. Coll. Cardiol. 2014, 64, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. J. Am. Med. Assoc. 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical Activity in the Prevention and Treatment of Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e007725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sport 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, P.; Ismond, K.P.; Riess, K.; Duarte-Rojo, A.; Al-Judaibi, B.; Dunn, M.A.; Holman, J.; Howes, N.; Haykowsky, M.J.F.; Josbeno, D.A.; et al. Exercise in cirrhosis: Translating evidence and experience to practice. J. Hepatol. 2018, 69, 1164–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.M.; Nilsen, T.S.; Gupta, D.; Jones, L.W. Exercise therapy and cardiovascular toxicity in cancer. Circulation 2018, 137, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Duregon, F.; Gobbo, S.; Bullo, V.; Roma, E.; Vendramin, B.; Bergamo, M.; Bocalini, D.S.; Di Blasio, A.; Cugusi, L.; Neunhaeuserer, D.; et al. Exercise prescription and tailored physical activity intervention in onco-hematology inpatients, a personalized bedside approach to improve clinical best practice. Hematol. Oncol. 2019, 37, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Foccardi, G.; Hansen, D.; Quinto, G.; Favero, C.; Coninx, K.; Ruiz, G.R.; Dendale, P.; Niebauer, J.; Ermolao, A.; Neunhaeuserer, D. How do General Practitioners assess physical activity and prescribe exercise in patients with different cardiovascular diseases? An Italian pilot study. Eur. J. Prev. Cardiol. 2020, 28, e20–e24. [Google Scholar] [CrossRef] [PubMed]
- Agiovlasitis, S.; Baruth, M.; Baynard, T.; Beck, D.T.; Brawner, C.A.; Bryant, M.S.; Castellani, J.W.; Chung, L.H.; Colberg-Ochs, S.R.; Colston, M.; et al. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Neunhäuserer, D.; Reich, B.; Mayr, B.; Kaiser, B.; Lamprecht, B.; Niederseer, D.; Ermolao, A.; Studnicka, M.; Niebauer, J. Impact of exercise training and supplemental oxygen on submaximal exercise performance in patients with COPD. Scand. J. Med. Sci. Sports 2021, 31, 710–719. [Google Scholar] [CrossRef]
- Neunhäuserer, D.; Patti, A.; Niederseer, D.; Kaiser, B.; Cadamuro, J.; Lamprecht, B.; Ermolao, A.; Studnicka, M.; Niebauer, J. Systemic Inflammation, Vascular Function, and Endothelial Progenitor Cells after an Exercise Training Intervention in COPD. Am. J. Med. 2021, 134, e171–e180. [Google Scholar] [CrossRef]
- Vecchiato, M.; Quinto, G.; Palermi, S.; Foccardi, G.; Mazzucato, B.; Battista, F.; Duregon, F.; Michieletto, F.; Neunhaeuserer, D.; Ermolao, A. Are Gyms a Feasible Setting for Exercise Training Interventions in Patients with Cardiovascular Risk Factors? An Italian 10-Years Cross-Sectional Survey Comparison. Int. J. Environ. Res. Public Health 2022, 19, 2407. [Google Scholar] [CrossRef]
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; van Mechelen, W.; Pratt, M.; Lancet Physical Activity Series 2 Executive Committee. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef]
- Neunhaeuserer, D.; Niebauer, J.; Degano, G.; Baioccato, V.; Borjesson, M.; Casasco, M.; Bachl, N.; Christodoulou, N.; Steinacker, J.M.; Papadopoulou, T.; et al. Sports and exercise medicine in Europe and the advances in the last decade. Br. J. Sports Med. 2021, 55, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Abu-Omar, K.; Rütten, A.; Burlacu, I.; Schätzlein, V.; Messing, S.; Suhrcke, M. The cost-effectiveness of physical activity interventions: A systematic review of reviews. Prev. Med. Rep. 2017, 8, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, P.; Murphy, E.; Smith, S.; Cupples, M.; Byrne, M.; Murphy, A. Long-term cost effectiveness of cardiac secondary prevention in primary care in the Republic of Ireland and Northern Ireland. Eur. J. Health Econ. 2016, 18, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Goryakin, Y.; Aldea, A.; Lerouge, A. Promoting sport and physical activity in Italy: A cost-effectiveness analysis of seven innovative public health policies. Ann. Ig. 2019, 31, 614–625. [Google Scholar] [CrossRef]
- Guillon, M.; Rochaix, L.; Dupont, J.C.K. Cost-effectiveness of interventions based on physical activity in the treatment of chronic conditions: A systematic literature review. Int. J. Technol. Assess. Health Care 2018, 34, 481–497. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T. Cost-effectiveness of exercise is medicine®. Curr. Sports Med. Rep. 2011, 10, 217–223. [Google Scholar] [CrossRef]
- Mattli, R.; Farcher, R.; Syleouni, M.-E.; Wieser, S.; Probst-Hensch, N.; Schmidt-Trucksäss, A.; Schwenkglenks, M. Physical Activity Interventions for Primary Prevention in Adults: A Systematic Review of Randomized Controlled Trial-Based Economic Evaluations. Sport Med. 2020, 50, 731–750. [Google Scholar] [CrossRef]
- Oldridge, N.; Taylor, R.S. Cost-effectiveness of exercise therapy in patients with coronary heart disease, chronic heart failure and associated risk factors: A systematic review of economic evaluations of randomized clinical trials. Eur. J. Prev. Cardiol. 2020, 27, 1045–1055. [Google Scholar] [CrossRef]
- Barbara De Mei, A.; Cadeddu, C.; Luzi, P.; Spinelli, A. Movimento, Sport e Salute: L’importanza Delle Politiche di Promozione Dell’attività Fisica e le Ricadute Sulla Collettività. 2018. Available online: https://www.epicentro.iss.it/attivita_fisica/pdf/18_9_web_rev.pdf (accessed on 27 October 2020).
- Ministero della Salute. Monitoraggio dei LEA—Il Nuovo Sistema di Garanzia. Dir Gen della Program Sanit Uff VI. 2020. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2970_allegato.pdf (accessed on 27 October 2020).
- ISTAT. Aspetti della Vita Quotidiana: Stato di Salute—Regioni e Tipo di Comune. Available online: http://dati.istat.it/Index.aspx?QueryId=15448 (accessed on 27 October 2020).
- Saia, F. Il Giornale Italiano di Cardiologia Invasiva. 2019. Available online: https://www.ser-veneto.it/public/File/documents/rapporti/FratturaFemore.pdf (accessed on 27 October 2020).
- Registro Tumori in Veneto. Registro Tumori Veneto. 2016. Available online: https://gecoopendata.registrotumoriveneto.it/prevalenza.php?sede=polmone&codSede=C33-C34.9%0Ahttps://gecoopendata.registrotumoriveneto.it/incidenza.php%0Ahttps://www.registrotumoriveneto.it/it/dati/dati-del-veneto (accessed on 27 October 2020).
- Fedeli, U.N.G. Le Fratture del Femore prossimale dell’Anziano nella Regione del Veneto. 2012. Available online: http://www.ser-veneto.it/public/File/documents/rapporti/FratturaFemore.pdf (accessed on 27 October 2020).
- Il Progetto Cuore. Dati su Rischio Cardiovascolare Sulla Popolazione Italiana. Available online: https://www.cuore.iss.it/indagini/CuoreData (accessed on 27 October 2020).
- EPICENTRO. Malattie Respiratorie Croniche. I Dati Della Sorveglianza. PASSI. Available online: https://www.epicentro.iss.it/passi/dati/croniche (accessed on 27 October 2020).
- EPICENTRO. Attività Fisica tra gli Anziani. Available online: https://www.epicentro.iss.it/passi-argento/info/archivio (accessed on 27 October 2020).
- Belardinelli, R.; Paolini, I.; Cianci, G.; Piva, R.; Georgiou, D.; Purcaro, A. Exercise training intervention after coronary angioplasty: The ETICA trial. J. Am. Coll. Cardiol. 2001, 37, 1891–1900. [Google Scholar] [CrossRef]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Anto, J.M. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: A population based cohort study. Thorax 2006, 61, 772–778. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.C.; Lee, I.-M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; de Gonzalez, A.B.; Hartge, P.; et al. Association of Leisure-Time Physical Activity with Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Barreto, P.D.S.; Rolland, Y.; Vellas, B.; Maltais, M. Association of Long-term Exercise Training with Risk of Falls, Fractures, Hospitalizations, and Mortality in Older Adults. JAMA Intern. Med. 2019, 179, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sistema Epidemiologico Regionale (SER). L’ospedalizzazione in Veneto dal 2006 al 2015. 2017. Available online: https://www.ser-veneto.it/public/File/documents/rapporti/RapportoSDO_2006_2015.pdf (accessed on 3 November 2020).
- Sallis, R.E. Exercise is medicine and physicians need to prescribe it! Br. J. Sports Med. 2009, 43, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavie, C.J.; Johannsen, N.; Swift, D.; Senechal, M.; Earnest, C.; Church, T.; Hutber, A.; Sallis, R.; Blair, S.N. Exercise is Medicine—The Importance of Physical Activity, Exercise Training, Cardiorespiratory Fitness, and Obesity in the Prevention and Treatment of Type 2 Diabetes. Eur. Endocrinol. 2014, 10, 18–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallis, R.E. Exercise in the management of chronic diseases: An underfilled prescription. Curr. Sports Med. Rep. 2017, 16, 225–226. [Google Scholar] [CrossRef]
- Pratt, M.; Norris, J.; Lobelo, F.; Roux, L.; Wang, G. The cost of physical inactivity: Moving into the 21st century. Br. J. Sports Med. 2014, 48, 171–173. [Google Scholar] [CrossRef] [Green Version]
- ISTAT. Italia in Cifre: Edizione 2015. 2015. Available online: https://www.istat.it/it/files//2015/08/ItaliaInCifre2015It.pdf (accessed on 3 November 2020).
- EPICENTRO. Attività Fisica—Sorveglianza Passi. Available online: https://www.epicentro.iss.it/passi/ (accessed on 27 October 2020).
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/272722/9789241514187-eng.pdf (accessed on 3 November 2020).
- World Health Organization. ACTIVE—A Technical Package for Increasing Physical Activity. 2018. Available online: https://www.who.int/publications/i/item/9789241514804 (accessed on 3 November 2020).
- EUPAP. Available online: https://www.eupap.org/ (accessed on 3 November 2020).
- Onerup, A.; Arvidsson, D.; Blomqvist, Å.; Daxberg, E.-L.; Jivegård, L.; Jonsdottir, I.H.; Lundqvist, S.; Mellén, A.; Persson, J.; Sjögren, P.; et al. Physical activity on prescription in accordance with the Swedish model increases physical activity: A systematic review. Br. J. Sports Med. 2019, 53, 383–388. [Google Scholar] [CrossRef]
- Lundqvist, S.; Börjesson, M.; Cider, Å.; Hagberg, L.; Ottehall, C.B.; Sjöström, J.; Larsson, M.E.H. Long-term physical activity on prescription intervention for patients with insufficient physical activity level—A randomized controlled trial. Trials 2020, 21, 793–804. [Google Scholar] [CrossRef]
- Exercise is Medicine. Available online: https://www.exerciseismedicine.org/ (accessed on 3 November 2020).
- Meyer, J.; McDowell, C.; Lansing, J.; Brower, C.; Smith, L.; Tully, M.; Herring, M. Changes in Physical Activity and Sedentary Behavior in Response to COVID-19 and Their Associations with Mental Health in 3052 US Adults. Int. J. Environ. Res. Public Health 2020, 17, 6469. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortolan, S.; Neunhaeuserer, D.; Quinto, G.; Barra, B.; Centanini, A.; Battista, F.; Vecchiato, M.; De Marchi, V.; Celidoni, M.; Rebba, V.; et al. Potential Cost Savings for the Healthcare System by Physical Activity in Different Chronic Diseases: A Pilot Study in the Veneto Region of Italy. Int. J. Environ. Res. Public Health 2022, 19, 7375. https://doi.org/10.3390/ijerph19127375
Ortolan S, Neunhaeuserer D, Quinto G, Barra B, Centanini A, Battista F, Vecchiato M, De Marchi V, Celidoni M, Rebba V, et al. Potential Cost Savings for the Healthcare System by Physical Activity in Different Chronic Diseases: A Pilot Study in the Veneto Region of Italy. International Journal of Environmental Research and Public Health. 2022; 19(12):7375. https://doi.org/10.3390/ijerph19127375
Chicago/Turabian StyleOrtolan, Sara, Daniel Neunhaeuserer, Giulia Quinto, Barbara Barra, Anna Centanini, Francesca Battista, Marco Vecchiato, Valentina De Marchi, Martina Celidoni, Vincenzo Rebba, and et al. 2022. "Potential Cost Savings for the Healthcare System by Physical Activity in Different Chronic Diseases: A Pilot Study in the Veneto Region of Italy" International Journal of Environmental Research and Public Health 19, no. 12: 7375. https://doi.org/10.3390/ijerph19127375
APA StyleOrtolan, S., Neunhaeuserer, D., Quinto, G., Barra, B., Centanini, A., Battista, F., Vecchiato, M., De Marchi, V., Celidoni, M., Rebba, V., & Ermolao, A. (2022). Potential Cost Savings for the Healthcare System by Physical Activity in Different Chronic Diseases: A Pilot Study in the Veneto Region of Italy. International Journal of Environmental Research and Public Health, 19(12), 7375. https://doi.org/10.3390/ijerph19127375






