Neurodevelopment in Normocephalic Children Exposed to Zika Virus in Utero with No Observable Defects at Birth: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Risk of Bias, Quality Assessment, Data Extraction and Analysis
2.4. Synthesis Methods
3. Results
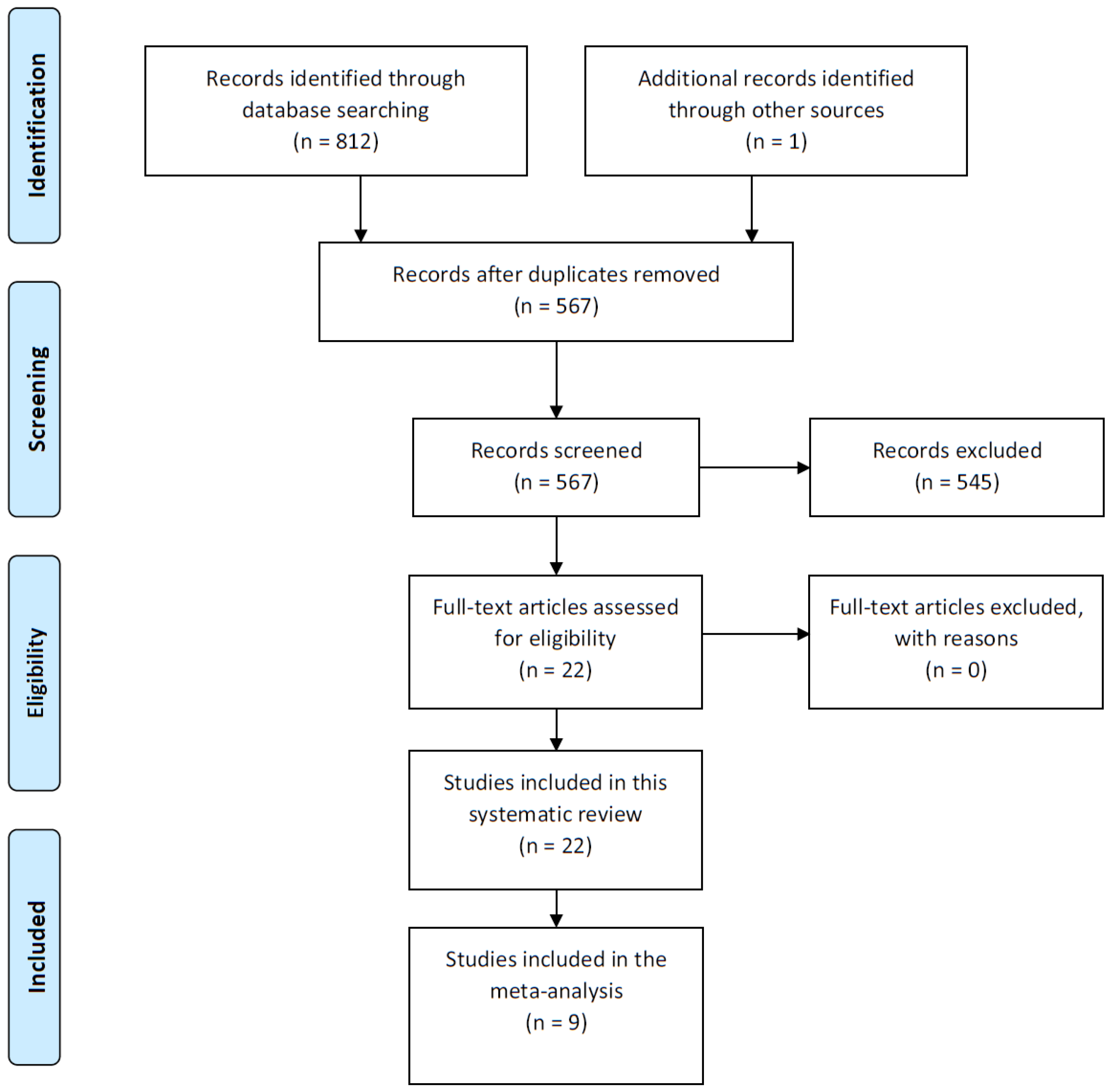
3.1. Study Selection and Characteristics
3.2. Maternal ZIKV Ascertainment
3.3. Infant ZIKV Ascertainment
3.4. Covariate Ascertainment
3.5. Neurodevelopmental Screening Tool Outcomes
3.6. Risk of Bias and Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Eijk, A.A.; van Genderen, P.J.; Verdijk, R.M.; Reusken, C.B.; Mögling, R.; van Kampen, J.J.A.; Widagdo, W.; Aron, G.I.; GeurtsvanKessel, C.H.; Pas, S.D.; et al. Miscarriage Associated with Zika Virus Infection. N. Engl. J. Med. 2016, 375, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Cranston, J.S.; Tiene, S.F.; Nielsen-Saines, K.; Vasconcelos, Z.; Pone, M.V.; Pone, S.; Zin, A.; Salles, T.S.; Pereira, J.P.J.; Orofino, D.; et al. Association between Antenatal Exposure to Zika Virus and Anatomical and Neurodevelopmental Abnormalities in Children. JAMA Netw. Open 2020, 3, e209303. [Google Scholar] [CrossRef] [PubMed]
- Goncé, A.; Martínez, M.J.; Marbán-Castro, E.; Saco, A.; Soler, A.; Alvarez-Mora, M.I.; Peiro, A.; Gonzalo, V.; Hale, G.; Bhatnagar, J.; et al. Spontaneous Abortion Associated with Zika Virus Infection and Persistent Viremia. Emerg. Infect. Dis. 2018, 24, 933–935. [Google Scholar] [CrossRef]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection-after the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Marbán-Castro, E.; Goncé, A.; Fumadó, V.; Romero-Acevedo, L.; Bardají, A. Zika virus infection in pregnant women and their children: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 265, 162–168. [Google Scholar] [CrossRef]
- Alvarado-Socarras, J.L.; Idrovo, Á.J.; Contreras-García, G.A.; Rodriguez-Morales, A.J.; Audcent, T.A.; Mogollon-Mendoza, A.C.; Paniz-Mondolfi, A. Congenital microcephaly: A diagnostic challenge during Zika epidemics. Travel Med. Infect. Dis. 2018, 23, 14–20. [Google Scholar] [CrossRef]
- Rice, M.E.; Galang, R.R.; Roth, N.M.; Ellington, S.R.; Moore, C.A.; Valencia-Prado, M.; Ellis, E.M.; Tufa, A.J.; Taulung, L.A.; Alfred, J.M.; et al. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection-U.S. Territories and Freely Associated States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 858–867. [Google Scholar] [CrossRef] [Green Version]
- Faiçal, A.V.; de Oliveira, J.C.; Oliveira, J.V.V.; de Almeida, B.L.; Agra, I.A.; Alcantara, L.C.J.; Acosta, A.X.; de Siqueira, I.C. Neurodevelopmental delay in normocephalic children with in utero exposure to Zika virus. BMJ Paediatr. Open 2019, 3, e000486. [Google Scholar] [CrossRef]
- Connery, A.K.; Colbert, A.M.; Lamb, M.M.; Hernández, S.; Martínez, M.A.; Bauer, D.; Arroyave, P.; El Sahly, H.M.; Paniagua-Avila, A.; Calvimontes, M.; et al. Receptive language skills among young children in rural Guatemala: The relationship between the Test de Vocabulario en Imagenes Peabody and a translated and adapted version of the Mullen Scales of Early Learning. Child Care. Health Dev. 2019, 45, 702–708. [Google Scholar] [CrossRef]
- Moreira, M.E.L.; Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Damasceno, L.; Pone, M.; Carvalho, L.M.A.; Pone, S.M.; Vasconcelos, Z.; Ribeiro, I.P.; et al. Neurodevelopment in Infants Exposed to Zika Virus in Utero. N. Engl. J. Med. 2018, 379, 2377–2379. [Google Scholar] [CrossRef]
- Aragao, M.F.V.V.; Holanda, A.C.; Brainer-Lima, A.M.; Petribu, N.C.L.; Castillo, M.; van der Linden, V.; Serpa, S.C.; Tenório, A.G.; Travassos, P.T.C.; Cordeiro, M.T.; et al. Nonmicrocephalic Infants with Congenital Zika Syndrome Suspected only after Neuroimaging Evaluation Compared with Those with Microcephaly at Birth and Postnatally: How Large Is the Zika Virus “Iceberg”? AJNR Am. J. Neuroradiol. 2017, 38, 1427–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabanathan, S.; Wills, B.; Gladstone, M. Child development assessment tools in low-income and middle-income countries: How can we use them more appropriately? Arch. Dis. Child. 2015, 100, 482–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boggs, D.; Milner, K.M.; Chandna, J.; Black, M.; Cavallera, V.; Dua, T.; Fink, G.; Kc, A.; Grantham-McGregor, S.; Hamadani, J.; et al. Rating early child development outcome measurement tools for routine health programme use. Arch. Dis. Child. 2019, 104, S22–S33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillamet, L.V.; Alonso, A.B.; Marban-Castro, E.; Casellas, A.; Mulkey, S.B.; Maxwell, L. Neurodevelopment of Children Exposed to ZIKV in Utero Who Had no Observable Effects of ZIKV at Birth: A Systematic Review with Meta-Analys. PROSPERO 2021 CRD42021242262. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021242262 (accessed on 23 February 2021).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013; The GRADE Working Group. 2013. Available online: www.guidelinedevelopment.org/handbook (accessed on 23 February 2021).
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (Developed by Evidence Prime, Inc.). Available online: gradepro.org (accessed on 23 February 2021).
- Freeman, M.F.; Tukey, J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950, 21, 607–611. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Lee, E.H.; Cooper, H.; Iwamoto, M.; Lash, M.; Conners, E.E.; Bloch, D.; Clark, S.; Hrusa, G.; Kubinson, H.; Paladini, M.; et al. First 12 Months of Life for Infants in New York City, New York, with Possible Congenital Zika Virus Exposure. J. Pediatr. Infect. Dis. Soc. 2020, 9, 311–319. [Google Scholar] [CrossRef]
- Pimentel, R.; Khosla, S.; Rondon, J.; Peña, F.; Sullivan, G.; Perez, M.; Mehta, S.D.; Brito, M.O. Birth Defects and Long-Term Neurodevelopmental Abnormalities in Infants Born during the Zika Virus Epidemic in the Dominican Republic. Ann. Glob. Heal. 2021, 87, 4. [Google Scholar] [CrossRef]
- Einspieler, C.; Utsch, F.; Brasil, P.; Aizawa, C.Y.P.; Peyton, C.; Hasue, R.H.; Genovesi, F.F.; Damasceno, L.; Moreira, M.E.; Adachi, K.; et al. Association of Infants Exposed to Prenatal Zika Virus Infection with Their Clinical, Neurologic, and Developmental Status Evaluated via the General Movement Assessment Tool. JAMA Netw. Open 2019, 2, e187235. [Google Scholar] [CrossRef] [Green Version]
- Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Vasconcelos, Z.; Gabaglia, C.R.; Damasceno, L.; Pone, M.; de Carvalho, L.M.A.; Pone, S.M.; Zin, A.A.; et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat. Med. 2019, 25, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.C.; Avvad-Portari, E.; Nascimento, A.C.M.; Machado, H.N.; Lobato, D.S.S.; Pereira, J.P.; Esquivel, M.S.; Vasconcelos, Z.C.; Zin, A.A.; Tsui, I.; et al. Discordant Zika Virus Findings in Twin Pregnancies Complicated by Antenatal Zika Virus Exposure: A Prospective Cohort. J. Infect. Dis. 2020, 221, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Fléchelles, O.; Tressières, B.; Dialo, M.; Elenga, N.; Mediamolle, N.; Mallard, A.; Hebert, J.-C.; Lachaume, N.; Couchy, E.; et al. In utero Zika virus exposure and neurodevelopment at 24 months in toddlers normocephalic at birth: A cohort study. BMC Med. 2021, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Familiar, I.; Boivin, M.; Magen, J.; Azcorra, J.A.; Phippen, C.; Barrett, E.A.; Miller, S.; Ruisenor-Escudero, H. Neurodevelopment outcomes in infants born to women with Zika virus infection during pregnancy in Mexico. Child Care Health Dev. 2021, 47, 311–318. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Arroyave-Wessel, M.; Peyton, C.; Bulas, D.I.; Fourzali, Y.; Jiang, J.; Russo, S.; McCarter, R.; Msall, M.E.; du Plessis, A.J.; et al. Neurodevelopmental Abnormalities in Children with in Utero Zika Virus Exposure without Congenital Zika Syndrome. JAMA Pediatr. 2020, 174, 269–276. [Google Scholar] [CrossRef]
- Vianna, R.A.D.O.; Lovero, K.L.; de Oliveira, S.A.; Fernandes, A.R.; Dos Santos, T.C.S.; Lima, L.C.S.D.S.; Carvalho, F.R.; Quintans, M.D.S.; Bueno, A.C.; Torbey, A.F.M.; et al. Children Born to Mothers with Rash during Zika Virus Epidemic in Brazil: First 18 Months of Life. J. Trop. Pediatr. 2019, 65, 592–602. [Google Scholar] [CrossRef]
- Coutinho, C.M.; Negrini, S.; Araujo, D.; Teixeira, S.R.; Amaral, F.R.; Moro, M.; Fernandes, J.; da Motta, M.; Negrini, B.; Caldas, C.; et al. Early maternal Zika infection predicts severe neonatal neurological damage: Results from the prospective Natural History of Zika Virus Infection in Gestation cohort study. BJOG 2021, 128, 317–326. [Google Scholar] [CrossRef]
- Gerzson, L.R.; de Almeida, C.S.; da Silva, J.H.; Feitosa, M.M.A.; de Oliveira, L.N.; Schuler-Faccini, L. Neurodevelopment of Nonmicrocephalic Children, after 18 Months of Life, Exposed Prenatally to Zika Virus. J. Child Neurol. 2020, 35, 278–282. [Google Scholar] [CrossRef]
- da Silva, P.F.S.; Eickmann, S.H.; de Alencar Ximenes, R.A.; Montarroyos, U.R.; de Carvalho Lima, M.; Martelli, C.M.T.; de Araújo, T.V.B.; Brickley, E.B.; Rodrigues, L.C.; da Silva Pastich Gonçalves, F.C.L.; et al. Pediatric neurodevelopment by prenatal Zika virus exposure: A cross-sectional study of the Microcephaly Epidemic Research Group Cohort. BMC Pediatr. 2020, 20, 472. [Google Scholar] [CrossRef]
- Valdes, V.; Zorrilla, C.D.; Gabard-Durnam, L.; Muler-Mendez, N.; Rahman, Z.I.; Rivera, D.; Nelson, C.A., 3rd. Cognitive Development of Infants Exposed to the Zika Virus in Puerto Rico. JAMA Netw. Open 2019, 2, e1914061. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.M.P.C.; Azevedo, C.D.S.L.; de Oliveira, R.D.M.A.B.; Barreto, F.K.A.; Rodrigues, A.S.R.; Simião, A.R.; Gomes, I.P.; Ribeiro, E.M.; Cavalcanti, L.P.D.G. Neurological growth and development of children asymptomatic at birth whose mothers had Zika during pregnancy. Rev. Soc. Bras. Med. Trop. 2021, 54, e01802020. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, P.M.; Junior, S.C.G.; Pone, S.M.; Pone, M.V.D.S.; Vasconcelos, Z.; Zin, A.; Vilibor, R.H.H.; Costa, R.P.; Meio, M.D.B.B.; Nielsen-Saines, K.; et al. Neurodevelopment of children exposed intra-uterus by Zika virus: A case series. PLoS ONE 2020, 15, e0229434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abtibol-Bernardino, M.R.; de Almeida Peixoto, L.D.F.A.; de Oliveira, G.A.; de Almeida, T.F.; Rodrigues, G.R.I.; Otani, R.H.; Chaves, B.C.S.; de Souza Rodrigues, C.; de Andrade, A.B.C.A.; de Fatima Redivo, E.; et al. Neurological Findings in Children without Congenital Microcephaly Exposed to Zika Virus in Utero: A Case Series Study. Viruses 2020, 12, 1335. [Google Scholar] [CrossRef]
- Soares-Marangoni, D.D.A.; Tedesco, N.M.; Nascimento, A.L.; De Almeida, P.R.; Pereira, C.N.S. Dos General movements and motor outcomes in two infants exposed to Zika virus: Brief report. Dev. Neurorehabil. 2019, 22, 71–74. [Google Scholar] [CrossRef]
- Marbán-Castro, E.; Goncé, A.; Fumadó, V.; Martínez, M.J.; López, M.; García-Otero, L.; Salazar, L.; Esteve, C.; Salvia, D.; Fortuny, C.; et al. Zika virus infection in pregnant travellers and impact on childhood neurodevelopment in the first two years of life: A prospective observational study. Travel Med. Infect. Dis. 2021, 40, 101985. [Google Scholar] [CrossRef]
- Peyton, C.; Msall, M.E.; Wroblewski, K.; Rogers, E.E.; Kohn, M.; Glass, H.C. Concurrent validity of the Warner Initial Developmental Evaluation of Adaptive and Functional Skills and the Bayley Scales of Infant and Toddler Development, Third Edition. Dev. Med. Child Neurol. 2021, 63, 349–354. [Google Scholar] [CrossRef]
- da Silva Rosa, B.C.; César, C.P.H.A.R.; Paranhos, L.R.; Guedes-Granzotti, R.B.; Lewis, D.R. Speech-language disorders in children with congenital Zika virus syndrome: A systematic review. Int. J. Pediatric Otorhinolaryngol. 2020, 138, 110309. [Google Scholar] [CrossRef]
- Power, G.M.; Francis, S.C.; Clemente, N.S.; Vasconcelos, Z.; Brasil, P.; Nielsen-Saines, K.; Brickley, E.B.; Moreira, M.E. Examining the Association of Socioeconomic Position with Microcephaly and Delayed Childhood Neurodevelopment among Children with Prenatal Zika Virus Exposure. Viruses 2020, 12, 1342. [Google Scholar] [CrossRef]
- Graham, K.A.; Fox, D.J.; Talati, A.; Pantea, C.; Brady, L.; Carter, S.L.; Friedenberg, E.; Vora, N.M.; Browne, M.L.; Lee, C.T. Prevalence and Clinical Attributes of Congenital Microcephaly-New York, 2013–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 125–129. [Google Scholar] [CrossRef]

| First Author | Countries (n of Children Included) | Study Design | Neuro-Developmental Tool | ZIKV Status-Related Inclusion Criteria (Pregnant Women) * | Maternal and Infant-Status Related Inclusion Criteria (Infants) | Children Age at Neurodevelopmental Assessment (Number of Assessments) | Control Group |
|---|---|---|---|---|---|---|---|
| Lopes Moreira ME | Brazil (94) | Prospective observational cohort study | BSID-III | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Included newborns with abnormal brain imaging (structural and nonstructural abnormalities), but none were ZIKV tested | Between 12 and 18 months (1) | No |
| Einspieler C | Brazil (56) | Prospective observational cohort study | BSID-III | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 12 months (1) | Yes, sex- and age-matched neurotypical controls without exposure to maternal ZIKV |
| Nielsen-Saines K | Brazil (146) | Prospective observational cohort study | BSID-III | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 12 months (1) | No |
| Rodrigues Gerzson L | Brazil (17) | Cross-sectional study with control group | BSID-III | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 18 months (1) | Yes, sex- and age-matched normocephalic children with no maternal history of ZIKV or other congenital infection. |
| Faiçal AV | Brazil (29) | Prospective child cohort | BSID-III | Probable by positive IgG at delivery | Infected children confirmed by PCR, but normocephalic | 19 months (1) | No |
| Peçanha PM | Brazil (84) | Longitudinal exploratory case series | BSID-III | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 9 and 15 months (2) | No |
| Sobhani NC | Brazil (3) | Prospective observational cohort | BSID-III | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 25 months (2 children), and at 39 months (one child) (1) | No |
| Cranston JS | Brazil (112) | Retrospective cohort of women and prospective cohort of children | BSID-III | Confirmed/probable (symptom referral, or abnormal US findings, or positive laboratory assay) | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 6 to 42 months (1) | No |
| Coutinho CM | Brazil (199) | Prospective population-based cohort study | BSID-III | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 3 months (1) | No |
| Abtibol-Bernardino MR | Brazil (26) | Case series | BSID-III | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 38 months (1) | No |
| Marbán-Castro E | Spain (21) | Prospective cohort ** | BSID-III | Confirmed (by PCR) and probable (by serological methods and microneutralization) | Born to ZIKV-confirmed or probable mothers. Three children presented abnormal brain findings (calcifications or cysts). Neonates were negative for ZIKV screening in placenta, cord blood and neonatal blood and urine | 24 months (1) | No |
| Soares-Marangoni DA | Brazil (2) | Case report | Prechtl’s GM assessment and AIMS | Confirmed by RT-PCR | Born to ZIKV-con-firmed mothers. Infants not tested for ZIKV | 4 and 12 months (1) | No |
| Rice ME | U.S. territories and freely associated states (1386) | Retrospective analysis of medical/surveillance collected data | Validated screening tools recommended by the American Academy of Pediatrics *** | Confirmed and probable (laboratory evidence of confirmed or possible ZIKV infection) | Born to ZIKV-confirmed and probable mothers. Infants not tested for ZIKV | 12 months (1) | No |
| Oliveira Vianna RA | Brazil (82) | Longitudinal observational study | DDST | Confirmed and probable/suspected (women with rash during pregnancy or three months before pregnancy coinciding with the PHENC in Brazil) | Group 1: Born to ZIKV-confirmed mothers; Group 2: Born to ZIKV-negative mothers; and Group 3: Born to mothers not tested for ZIKV but who tested negative for other congenital infections. Infants not tested for ZIKV | 6, 12 and 18 months (3) | Yes, 26 children whose mothers tested negative by RT-qPCR for ZIKV (Group 2) |
| Valdes V | Puerto Rico (65) | Cross-sectional study | MSEL (translated to Spanish and adapted for Puerto Rico) | Confirmed/probable/suspected (by PCR or serology) | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 3 to 6 months; or 9 to 12 months (±2 weeks) (1) | Yes, 36 children born from mothers with negative PCR or ELISA for ZIKV |
| Lee EH | USA (148) | Retrospective analysis of medical/surveillance collected data | No test specified. Neurodevelopmental abnormalities possibly associated with ZIKV. | Confirmed and probable (Laboratory evidence of ZIKV infection during pregnancy) | Born to ZIKV-confirmed or probable mothers. Infants not tested for ZIKV | At birth, 2, 6, and 12 months (4) | No |
| Mulkey SB | Colombia and USA (70) | Prospective cohort | WIDEA and AIMS | Confirmed and probable (CDC clinical criteria for probable ZIKV infection and laboratory evidence of ZIKV confirmed by one or more tests, including PCR, IgM, IgG, and PRNT) | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | One or two assessments between 4 and 18 months (1–2) | No |
| Da Silva PFS | Brazil (140) | Cross-sectional study, nested in a cohort study | SWYC | Confirmed by RT-PCR | Group 1: Severe microcephaly; Group 2: Moderate microcephaly; Group 3: Prenatal ZIKV exposure confirmed by maternal RT-PCR testing but no microcephaly; Group 4: Neurotypical control group. For this review, only Groups 3 and 4 were considered. Infants not tested for ZIKV | 28 months (1) | Yes, 46 neurotypical children with neither microcephaly nor any other brain abnormalities detectable by brain US at birth who were born to mothers with no laboratory evidence of ZIKV infection during pregnancy (Group 4) |
| Familiar I | Mexico (59) | Prospective cohort | MSEL and FTII | Confirmed by RT-PCR | Born to mothers with confirmed infection, normocephalic and asymptomatic. Infants not tested for ZIKV | 6 months (1) | Yes, 45 healthy children without ZIKV exposure |
| Cabral Maia AMP | Brazil (17) | Cross-sectional case series study | None (Child Health Booklet developed by the Brazilian Ministry of Health) | Confirmed by laboratory (diagnostic tool not reported) | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV | 10–25 months (1) | No |
| Pimentel R | The Dominican Republic (42) | Retrospective cohort analysis of children | DDST | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Infants not tested for ZIKV. Only neonates with microcephaly were included | 1, 2, 3, 6, 9, 12, 15, and 18 months (8) | No |
| Grant R | Guadeloupe, Martinique, and French Guiana (235) | Population-based mother–child cohort | ASQ, M-CHAT and IFDC | Confirmed by RT-PCR | Born to ZIKV-confirmed mothers. Only included infants who presented positive ZIKV serologies in cord and/or neonatal/infant blood | 24 months (1) | Yes, children born to mothers with negative IgG at delivery |
| First Author | Characteristics: Children Evaluated (n), Inclusion Criteria, Tool Used and Main Study Objective | Socioeconomic Characteristics | Main Results | Neurodevelopment Affected in ZIKV Exposed Children |
|---|---|---|---|---|
| Soares-Marangoni D.A. | n= 2. Normocephalic children with negative serologies for other congenital infections, and negative ZIKV-PCR on urine, cerebrospinal fluid and umbilical cord samples; born to women with positive ZIKV-PCR. Prechtl’s General Movement assessment and the Alberta Infant Motor Scale. To describe the GMs in the fidgety * period and the motor performance of two infants who were exposed to ZIKV during distinct trimesters of gestation. | Not reported. | GMs in the fidgety period are early markers of motor performance at 12 months of age. In Case 1, fidgety movements were absent at 16 weeks after term and motor development was severely impaired at 12 months of age. In Case 2, fidgety movements were normal at 13 weeks and the motor outcome was typical at 12 months | Yes |
| Rice M.E. | n = 1386. Validated screening tools recommended by the American Academy of Pediatrics. Normocephalic children with no Zika-associated birth defects, born to women with laboratory evidence of confirmed or possible ZIKV infection. To report ZIKV-associated birth defects and/or neurodevelopmental abnormality possibly associated with congenital ZIKV, among one-year-old children born to mothers with confirmed or possible infection. | Not reported | Among 1450 children, 76% had developmental screening or evaluation, 60% had postnatal neuroimaging, 48% had automated auditory brainstem response-based hearing screen or evaluation, and 36% had an ophthalmologic evaluation. Among evaluated children, 6% had at least one ZIKV-associated birth defect, 9% had at least one neurodevelopmental abnormality possibly associated with congenital ZIKV infection, and 1% had both | Yes |
| Oliveira Vianna R.A. | n = 82. Children whose mothers had a rash and tested positive to ZIKV by PCR (Group 1); children whose mothers tested negative by PCR (Group 2); and children whose mothers did not undergo any testing for ZIKV but tested negative for other congenital infections (Group 3). DDST. To better understand the clinical spectrum and course of CZS during the first 18 months of life of children whose mothers had rash during pregnancy. | Most women were less than 30 years old, had at least 9 years of schooling; 37% of families earned one or less Brazilian monthly minimum wage, and 54% were residents of informal human settlements ** | From the 108 children in the study, 26 developed CZS; thus, only 82 were healthy asymptomatic children. At 12 months, 7 of 82 children with no CZS (8.5%) had isolated abnormalities by physical examination that did not fulfil the criteria for CZS. | Yes |
| Valdes V. | n = 65. Children born to mothers with at least one prenatal or postnatal positive ZIKV-PCR. MSEL. To determine whether infants of mothers with at least one positive ZIKV test during pregnancy show differences in cognitive scores at ages 3 to 6 months and ages 9 to 12 months. | Mothers and fathers in the study had high levels of education (93.8%, and 75% with high school level education or higher, respectively), while 58.3% of mothers and 13.9% of fathers were unemployed or worked from home. Most of the children (88.9%) spoke Spanish, while the others were bilingual (Spanish and English). Regarding home status, 44.4% of families owned a house, 22.2% rented a house, 22.2% lived in public housing, and 11.1% occupied a house for free. | Prenatal maternal ZIKV infection is associated with lower receptive language scores during the first year of life; however, exposure to ZIKV does not appear to be associated with other domains of cognitive development. Maternal education, paternal education, maternal employment, paternal employment, and home status were tested to assess a possible association with ZIKV status. Maternal employment was the only variable significantly associated with ZIKV status (χ2 = 6.72; Cramér V = 0.32; p = 0.04). | Yes (only the language function) |
| Lee E.H. | n= 148 Children without birth defects, nor laboratory evidence of congenital ZIKV infection, born to women with laboratory evidence of ZIKV infection. No test specified. To characterize the epidemiology and clinical significance of congenital ZIKV exposure by prospectively following a cohort of infants with possible congenital exposure through their first year of life. | Not reported. | Most children, 95.3% (385), appeared well, whereas 19 (4.7%) had a possible ZIKV-associated birth defect. From 370 infants with neither birth defects nor laboratory evidence of congenital infection, or with no ZIKV testing, information at 12 months of age was available for 148 cases. Overall, 4 of 148 infants were reported to have a developmental delay; 2 infants demonstrated gross motor and speech delays, and 2 had isolated speech delay. Of the 22 infants younger than 12 months, only 13 had follow-up, and all of them had normal neurodevelopment. | Yes |
| Mulkey S.B. | n = 70 Normocephalic live-born with normal fetal brain findings on MRI, and average examination results without clinical evidence of CZS, born to women with laboratory evidence of ZIKV infection. WIDEA and AIMS. To assess the neurodevelopment of children exposed to ZIKV in utero born without CZS. | Not reported. | Infants with in utero ZIKV exposure without CZS appeared at risk for abnormal neurodevelopmental outcomes in the first 18 months of life. The WIDEA total score (coefficients: age = –0.227 vs. age2 = 0.006; p < 0.003) and self-care domain score (coefficients: age = –0.238 vs. age2 = 0.01; p < 0.008) showed curvilinear associations with age. Other domain scores showed linear declines with increasing age based on coefficients for communication (–0.036; p = 0.001), social cognition (–0.10; p < 0.001), and mobility (–0.14; p < 0.001). The AIMS scores were similar to the normative sample over time (95% CI, –0.107 to 0.037; p = 0.34). Overall, 19 of 57 infants (33%) who underwent postnatal cranial ultrasonography had a nonspecific, mild finding. No difference was found in the decline of WIDEA z scores between infants with and those without cranial ultrasonography findings except for a complex interactive relationship involving the social cognition domain (p < 0.049). The AIMS z scores were lower in infants with nonspecific cranial ultrasonography findings (–0.49; p = 0.07). | Yes |
| Da Silva P.F.S. | n = 140 Normocephalic children born to women with confirmed ZIKV-PCR. SWYC. To investigate patterns of neurodevelopment and behavior in groups of children with different severities of ZIKV-related microcephaly and children with prenatal ZIKV exposure in the absence of microcephaly. | Not reported. | ZIKV-exposed children without microcephaly and neurotypical controls had similar frequencies of risk of development delay. In comparison, 13.8% of ZIKV-exposed normocephalic children and 21.7% of control group children were identified by SWYC assessment as being ‘at risk’. | No |
| Familiar I. | n = 59 Normocephalic and asymptomatic children, born to women with confirmed ZIKV-PCR. MSEL and FTII. To assess neurodevelopmental outcomes in normocephalic infants born to women with ZIKV infection during pregnancy in Mexico. | Maternal educational level was high in the ZIKV-exposed group (95%), and in the unexposed group (89%). Overall, 78% and 80% of women were unemployed or worked from home in the ZIKV-exposed and -unexposed groups, respectively. No significant differences in demographic or anthropometric characteristics were observed. | All MSEL sub-scale scores, except expressive language, were significantly lower among ZIKV-exposed children compared to controls, including the overall standard composite (80 ± 10 vs. 87 ± 7.4, respectively; p < 0.001). In comparison with their peers, infants born to women with confirmed ZIKV infection during pregnancy showed poorer neurodevelopmental performance in language and motor domains and worse visual recognition memory at six months of age. | Yes |
| Cabral Maia A.M.P. | n = 17 Normocephalic children born to women with laboratory confirmed ZIKV infection. Child Health Booklet developed by the Brazilian Ministry of Health To evaluate the developmental and anthropometric milestones of asymptomatic children whose mothers had ZIKV infection. | Only one-third of mothers had completed high school (7/17, 41.2%); 7/17 (41.2%) were married, and 8/17 (47.1%) were housewives. The average income was one minimum wage (954.00 BRL). Among the women who were housewives, 3/8 (37.5%) had quit their jobs to take care of their children. | Most children, 15/17 (88.2%), presented with at least one delayed developmental milestone with respect to the standards for the age group. Among these children, 5/15 (33.3%) reached three developmental milestones, 5/15 (33.3%) reached two, and 5/15 (33.3%) reached only one. | Yes |
| Pimentel R. | n = 42 Children born without obvious ZIKV-associated birth defects, to symptomatic women with confirmed ZIKV-PCR.DDST. To assess the clinical and epidemiological characteristics of infants with ZIKV-associated microcephaly, and the neurodevelopmental abnormalities during the first 18 months of life for a group of infants with possible congenital ZIKV exposure. | Authors compared sociodemographic characteristics and clinical presentation of mothers with or without an infant with abnormal developmental screening, and found no significant differences between the two groups except for higher-frequency abdominal pain during pregnancy in women whose infants had an abnormal developmental screen (85% vs. 38%, p = 0.007). Although the sample size is small, maternal alcohol use and smoking history were not associated with infant’s developmental delay. | Of 42 infants with possible congenital ZIKV exposure followed longitudinally, 52% exhibited possible developmental delay in at least one visit throughout the 18-month observation period. Interestingly, most of the observed neurodevelopmental abnormalities resolved over time and only four infants were noted to have abnormalities that persisted for 15–18 months. If the two infants who developed postnatal microcephaly were excluded, 5% (2/42) of infants had neurodevelopmental abnormalities possibly associated with congenital ZIKV infection. | Yes |
| Grant R. | n= 235 Normocephalic children born with normal transfontanelle cerebral ultrasound findings, or normal ultrasound findings on the last ultrasound performed during the third trimester of the mother’s pregnancy, born to women with confirmed ZIKV-PCR. ASQ, M-CHAT, and IFDC. To determine the impact of ZIKV exposure on neurodevelopment at 24 months of age among toddlers who were born normocephalic to women who were pregnant during the 2016 ZIKV outbreak in French territories in the Americas. | Comparisons between ZIKV-exposed and unexposed toddlers indicated a lower maternal age (p= 0.01), higher maternal education (p = 0.04), and higher paternal education (p = 0.04) in the unexposed; a higher proportion of toddlers from Guadeloupe in the exposed group and a higher proportion of toddlers from Martinique in the unexposed group (p≤ 0.001); higher parity in the ZIKV exposed (p = 0.04); and greater use of mosquito repellents in the exposed group (p = 0.05). | In one of the largest population-based, mother–child cohorts of in utero ZIKV-exposed normocephalic at birth to date. Authors found that 15.3% of toddlers exposed to ZIKV have abnormal neurodevelopment findings at 24 months of age. However, differences were not statistically significant when compared to not-exposed toddlers. | No |
| First Author | Number of Children Evaluated | Cognitive Delay | Language Delay | Motor Delay | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Affected Children | Prevalence (95% CI) | % Weight | Affected Children | Prevalence (95% CI) | % Weight | Affected Children | Prevalence (95% CI) | % Weight | ||
| Nielsen-Saines K. | 146 | 14 | 9.6 (5.8, 15.5) | 30.49 | 51 | 34.9 (27.7, 43.0) | 16.73 | 24 | 16.4 (11.3, 23.3) | 14.78 |
| Lopes Moreira M.E. | 94 | 11 | 11.7 (6.7, 19.8) | 19.67 | 25 | 26.6 (18.7, 36.3) | 15.43 | 18 | 19.1 (12.5, 28.3) | 14.12 |
| Peçanha P.M. | 84 | 4 | 4.8 (1.9, 11.6) | 17.59 | 31 | 36.9 (27.4, 47.6) | 15.05 | 20 | 23.8 (16.0, 33.9) | 13.92 |
| Einspieler C. | 56 | 1 | 1.8 (0.3, 9.4) | 11.76 | 6 | 10.7 (5.0, 21.5) | 13.46 | 1 | 1.8 (0.3, 9.4) | 13.03 |
| Faiçal A.V. | 29 | 4 | 13.8 (5.5, 30.6) | 6.14 | 9 | 31.0 (17.3, 49.2) | 10.41 | 1 | 3.4 (0.6, 17.2) | 11.08 |
| Abtibol-Bernardino M.R. | 26 | 5 | 19.2 (8.5, 37.9) | 5.52 | 10 | 38.5 (22.4, 57.5) | 9.89 | 9 | 34.6 (19.4, 53.8) | 10.70 |
| Marbán-Castro E. ** | 21 | 1 | 4.8 (0.8, 22.7) | 4.47 | 7 | 33.3 (17.2, 54.6) | 8.86 | 0 | 0.0 (0.0, 15.5) | 9.92 |
| Rodrigues Gerzson L. | 17 | 1 | 5.9 (1.0, 27.0) | 3.64 | 9 | 52.9 (31.0, 73.8) | 7.86 | 4 | 23.5 (9.6, 47.3) | 9.12 |
| Sobhani N.C. | 3 | 0 | 0.0 (0.0, 56.1) | 0.73 | 0 | 0.0 (0.0, 56.1) | 2.31 | 0 | 0.0 (0.0, 56.1) | 3.33 |
| Pooled prevalence * | 6.5 (4.1, 9.3) Q Heterogeneity chi-squared = 11.60 (d.f. = 8) p = 0.1701; I2 = 31.0% | 29.7 (0.217, 0.382) Q Heterogeneity chi-squared = 22.77 (d.f. = 8) p = 0.0037; I2: = 64.9% | 11.5 (4.8, 20.1) Q Heterogeneity chi-squared = 37.02 (d.f. = 8) p <0.0001; I2 = 78.4% | |||||||
| First Author | Number of Children Evaluated | Moderate and Severe Cognitive Delay | Moderate and Severe Language Delay | Moderate And Severe Motor Delay | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Affected Children | Prevalence (95% CI) | % Weight | Affected Children | Prevalence (95% CI) | % Weight | Affected Children | Prevalence (95% CI) | % Weight | ||
| Nielsen-Saines K. | 146 | 8 | 5.5 (2.8, 10.4) | 36.99 | 17 | 11.6 (7.4, 17.9) | 36.99 | 7 | 4.8 (2.3, 9.6) | 36.99 |
| Lopes Moreira M.E. | 94 | 6 | 6.4 (3.0, 13.2) | 23.86 | 10 | 10.6 (5.9, 18.5) | 23.86 | 7 | 7.4 (3.7, 14.6) | 23.86 |
| Einspieler C. | 56 | 0 | 0.0 (0.0, 6.4) | 14.27 | 3 | 5.4 (1.8, 14.6) | 14.27 | 1 | 1.8 (0.3, 9.4) | 14.27 |
| Faiçal A.V. | 29 | 4 | 13.8 (5.5, 30.6) | 7.45 | 2 | 6.9 (1.9, 22.0) | 7.45 | 0 | 0.0 (0.0, 11.7) | 7.45 |
| Abtibol-Bernardino M.R. | 26 | 0 | 0.0 (0.0, 12.9) | 6.69 | 6 | 23.1 (11.0, 42.1) | 6.69 | 3 | 11.5 (4.0, 29.0) | 6.69 |
| Marbán-Castro E. ** | 21 | 0 | 0.0 (0.0, 15.5) | 5.43 | 1 | 4.8 (0.8, 22.7) | 5.43 | 0 | 0.0 (0.0, 15.5) | 5.43 |
| Rodrigues Gerzson L. | 17 | 0 | 0.0 (0.0, 18.4) | 4.42 | 2 | 11.8 (3.3, 34.3) | 4.42 | 0 | 0.0 (0.0, 18.4) | 4.42 |
| Sobhani N.C. | 3 | 0 | 0.0 (0.0, 56.1) | 0.88 | 0 | 0.0 (0.0, 56.1) | 0.88 | 0 | 0.0 (0.0, 56.1) | 0.88 |
| Pooled prevalence * | 1.9 (0.4, 4.1) Q Heterogeneity chi-squared = 13.42 (d.f. = 7) p = 0.0626; I2 = 47.8% | 8.4 (5.4, 11.8) Q Heterogeneity chi-squared = 6.08 (d.f. = 7) p = 0.5306; I2= 0.0% | 2.2 (0.6, 4.5) Q Heterogeneity chi-squared = 8.49 (d.f. = 7) p = 0.2918; I2 = 17.5% | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marbán-Castro, E.; Vazquez Guillamet, L.J.; Pantoja, P.E.; Casellas, A.; Maxwell, L.; Mulkey, S.B.; Menéndez, C.; Bardají, A. Neurodevelopment in Normocephalic Children Exposed to Zika Virus in Utero with No Observable Defects at Birth: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 7319. https://doi.org/10.3390/ijerph19127319
Marbán-Castro E, Vazquez Guillamet LJ, Pantoja PE, Casellas A, Maxwell L, Mulkey SB, Menéndez C, Bardají A. Neurodevelopment in Normocephalic Children Exposed to Zika Virus in Utero with No Observable Defects at Birth: A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(12):7319. https://doi.org/10.3390/ijerph19127319
Chicago/Turabian StyleMarbán-Castro, Elena, Laia J. Vazquez Guillamet, Percy Efrain Pantoja, Aina Casellas, Lauren Maxwell, Sarah B. Mulkey, Clara Menéndez, and Azucena Bardají. 2022. "Neurodevelopment in Normocephalic Children Exposed to Zika Virus in Utero with No Observable Defects at Birth: A Systematic Review with Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 12: 7319. https://doi.org/10.3390/ijerph19127319
APA StyleMarbán-Castro, E., Vazquez Guillamet, L. J., Pantoja, P. E., Casellas, A., Maxwell, L., Mulkey, S. B., Menéndez, C., & Bardají, A. (2022). Neurodevelopment in Normocephalic Children Exposed to Zika Virus in Utero with No Observable Defects at Birth: A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health, 19(12), 7319. https://doi.org/10.3390/ijerph19127319









