Short-Term High-Intensity Circuit Training Does Not Modify Resting Heart Rate Variability in Adults during the COVID-19 Confinement
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
2.3. Heart Rate Variability Assessment
2.4. High-Intensity Circuit Training
2.5. Statistical Analysis
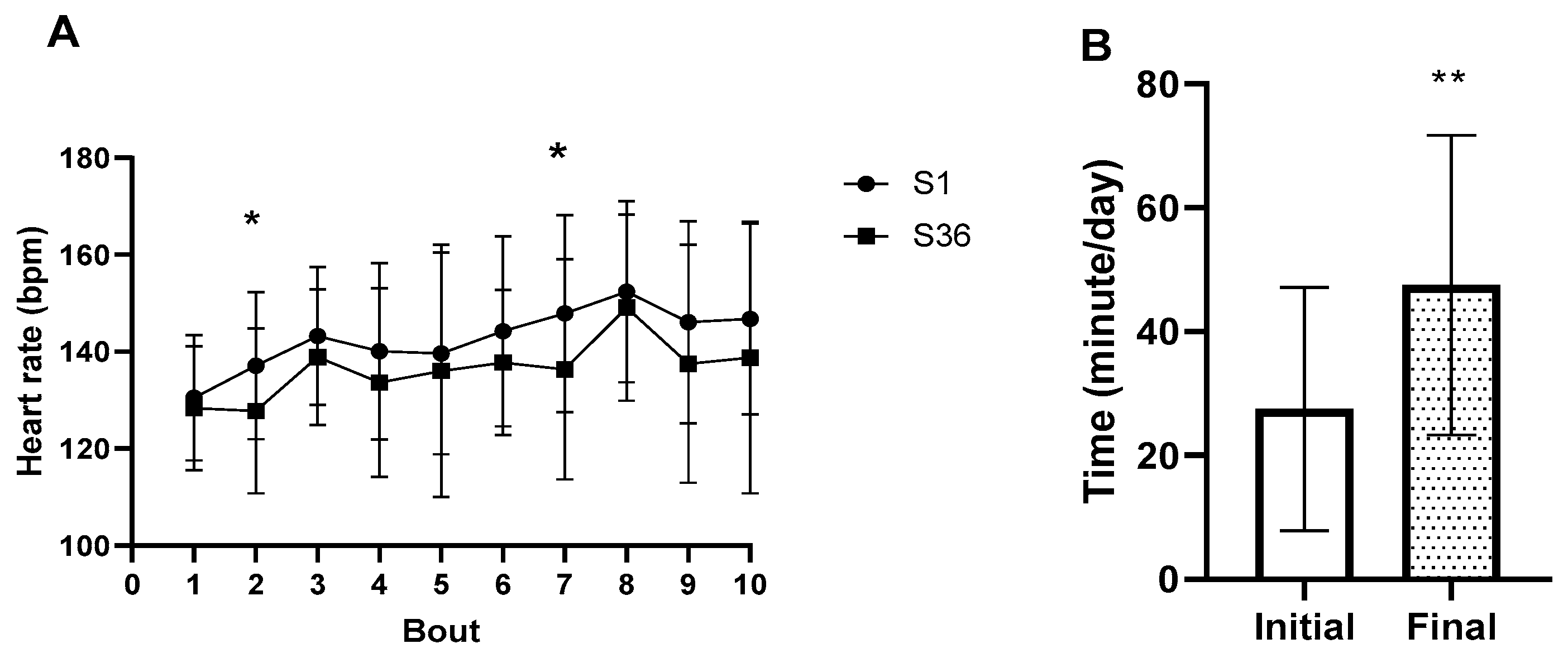
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suárez, V.; Quezada, M.S.; Ruiz, S.O.; De Jesús, E.R. Epidemiology of COVID-19 in Mexico: From the 27th of February to the 30th of April 2020. Rev. Clínica Española 2020, 220, 463–471. [Google Scholar] [CrossRef]
- Wackerhage, H.; Everett, R.; Krüger, K.; Murgia, M.; Simon, P.; Gehlert, S.; Neuberger, E.; Baumert, P.; Schönfelder, M. Sport, Exercise and COVID-19, the Disease Caused by the SARS-CoV-2 Coronavirus. Dtsch. Z. Sportmed. 2020, 71, E1–E12. [Google Scholar] [CrossRef]
- Ricci, F.; Izzicupo, P.; Moscucci, F.; Sciomer, S.; Maffei, S.; Di Baldassarre, A.; Mattioli, A.V.; Gallina, S. Recommendations for Physical Inactivity and Sedentary Behavior During the Coronavirus Disease (COVID-19) Pandemic. Front. Public Health 2020, 8, 199. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Costa, F.F.; Rosário, W.R.; Farias, A.C.R.; de Souza, R.G.; Gondim, R.S.D.; Barroso, W.A. Metabolic syndrome and COVID-19: An update on the associated comorbidities and proposed therapies. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 809–814. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar] [CrossRef]
- Narici, M.; De Vito, G.; Franchi, M.; Paoli, A.; Moro, T.; Marcolin, G.; Grassi, B.; Baldassarre, G.; Zuccarelli, L.; Biolo, G.; et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2021, 21, 614–635. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef]
- Aubert, A.E.; Seps, B.; Beckers, F. Heart Rate Variability in Athletes. Sports Med. 2003, 33, 889–919. [Google Scholar] [CrossRef]
- Klika, B.; Jordan, C. High-Intensity Circuit Training Using Body Weight: Maximum Results with Minimal Investment. ACSM’S Health Fit. J. 2013, 17, 8–13. [Google Scholar] [CrossRef]
- Gist, N.H.; Freese, E.C.; Cureton, K.J. Comparison of Responses to Two High-Intensity Intermittent Exercise Protocols. J. Strength Cond. Res. 2014, 28, 3033–3040. [Google Scholar] [CrossRef]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef] [PubMed]
- Alvares, G.A.; Quintana, D.S.; Hickie, I.B.; Guastella, A.J. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. J. Psychiatry Neurosci. 2016, 41, 89–104. [Google Scholar] [CrossRef]
- Nardelli, M.; Valenza, G.; Cristea, I.A.; Gentili, C.; Cotet, C.; David, D.; Lanata, A.; Scilingo, E.P. Characterizing psychological dimensions in non-pathological subjects through autonomic nervous system dynamics. Front. Comput. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-Intensity Interval Training, Solutions to the Programming Puzzle: Part II: Anaerobic Energy, Neuromuscular Load and Practical Applications. Sport. Med. 2013, 43, 313–338. [Google Scholar] [CrossRef] [PubMed]
- Rickards, C.A.; Ryan, K.L.; Convertino, V.A. Characterization of common measures of heart period variability in healthy human subjects: Implications for patient monitoring. Int. J. Clin. Monit. Comput. 2010, 24, 61–70. [Google Scholar] [CrossRef]
- Vrachimis, A.; Hadjicharalambous, M.; Tyler, C. The Effect of Circuit Training on Resting Heart Rate Variability, Cardiovascular Disease Risk Factors and Physical Fitness in Healthy Untrained Adults. Health 2016, 08, 144–155. [Google Scholar] [CrossRef]
- Bechke, E.; Kliszczewicz, B.; Feito, Y.; Kelemen, H.; Nickerson, B. Resting cardiac autonomic activity and body composition following a 16-week high-intensity functional training intervention in women: A pilot study. J. Hum. Sport Exerc. 2017, 12, 680–688. [Google Scholar] [CrossRef]
- Amatriain-Fernández, S.; Gronwald, T.; Murillo-Rodríguez, E.; Imperatori, C.; Solano, A.F.; Latini, A.; Budde, H. Physical Exercise Potentials against Viral Diseases Like COVID-19 in the Elderly. Front. Med. 2020, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.; Irigoyen, M.C.; Consolim-Colombo, F.; Saraiva, J.F.K.; De Angelis, K. Physically Active Lifestyle as an Approach to Confronting COVID-19. Arq. Bras. Cardiol. 2020, 114, 601–602. [Google Scholar]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. PAR-Q, Canadian Home Fitness Test and Exercise Screening Alternatives. Sports Med. 1988, 5, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Schleppenbach, L.N.; Ezer, A.B.; Gronemus, S.A.; Widenski, K.R.; Braun, S.I.; Janot, J.M. Speed- and Circuit-Based High-Intensity Interval Training on Recovery Oxygen Consumption. Int. J. Exerc. Sci. 2017, 10, 942–953. [Google Scholar] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef]
- López-Bueno, R.; Calatayud, J.; Andersen, L.; Balsalobre-Fernández, C.; Casaña, J.; Casajús, J.; Smith, L.; López-Sánchez, G. Immediate Impact of the COVID-19 Confinement on Physical Activity Levels in Spanish Adults. Sustainability 2020, 12, 5708. [Google Scholar] [CrossRef]
- Brand, R.; Timme, S.; Nosrat, S. When Pandemic Hits: Exercise Frequency and Subjective Well-Being during COVID-19 Pandemic. Front. Psychol. 2020, 11, 2391. [Google Scholar] [CrossRef] [PubMed]
- Antunes, B.M.; Rossi, F.E.; Teixeira, A.; Lira, F.S. Short-time high-intensity exercise increases peripheral BDNF in a physical fitness-dependent way in healthy men. Eur. J. Sport Sci. 2019, 20, 43–50. [Google Scholar] [CrossRef]
- Ingram, J.; Maciejewski, G.; Hand, C.J. Changes in Diet, Sleep, and Physical Activity Are Associated with Differences in Negative Mood during COVID-19 Lockdown. Front. Psychol. 2020, 11, 241–252. [Google Scholar] [CrossRef]
- Cheval, B.; Sivaramakrishnan, H.; Maltagliati, S.; Fessler, L.; Forestier, C.; Sarrazin, P.; Orsholits, D.; Chalabaev, A.; Sander, D.; Ntoumanis, N.; et al. Relationships between changes in self-reported physical activity, sedentary behaviour and health during the coronavirus (COVID-19) pandemic in France and Switzerland. J. Sports Sci. 2021, 39, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Simpson, M.B.; Al Haddad, H.; Bourdon, P.C.; Mendez-Villanueva, A. Monitoring changes in physical performance with heart rate measures in young soccer players. Eur. J. Appl. Physiol. 2012, 112, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Mendez-Villanueva, A.; Quod, M.J.; Poulos, N.; Bourdon, P. Determinants of the variability of heart rate measures during a competitive period in young soccer players. Eur. J. Appl. Physiol. 2010, 109, 869–878. [Google Scholar] [CrossRef]
- Little, J.P.; Langley, J.; Lee, M.; Myette-Côté, E.; Jackson, G.; Durrer, C.; Gibala, M.J.; Jung, M.E. Sprint exercise snacks: A novel approach to increase aerobic fitness. Eur. J. Appl. Physiol. 2019, 119, 1203–1212. [Google Scholar] [CrossRef]
- Caldwell, H.G.; Coombs, G.B.; Rafiei, H.; Ainslie, P.N.; Little, J.P. Hourly staircase sprinting exercise “snacks” improve femoral artery shear patterns but not flow-mediated dilation or cerebrovascular regulation: A pilot study. Appl. Physiol. Nutr. Metab. 2021, 46, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.; Antunes, B.; Figueiredo, C.; Campos, E.; Panissa, V.; St-Pierre, D.; Lavoie, J.-M.; Magri-Tomaz, L. Impact of 5-week high-intensity interval training on indices of cardio metabolic health in men. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1359–1364. [Google Scholar] [CrossRef]
- Alansare, A.; Alford, K.; Lee, S.; Church, T.; Jung, H.C. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Heart Rate Variability in Physically Inactive Adults. Int. J. Environ. Res. Public Health 2018, 15, 1508. [Google Scholar] [CrossRef]
- Figueiredo, C.; Antunes, B.M.; Giacon, T.R.; Vanderlei, L.C.M.; Campos, E.Z.; Peres, F.P.; Clark, N.W.; Panissa, V.L.G.; Lira, F.S. Influence of Acute and Chronic High-Intensity Intermittent Aerobic Plus Strength Exercise on BDNF, Lipid and Autonomic Parameters. J. Sports Sci. Med. 2019, 18, 359–368. [Google Scholar]
- Schaun, G.Z.; Pinto, S.S.; Silva, M.R.; Dolinski, D.B.; Alberton, C.L. Whole-Body High-Intensity Interval Training Induce Similar Cardiorespiratory Adaptations Compared with Traditional High-Intensity Interval Training and Moderate-Intensity Continuous Training in Healthy Men. J. Strength Cond. Res. 2018, 32, 2730–2742. [Google Scholar] [CrossRef]
- Castrillón, C.I.M.; Miranda, R.A.T.; Cabral-Santos, C.; Vanzella, L.M.; Rodrigues, B.; Vanderlei, L.C.M.; Lira, F.S.; Campos, E.Z. High-Intensity Intermittent Exercise and Autonomic Modulation: Effects of Different Volume Sessions. Int. J. Sports Med. 2017, 38, 468–472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buchheit, M.; Simon, C.; Charloux, A.; Doutreleau, S.; Piquard, F.; Brandenberger, G. Relationship Between Very High Physical Activity Energy Expenditure, Heart Rate Variability and Self-Estimate of Health Status in Middle-Aged Individuals. Int. J. Sports Med. 2005, 27, 697–701. [Google Scholar] [CrossRef]
- Buchheit, M.; Simon, C.; Charloux, A.; Doutreleau, S.; Piquard, F.; Brandenberger, G. Heart Rate Variability and Intensity of Habitual Physical Activity in Middle-Aged Persons. Med. Sci. Sports Exerc. 2005, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Antelmi, I.; De Paula, R.S.; Shinzato, A.R.; Peres, C.A.; Mansur, A.J.; Grupi, C.J. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am. J. Cardiol. 2004, 93, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Abhishekh, H.A.; Nisarga, P.; Kisan, R.; Meghana, A.; Chandran, S.; Raju, T.; Sathyaprabha, T.N. Influence of age and gender on autonomic regulation of heart. J. Clin. Monit. Comput. 2013, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Mourot, L.; Bouhaddi, M.; Perrey, S.; Cappelle, S.; Henriet, M.-T.; Wolf, J.-P.; Rouillon, J.-D.; Regnard, J. Decrease in heart rate variability with overtraining: Assessment by the Poincare plot analysis. Clin. Physiol. Funct. Imaging 2004, 24, 10–18. [Google Scholar] [CrossRef]


| Variable | CON (n = 4) m/f (0/4) | HICT (n = 9) m/f (4/5) | p≤ |
|---|---|---|---|
| Age (yr.) | 29.5 ± 1.7 | 31.9 ± 4.4 | 0.320 |
| Weight (kg) | 68.6 ± 6.9 | 80 ± 18.1 | 0.250 |
| Height (cm) | 160.0 ± 4.0 | 167.0 ± 8.0 | 0.001 |
| BMI (kg/m2) | 26.39 ± 2.6 | 28.47 ± 4.8 | 0.440 |
| IPAQ (METs) * | 2253.25 ± 1933 | 1240.81 ± 1104 | 0.260 |
| Vigorous activity (min/day) | 7.5 ± 15.0 | 27.5 ± 19.6 | 0.106 |
| Variable | Group | Mean ± sd | p= | Cohen’s d | Power (1 − ß) | Smallest Worthwhile Change (Threshold) |
|---|---|---|---|---|---|---|
| Mean RR (ms) | CON | 910.0 ± 113.9 | 0.796 | −0.248 | 0.99 | 45.3 |
| HICT | 965.3 ± 251.7 | |||||
| SDNN (ms) | CON | 50.4 ± 19.3 | 0.587 | −0.368 | 0.99 | 5.5 |
| HICT | 59.2 ± 25.3 | |||||
| Mean HR (bpm) | CON | 66.7 ± 8.6 | 0.710 | −0.033 | 1.00 | 6.3 |
| HICT | 67.4 ± 23.5 | |||||
| SDHR (bpm) | CON | 3.8 ± 1.2 | 0.642 | −0.218 | 0.99 | 1.4 |
| HICT | 4.6 ± 4.5 | |||||
| Min HR (bpm) | CON | 58.4 ± 5.1 | 0.236 | 0.225 | 1.00 | 3.0 |
| HICT | 55.8 ± 13.1 | |||||
| Max HR (bpm) | CON | 82.6 ± 12.3 | 0.593 | 0.053 | 0.99 | 7.6 |
| HICT | 81.4 ± 25.2 | |||||
| RMSSD (ms) | CON | 47.2 ± 28.4 | 0.983 | −0.552 | 0.98 | 8.4 |
| HICT | 66.5 ± 37.2 | |||||
| NN50 (beats) | CON | 71.3 ± 67.0 | 0.375 | −0.212 | 1.00 | 20.4 |
| HICT | 85.6 ± 67.6 | |||||
| pNN50 (%) | CON | 23.3 ± 24.0 | 0.109 | −0.318 | 1.00 | 8.4 |
| HICT | 31.4 ± 25.7 | |||||
| LF (n.u.) | CON | 58.7 ± 23.6 | 0.205 | 0.136 | 1.00 | 6.7 |
| HICT | 55.6 ± 22.7 | |||||
| HF (n.u.) | CON | 41.2 ± 23.6 | 0.204 | −0.136 | 1.00 | 6.7 |
| HICT | 44.3 ± 22.7 | |||||
| Total power (ms2) | CON | 2657.2 ± 1837.3 | 0.787 | −0.487 | 1.00 | 935.1 |
| HICT | 7506.1 ± 11,630.5 | |||||
| LF/HF | CON | 2.2 ± 1.9 | 0.623 | −0.053 | 1.00 | 1.3 |
| HICT | 2.3 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Suárez, P.C.; Aburto-Corona, J.A.; Rentería, I.; Gómez-Miranda, L.M.; Moncada-Jiménez, J.; Lira, F.S.; Antunes, B.M.; Jiménez-Maldonado, A. Short-Term High-Intensity Circuit Training Does Not Modify Resting Heart Rate Variability in Adults during the COVID-19 Confinement. Int. J. Environ. Res. Public Health 2022, 19, 7367. https://doi.org/10.3390/ijerph19127367
García-Suárez PC, Aburto-Corona JA, Rentería I, Gómez-Miranda LM, Moncada-Jiménez J, Lira FS, Antunes BM, Jiménez-Maldonado A. Short-Term High-Intensity Circuit Training Does Not Modify Resting Heart Rate Variability in Adults during the COVID-19 Confinement. International Journal of Environmental Research and Public Health. 2022; 19(12):7367. https://doi.org/10.3390/ijerph19127367
Chicago/Turabian StyleGarcía-Suárez, Patricia C., Jorge A. Aburto-Corona, Iván Rentería, Luis M. Gómez-Miranda, José Moncada-Jiménez, Fábio Santos Lira, Barbara Moura Antunes, and Alberto Jiménez-Maldonado. 2022. "Short-Term High-Intensity Circuit Training Does Not Modify Resting Heart Rate Variability in Adults during the COVID-19 Confinement" International Journal of Environmental Research and Public Health 19, no. 12: 7367. https://doi.org/10.3390/ijerph19127367
APA StyleGarcía-Suárez, P. C., Aburto-Corona, J. A., Rentería, I., Gómez-Miranda, L. M., Moncada-Jiménez, J., Lira, F. S., Antunes, B. M., & Jiménez-Maldonado, A. (2022). Short-Term High-Intensity Circuit Training Does Not Modify Resting Heart Rate Variability in Adults during the COVID-19 Confinement. International Journal of Environmental Research and Public Health, 19(12), 7367. https://doi.org/10.3390/ijerph19127367









