Association of Hyperuricemia with Impaired Left Ventricular Systolic Function in Patients with Atrial Fibrillation and Preserved Kidney Function: Analysis of the POL-AF Registry Cohort
Abstract
1. Introduction
2. Materials and Methods
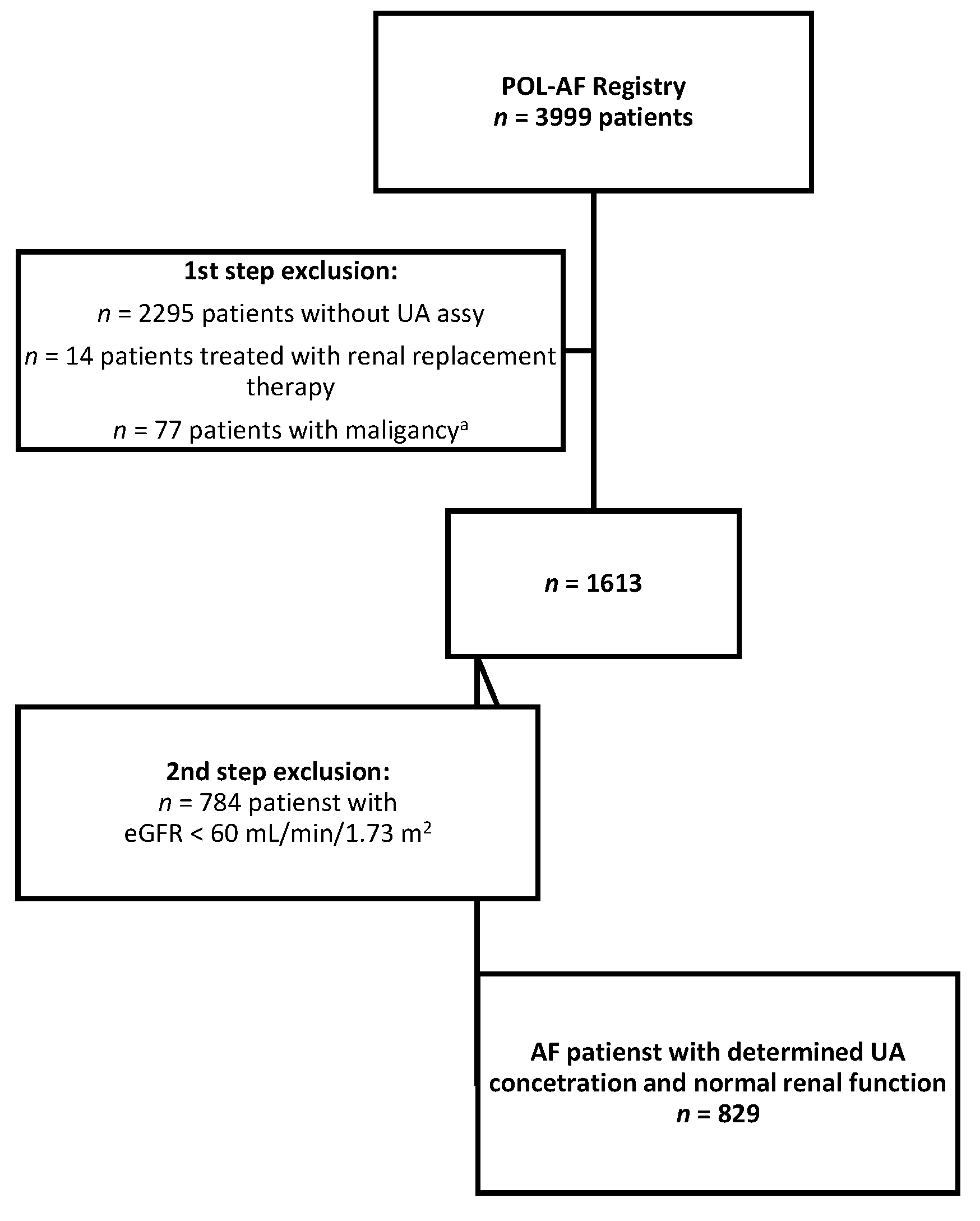
2.1. Study Design and Participants
2.2. Patient Grouping
2.3. Statistical Analysis
3. Results
3.1. General Characteristics
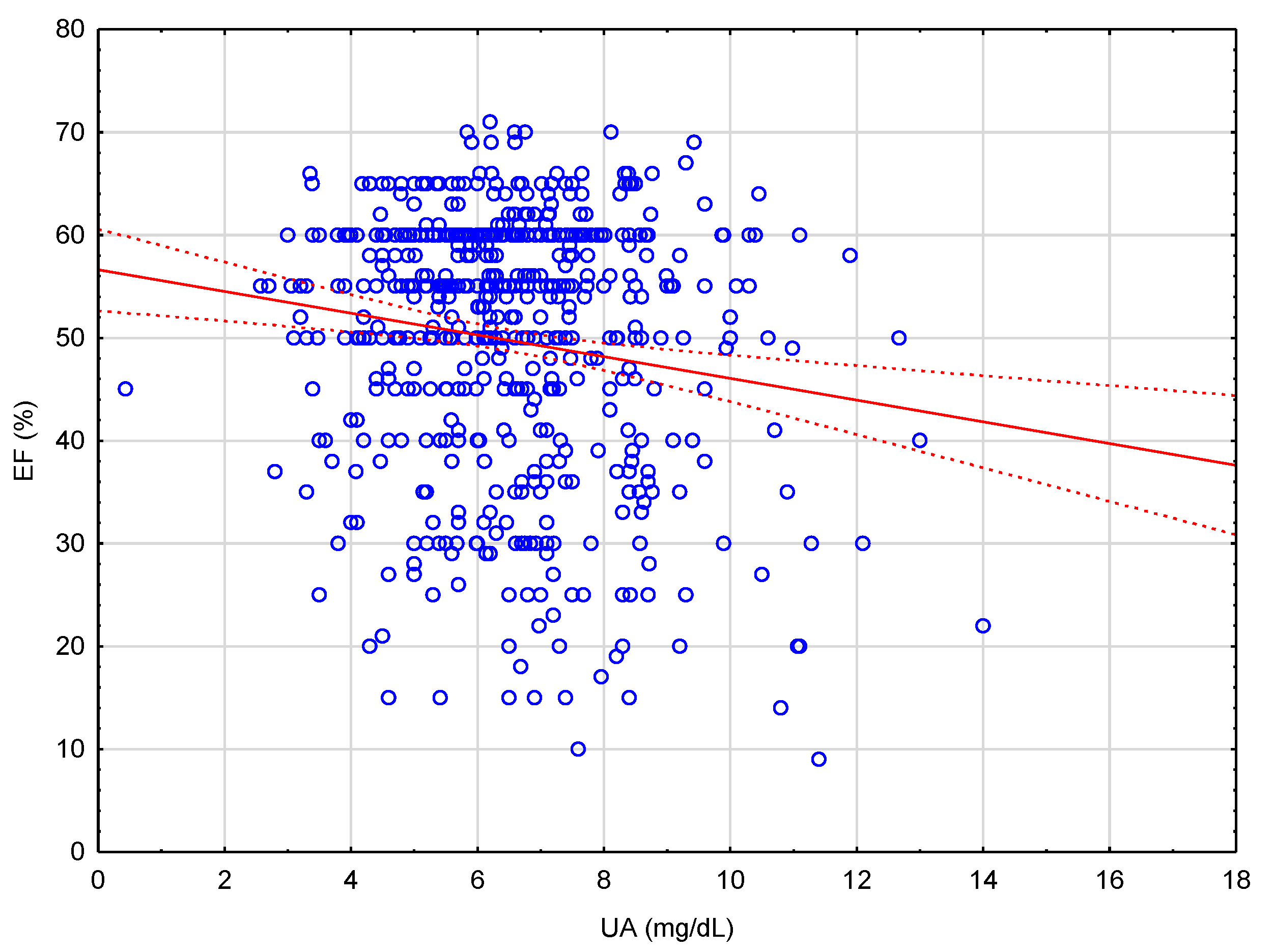
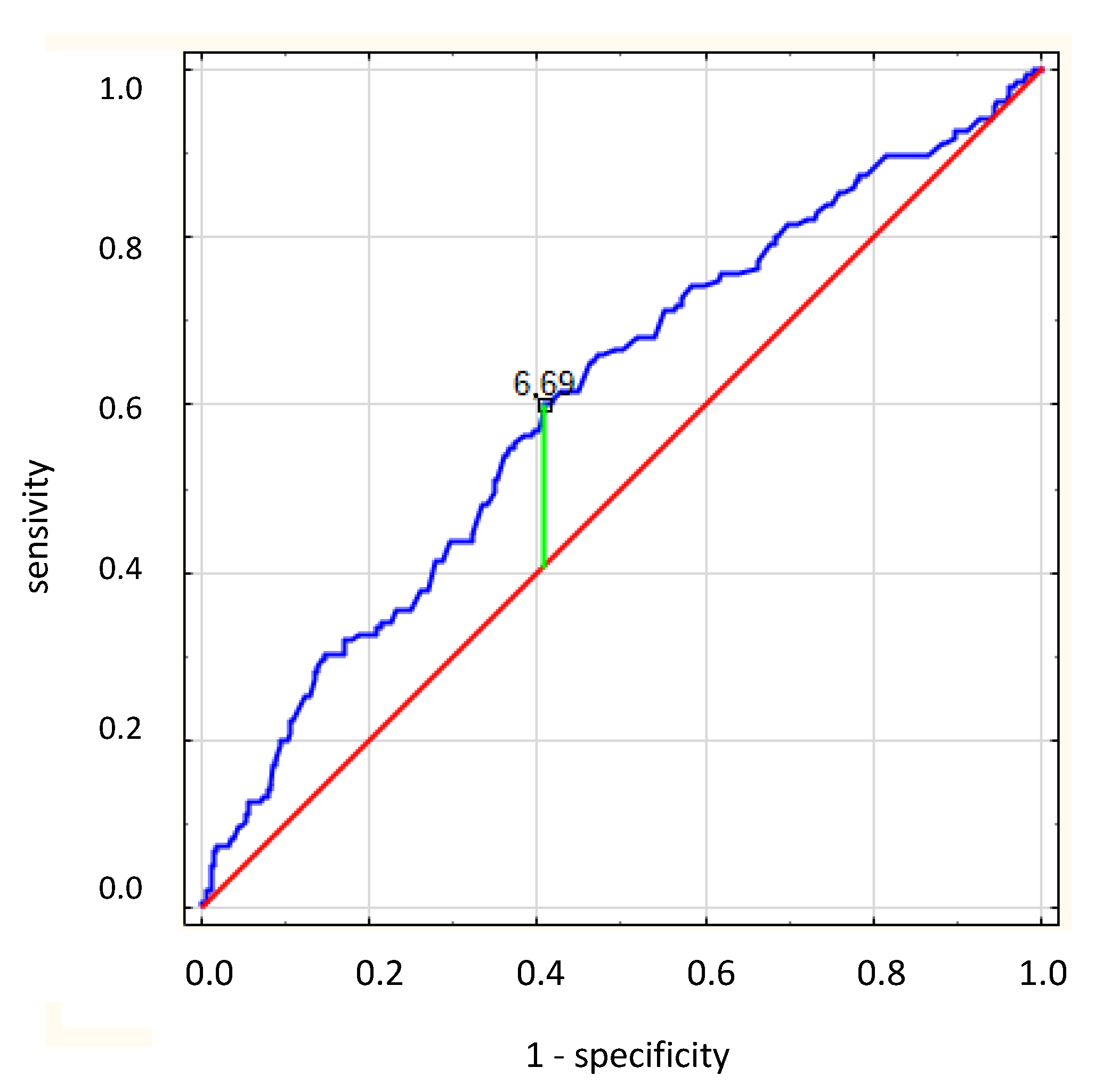
3.2. Uric Acid
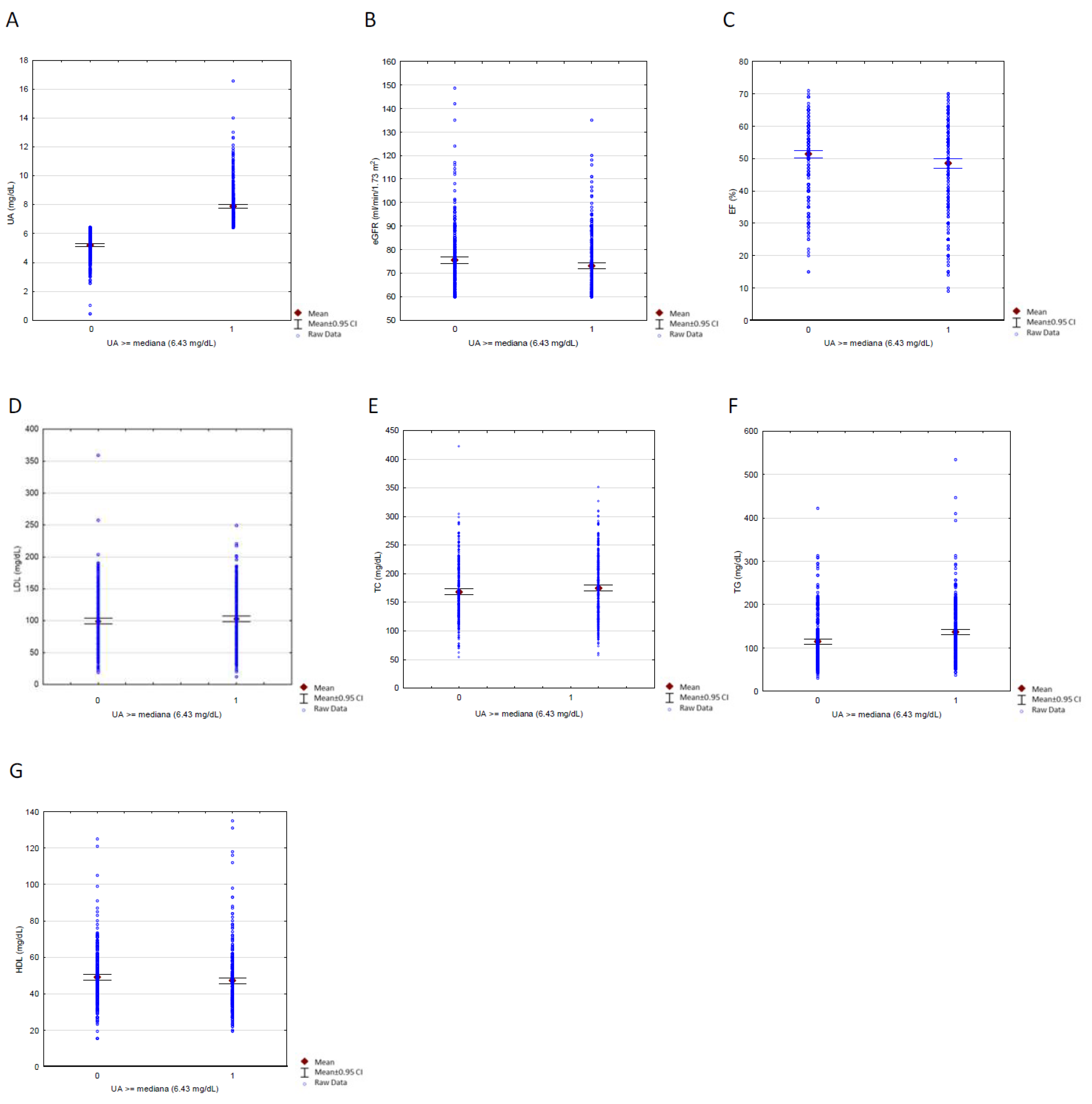
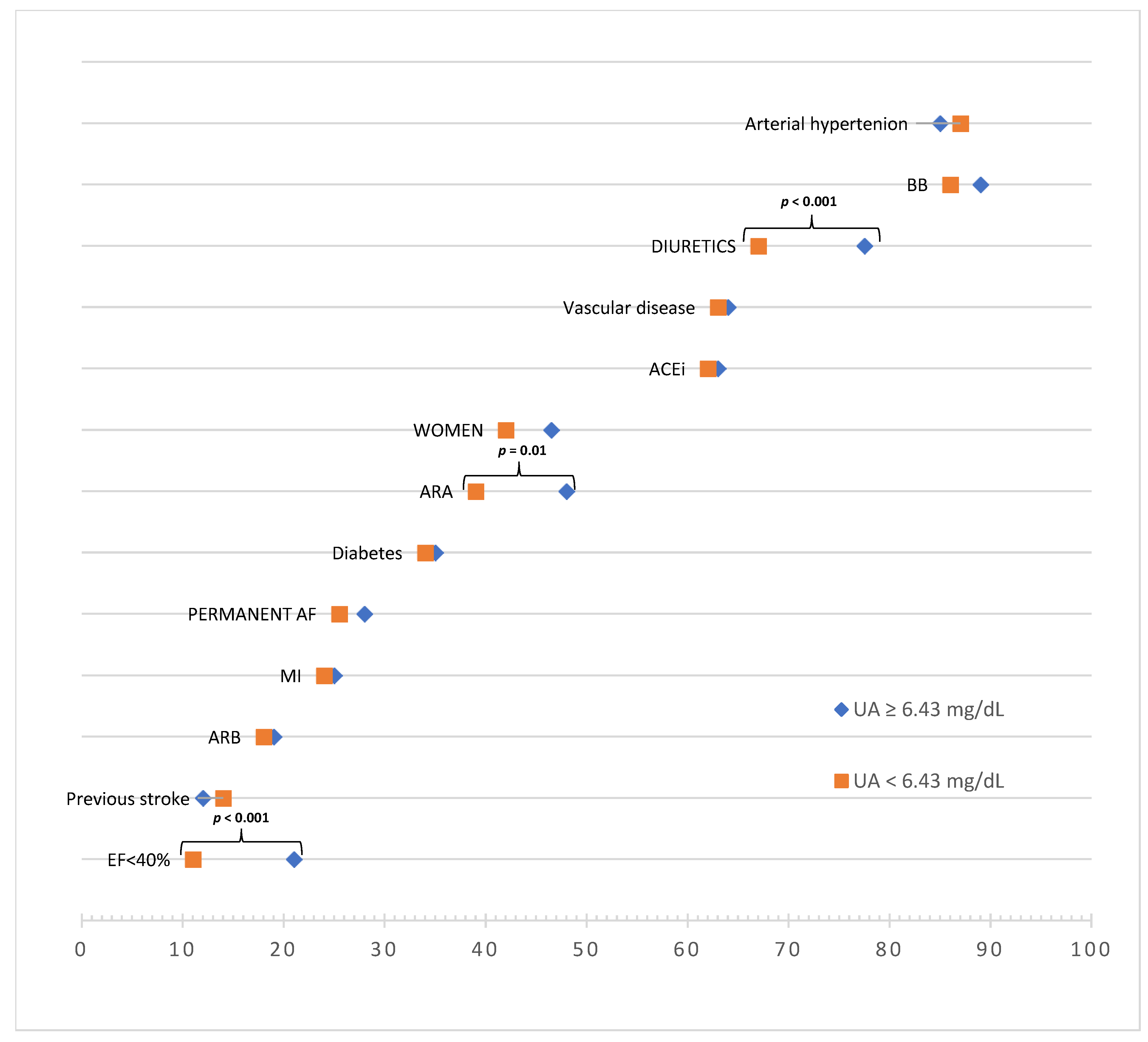
3.3. Comparison of Patients with High vs. Low Uric Acid Concentrations
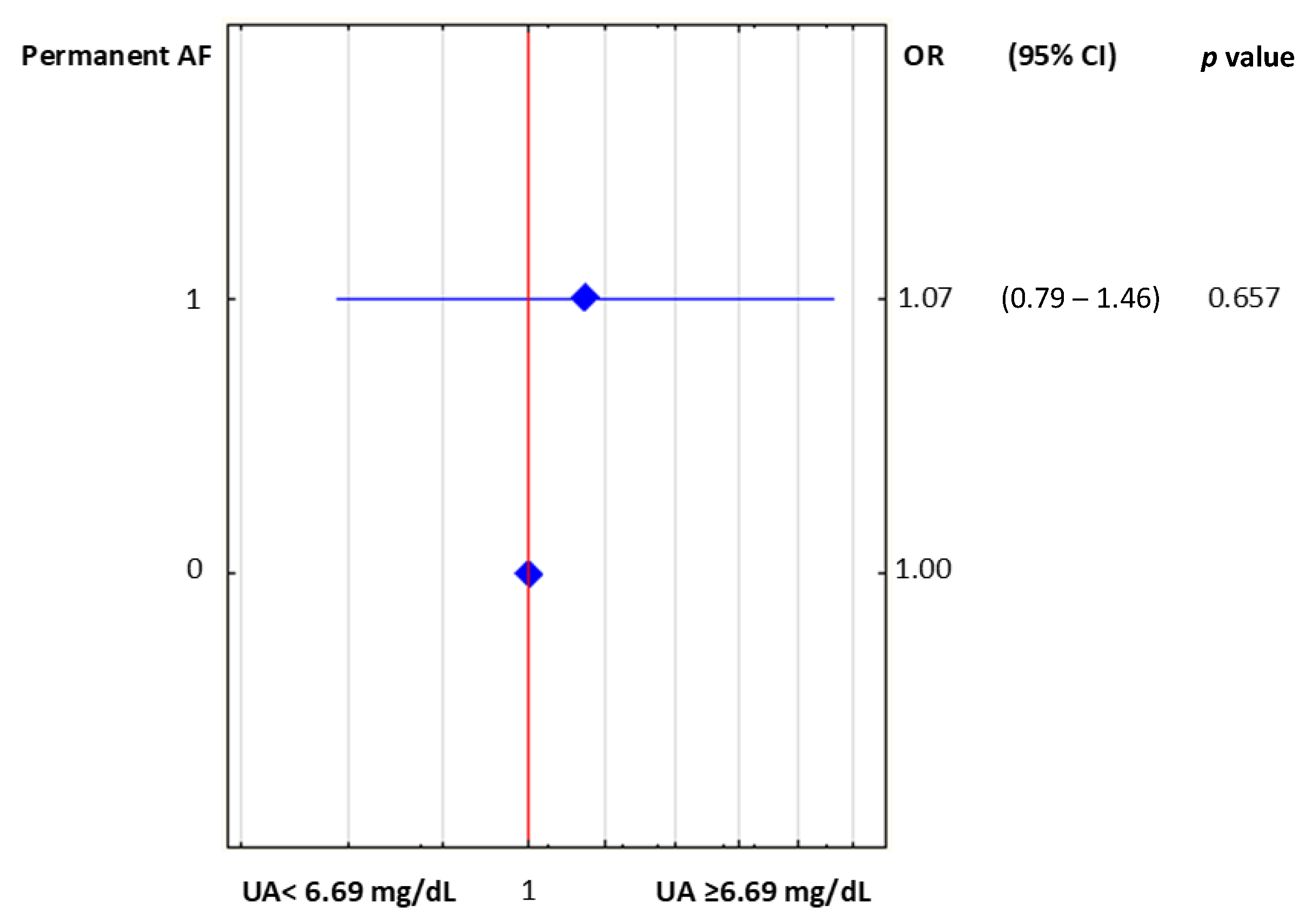
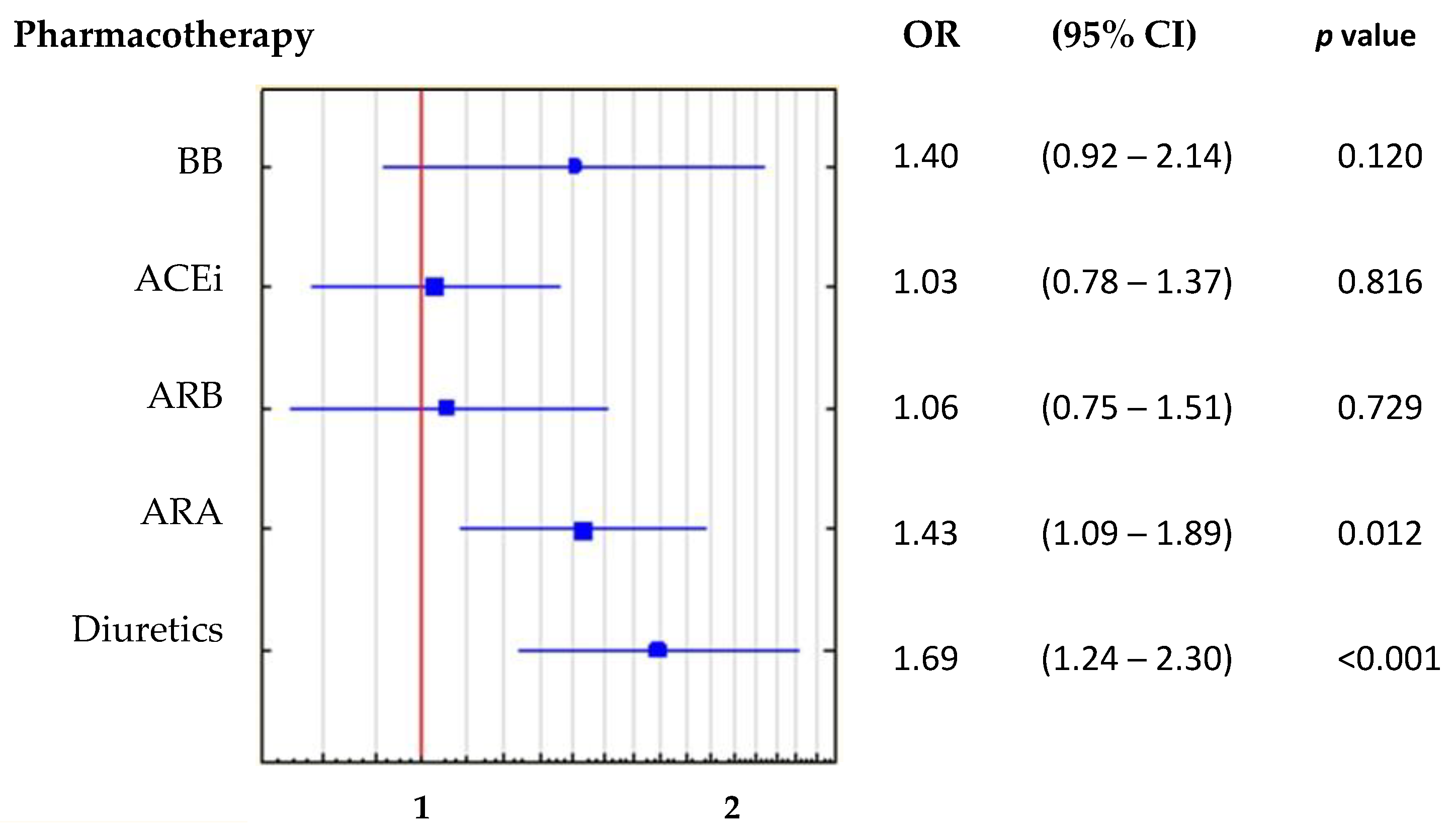
3.4. Drug Effects
4. Discussion
4.1. Heart Failure, Renal Failure, and Hyperuricemia—A Toxic Triangle
4.2. Uric Acid Separate from Kidney Function
4.3. Uric Acid Separate from Potential Drug Effects
4.4. Cause or Effect, Marker, or Risk Factor?
4.5. Limitations
5. Conclusions
- In patients with AF, hyperuricemia is a marker of reduced EF.
- In patients with AF and eGFR >60 mL/min/1.73 m2, the relationship between UA and EF
- (a)
- Is independent of renal function; and
- (b)
- Is independent of the use of drugs typical for treating heart failure
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498, Erratum in Eur. Heart J. 2021, 42, 507; Erratum in Eur. Heart J. 2021, 42, 546–547; Erratum in Eur. Heart J. 2021, 42, 4191. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Tykarski, A.; Widecka, K.; Filipiak, K.J.; Domienik-Karłowicz, J.; Kostka-Jeziorny, K.; Varga, A.; Jaguszewski, M.; Narkiewicz, K.; Mancia, G. Expert consensus for the diagnosis and treatment of patient with hyperuricemia and high cardiovascular risk. Cardiol. J. 2018, 25, 545–563. [Google Scholar] [CrossRef]
- Borghi, C.; Domienik-Karłowicz, J.; Tykarski, A.; Widecka, K.; Filipiak, K.J.; Jaguszewski, M.J.; Narkiewicz, K.; Mancia, G. Expert consensus for the diagnosis and treatment of patient with hyperuricemia and high cardiovascular risk: 2021 update. Cardiol. J. 2021, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Norvik, J.V.; Storhaug, H.M.; Ytrehus, K.; Jenssen, T.G.; Zykova, S.N.; Eriksen, B.O.; Solbu, M.D. Overweight modifies the longitudinal association between uric acid and some components of the metabolic syndrome: The Tromsø Study. BMC Cardiovasc. Disord. 2016, 16, 85. [Google Scholar] [CrossRef]
- Shirasawa, T.; Ochiai, H.; Yoshimoto, T.; Nagahama, S.; Watanabe, A.; Yoshida, R.; Kokaze, A. Cross-sectional study of associations between normal body weight with central obesity and hyperuricemia in Japan. BMC Endocr. Disord. 2020, 20, 2. [Google Scholar]
- Bombelli, M.; Ronchi, I.; Volpe, M.; Facchetti, R.; Carugo, S.; Dell’oro, R.; Cuspidi, C.; Grassi, G.; Mancia, G. Prognostic value of serum uric acid: New-onset in and out-of-office hypertension and long-term mortality. J. Hypertens. 2014, 32, 1237–1244. [Google Scholar] [CrossRef]
- Grayson, P.C.; Kim, S.Y.; LaValley, M.; Choi, H.K. Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res. 2011, 63, 102–110. [Google Scholar] [CrossRef]
- Kim, S.Y.; Guevara, J.P.; Kim, K.M.; Choi, H.K.; Heitjan, D.F.; Albert, D.A. Hyperuricemia and risk of stroke: A systematic review and meta-analysis. Arthritis Care Res. 2009, 61, 885–892. [Google Scholar] [CrossRef]
- Zhong, C.; Zhong, X.; Xu, T.; Xu, T.; Zhang, Y. Sex-Specific relationship between serum uric acid and risk of stroke: A dose-response me-ta-analysis of prospective studies. J. Am. Heart Assoc. 2017, 6, e005042. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Braun, S.; Haase, H.-U.; Schulz, S.; Ranftl, S.; Hadamitzky, M.; Mehilli, J.; Schömig, A.; Kastrati, A. Prognostic Value of Uric Acid in Patients with Acute Coronary Syndromes. Am. J. Cardiol. 2012, 109, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Ilundain-González, A.I.; Gimeno-Orna, J.A.; Sáenz-Abad, D.; Pons-Dolset, J.; Hoyo, J.C.-D.; Lahoza-Pérez, M.D.C. Impact of uric acid levels on the risk of long-term cardiovascular mortality in patients with type 2 diabetes mellitus. Endocrinol. Diabetes Nutr. 2018, 65, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Tamariz, L.; Agarwal, S.; Soliman, E.Z.; Chamberlain, A.M.; Prineas, R.; Folsom, A.R.; Ambrose, M.; Alonso, A. Association of Serum Uric Acid with Incident Atrial Fibrillation (from the Atherosclerosis Risk in Communities [ARIC] Study). Am. J. Cardiol. 2011, 108, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-H.; Huang, D.-S.; Dang-Sheng, H.; Zhang, L.-W.; Ma, Y.-J.; Wang, Y.-M.; Sun, H.-Y. Association Between Serum Uric Acid Levels and Atrial Fibrillation Risk. Cell. Physiol. Biochem. 2016, 38, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, N.; Wang, R.; Wang, Y.; Cai, S. Hyperuricemia is independently associated with increased risk of atrial fibrillation: A meta-analysis of cohort studies. Int. J. Cardiol. 2015, 184, 699–702. [Google Scholar] [CrossRef]
- Doehner, W.; Springer, J.; Anker, S.D.; Landmesser, U.; Struthers, A.D. Uric acid in chronic heart failure-current pathophysiological concepts. Eur. J. Heart Fail. 2008, 10, 1269–1270. [Google Scholar] [CrossRef][Green Version]
- Huang, H.; Huang, B.; Li, Y.; Huang, Y.; Li, J.; Yao, H.; Jing, X.; Chen, J.; Wang, J. Uric acid and risk of heart failure: A systematic review and meta-analysis. Eur. J. Heart Fail. 2013, 16, 15–24. [Google Scholar] [CrossRef]
- Wełnicki, M.; Gorczyca, I.; Wójcik, W.; Jelonek, O.; Maciorowska, M.; Uzie˛bło-Życzkowska, B.; Wójcik, M.; Błaszczyk, R.; Rajtar-Salwa, R.; Tokarek, T.; et al. Hyperuricemia as a Marker of Reduced Left Ventricular Ejection Fraction in Patients with Atrial Fibrillation: Results of the POL-AF Registry Study. J. Clin. Med. 2021, 10, 1829. [Google Scholar] [CrossRef]
- Adamska-Wełnicka, A.; Wełnicki, M.; Mamcarz, A.; Gellert, R. Chronic Kidney Disease and Heart Failure–Everyday Diagnostic Challenges. Diagnostics 2021, 11, 2164. [Google Scholar] [CrossRef]
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; Van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef]
- McAlister, F.A.; Ezekowitz, J.; Tarantini, L.; Squire, I.; Komajda, M.; Bayes-Genis, A.; Gotsman, I.; Whalley, G.; Earle, N.; Poppe, K.K.; et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: Impact of the new chronic kidney disease—Epidemiology collaboration group formula. Circ. Heart Fail. 2012, 5, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Benn, M. Atrial Fibrillation and Chronic Kidney Disease. Eur. Heart J. 2021, 42, 2824–2826. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Y.; Gupta, D.; Wong, C.F.; Lip, G.Y.H. Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc. Res. 2020, 117, 1046–1059. [Google Scholar] [CrossRef]
- Soliman, E.Z.; Prineas, R.J.; Go, A.S.; Xie, D.; Lash, J.P.; Rahman, M.; Ojo, A.; Teal, V.L.; Jensvold, N.G.; Robinson, N.L.; et al. Chronic kidney disease and prevalent atrial fibrillation: The Chronic Renal Insufficiency Cohort (CRIC). Am. Heart J. 2010, 159, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Laroche, C.; Diemberger, I.; Popescu, M.I.; Rasmussen, L.H.; Petrescu, L.; Crijns, H.J.G.M.; Tavazzi, L.; Maggioni, A.P.; Lip, G.Y.H. Glomerular filtration rate in patients with atrial fibrillation and 1-year outcomes. Sci. Rep. 2016, 6, 30271. [Google Scholar] [CrossRef]
- Banerjee, A.; Halimi, J.; Vourc’H, P.; Andres, C.R.; Taillandier, S.; Lip, G.Y.H.; Fauchier, L. A prospective study of estimated glomerular filtration rate and outcomes in patients with atrial fibrillation: The Loire Valley Atrial Fibrillation Project. Eur. Heart J. 2013, 34, P5614. [Google Scholar] [CrossRef]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Sanchez-Lozada, L.-G.; et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Pontremoli, R.; Burnier, M. Management of Hyperuricemia in Patients with Chronic Kidney Disease: A Focus on Renal Protection. Curr. Hypertens. Rep. 2020, 22, 102. [Google Scholar] [CrossRef]
- Mewton, N.; Girerd, N.; Boffa, J.-J.; Courivaud, C.; Isnard, R.; Juillard, L.; Lamblin, N.; Legrand, M.; Logeart, D.; Mariat, C.; et al. Practical management of worsening renal function in outpatients with heart failure and reduced ejection fraction: Statement from a panel of multidisciplinary experts and the Heart Failure Working Group of the French Society of Cardiology. Arch. Cardiovasc. Dis. 2020, 113, 660–670. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Kumrić, M.; Borovac, J.; Kurir, T.; Božić, J. Clinical Implications of Uric Acid in Heart Failure: A Comprehensive Review. Life 2021, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; De Vivo, O.; Nuti, R.; McCullough, P.A. Prevalence of Hyperuricemia in Patients with Acute Heart Failure with Either Reduced or Preserved Ejection Fraction. Am. J. Cardiol. 2017, 120, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Yoshihisa, A.; Kanno, Y.; Takiguchi, M.; Sato, A.; Miura, S.; Nakamura, Y.; Yamauchi, H.; Owada, T.; Abe, S.; et al. Relationship of hyperuricemia with mortality in heart failure patients with preserved ejection fraction. Am. J. Physiol. Circ. Physiol. 2015, 309, H1123–H1129. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-A.; Lin, H.; Wang, C.-Q.; Zhang, J.-F.; Gu, J. Association between long-term prescription of febuxostat and the progression of heart failure with preserved ejection fraction in patients with hypertension and asymptomatic hyperuricemia. Heart Vessel. 2020, 35, 1446–1453. [Google Scholar] [CrossRef]
- Suzuki, S.; Yoshihisa, A.; Yokokawa, T.; Kobayashi, A.; Yamaki, T.; Kunii, H.; Nakazato, K.; Tsuda, A.; Tsuda, T.; Ishibashi, T.; et al. Comparison between febuxostat and allopurinol uric acid-lowering therapy in patients with chronic heart failure and hyperuricemia: A multicenter randomized controlled trial. J. Int. Med Res. 2021, 49, 3000605211062770. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Ratner, B. The correlation coefficient: Its values range between +1/−1, or do they? J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
| Demographics and Comorbidities | Number (%) or Mean (±SD) | |
|---|---|---|
| Women | 366 (44%) | |
| Age | 72 (±11) | |
| BMI | 29.5 (±5.5) | |
| Arterial hypertension | 175 (86%) | |
| Diabetes | 284 (34%) | |
| MI | 204 (24.6%) | |
| Previous stroke | 107 (13%) | |
| Vascular disease a | 526 (63%) | |
| Permanent AF | 233 (27%) | |
| EF < 40% | 135 (16%) | |
| Pharmacotherapy | Number (%) | |
| BB | 726 (87.6%) | |
| ACEi | 519 (62.6%) | |
| ARB | 155 (18.7%) | |
| ARA | 359 (43%) | |
| Diuretics b | 599 (72%) | |
| Laboratory Results | Mean (median) | Standard Deviation (min–max) |
| eGFR (mL/min/1.73 m2) | 74.3 | 13.75 |
| UA (mg/dL) | 6.56 (6.43) | 1.77 (0.44–16.55) |
| TC (mg/dL) | 171.8 | 52.68 |
| LDL (mg/dL) | 100.91 | 44.59 |
| TG (mg/dL) | 125.84 | 60.88 |
| HDL (mg/dL) | 49.69 | 21.45 |
| Variable | eGFR (mL/min/1.73 m2) | UA (mg/dL) | EF (%) | Age (years) | BMI (kg/m2) |
|---|---|---|---|---|---|
| eGFR (mL/min/1.73 m2) | 1.00 | −0.07 | −0.05 | 0.05 | −0.12 |
| UA (mg/dL) | −0.07 | 1.00 | −0.15 | 0.00 | −0.04 |
| EF (%) | −0.05 | −0.15 | 1.00 | −0.03 | 0.02 |
| Age (years) | 0.05 | 0.00 | −0.03 | 1.00 | −0.20 |
| BMI (kg/m2) | −0.12 | −0.04 | 0.02 | −0.20 | 1.00 |
| Variable | Correlation Coefficient (beta) | Regression Coefficient | T | Significance—p |
|---|---|---|---|---|
| Age (years) | −0.026 | −0.030 | −0.622 | 0.534 |
| BMI (kg/m2) | 0.001 | 0.002 | 0.020 | 0.984 |
| eGFR (mL/min/1.73 m2) | −0.055 | −0.048 | −1.314 | 0.189 |
| UA (mg/dL) | −0.150 | −1.082 | −3.597 | 0.000 |
| Demographics and Comorbidities | UA < 6.43 mg/dL n = 412 | UA ≥ 6.43 mg/dL n = 417 | p Value |
|---|---|---|---|
| Number (%) or Mean (±SD) | Number (%) or Mean (±SD) | ||
| Women | 172 (42%) | 194 (46.5%) | p = 0.166 |
| Age (years) | 72.46 (±10.77) | 72.86 (±11.39) | p = 0.608 |
| BMI (kg/m2) | 29.89 (±5.34) | 29.08 (±5.7) | p = 0.055 |
| Arterial hypertension | 361 (87%) | 354 (85%) | p = 0.254 |
| Diabetes | 139 (34%) | 145 (35%) | p = 0.754 |
| MI | 100 (24%) | 104 (25%) | p = 0.823 |
| Previous stroke | 57 (14%) | 50 (12%) | p = 0.424 |
| Vascular disease a | 261 (63%) | 265 (64%) | p = 0.952 |
| Permanent AF | 105 (25.5%) | 118 (28%) | p = 0.361 |
| EF < 40% | 46 (11%) | 89 (21%) | p< 0.001 |
| Pharmacotherapy | Number (%) | Number (%) | |
| BB | 353 (86%) | 373 (89%) | p = 0.116 |
| ACEi | 256 (62%) | 263 (63%) | p = 0.816 |
| ARB | 75 (18%) | 80 (19%) | p = 0.729 |
| ARA | 160 (39%) | 199 (48%) | p= 0.01 |
| Diuretics b | 276 (67%) | 323 (77.5%) | p< 0.001 |
| Laboratory Results | Mean (±SD) | Mean (±SD) | |
| eGFR (mL/min/1.73 m2) | 75.5 (±13.9) | 73.05 (±13.91) | p= 0.01 |
| UA (mg/dL) | 5.21 (±0.96) | 7.89 (±1.34) | p< 0.001 |
| TC (mg/dL) | 168.36 (±51.63) | 175.08 (±53.51) | p = 0.076 |
| LDL (mg/dL) | 99.22 (±44.08) | 102.53 (±44.08) | p = 0.303 |
| TG (mg/dL) | 114.65 (±56.54) | 136.49 (±63.07) | p< 0.001 |
| HDL (mg/dL) | 50.23 (±25.94) | 47.23 (±16.0) | p = 0.054 |
| EF (%) | 51.43 (±10.87) | 48.53 (±13.79) | p= 0.002 |
| Variables | eGFR (mL/min/1.73 m2) | UA (mg/dL) | EF (%) | Age (years) | BMI (kg/m2) |
|---|---|---|---|---|---|
| eGFR (mL/min/1.73 m2) | 1.00 | −0.05 | −0.02 | 0.06 | −0.13 |
| UA (mg/dL) | −0.05 | 1.00 | −0.15 | 0.02 | −0.08 |
| EF (%) | −0.02 | −0.15 | 1.00 | −0.06 | 0.01 |
| Age (years) | 0.06 | 0.02 | −0.06 | 1.00 | −0.17 |
| BMI (kg/m2) | −0.13 | −0.08 | 0.01 | −0.17 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wełnicki, M.; Gorczyca-Głowacka, I.; Lubas, A.; Wójcik, W.; Jelonek, O.; Maciorowska, M.; Uziębło-Życzkowska, B.; Wójcik, M.; Błaszczyk, R.; Rajtar-Salwa, R.; et al. Association of Hyperuricemia with Impaired Left Ventricular Systolic Function in Patients with Atrial Fibrillation and Preserved Kidney Function: Analysis of the POL-AF Registry Cohort. Int. J. Environ. Res. Public Health 2022, 19, 7288. https://doi.org/10.3390/ijerph19127288
Wełnicki M, Gorczyca-Głowacka I, Lubas A, Wójcik W, Jelonek O, Maciorowska M, Uziębło-Życzkowska B, Wójcik M, Błaszczyk R, Rajtar-Salwa R, et al. Association of Hyperuricemia with Impaired Left Ventricular Systolic Function in Patients with Atrial Fibrillation and Preserved Kidney Function: Analysis of the POL-AF Registry Cohort. International Journal of Environmental Research and Public Health. 2022; 19(12):7288. https://doi.org/10.3390/ijerph19127288
Chicago/Turabian StyleWełnicki, Marcin, Iwona Gorczyca-Głowacka, Arkadiusz Lubas, Wiktor Wójcik, Olga Jelonek, Małgorzata Maciorowska, Beata Uziębło-Życzkowska, Maciej Wójcik, Robert Błaszczyk, Renata Rajtar-Salwa, and et al. 2022. "Association of Hyperuricemia with Impaired Left Ventricular Systolic Function in Patients with Atrial Fibrillation and Preserved Kidney Function: Analysis of the POL-AF Registry Cohort" International Journal of Environmental Research and Public Health 19, no. 12: 7288. https://doi.org/10.3390/ijerph19127288
APA StyleWełnicki, M., Gorczyca-Głowacka, I., Lubas, A., Wójcik, W., Jelonek, O., Maciorowska, M., Uziębło-Życzkowska, B., Wójcik, M., Błaszczyk, R., Rajtar-Salwa, R., Tokarek, T., Bil, J., Wojewódzki, M., Szpotowicz, A., Krzciuk, M., Gawałko, M., Kapłon-Cieślicka, A., Tomaszuk-Kazberuk, A., Szyszkowska, A., ... Mamcarz, A. (2022). Association of Hyperuricemia with Impaired Left Ventricular Systolic Function in Patients with Atrial Fibrillation and Preserved Kidney Function: Analysis of the POL-AF Registry Cohort. International Journal of Environmental Research and Public Health, 19(12), 7288. https://doi.org/10.3390/ijerph19127288











