SARS-CoV-2 Risk Quantification Model and Validation Based on Large-Scale Dutch Test Events
Abstract
:1. Introduction
1.1. Research Question
- Type I: Indoor, passive (theater show or conference),
- Type II: Indoor, active (concert or dance events),
- Type III: Outdoor, active (public sports events),
- Type IV: Outdoor, active festival (festivals).
1.2. The Infection Risk per Hour
2. Methodology: SARS-CoV-2 Risk Quantification Model
2.1. Methodology
- Two types of individuals, j, who can attend an event and for which different test regimes can apply. j = 1 corresponds to unvaccinated individuals without a documented infection, j = 2 corresponds to vaccinated individuals or people with a documented infection. Vj is the proportion of the people in each group. The sum of V1 and V2 is always 100%. At each event, we assume a homogeneous mixing of all people. Therefore, is the risk that a j = 1 individual infect a j = 1 individual, is the risk that a j = 1 individual infect a j = 2 individual, is the risk that a j = 2 individual infects a j = 1 individual, and is the risk that a j = 2 individual infect a j = 2 individual.
- The prevalence P describes the proportion of infectious people in the community during the event.
- The complete role of virus-laden droplets and aerosol transmission is poorly understood [13]. We distinguish between two contact classes that are most significant: C1 is the number of contacts per hour within 1.5 m (droplets) and C2 is the number of contacts per hour within 10 m (aerosols). Smart logistics and crowd control at an event reduce contacts and help avoid gatherings of large groups of people. Therefore, specific data were collected at test events because the duration of events is limited the latency period is shorter than the duration of stay at each location.
- Factor FT,j describes the effectiveness of testing for group j in a certain window prior to the event.
- Vaccination and earlier infections result in a level of immunity and reduce the infectiousness I and susceptibility S of an individual. Because people without a documented infection might have an earlier infection the weighted I and S have to be defined for individuals of type j. and are the relative infectiousness and susceptibility for an individual of type j related to a naïve person. During the test events used to validate the model, all people were considered to be naïve (which means that they do not have any immunity), nobody was vaccinated, and a limited (not significant) number of people have had an earlier infection.
- FY,x is the percentage of reduction of aerosols because of ventilation. When x = 0, locations are ventilated according to building codes, x = 1 means very well ventilated and x = 2 is outdoor.
- FM is the risk reduction factor for personal protection measures such as mouth-nose masks.
- Z is a multiplier for variants that are more infectious than the Alpha variant which was dominant during the data collection for the transmission coefficients.
2.2. Transmission Coefficients A1 and A2
- The time spent at a certain location.
- The number of persons in a range of 10 m.
- The number of contacts within a distance of <0.5 m, between 0.5 and 1.5, and between 1.5 and 2.0 m for less than a minute, between 1 and 15 min, and more than 15 min.
- The proportion of the time which was spent indoors, indoors and well ventilated or outdoors, given a type of location.
2.3. Total Number of Infections at an Event
3. Validation of the Model
3.1. Internal Validation: Reproduction of Infections at Different Settings
3.2. External Validation: Test Events
- P is based on the prevalence of the date of the events as published by RIVM [13].
- V1 = 100%. Because vaccination was not available yet and earlier infections were very limited and not significant, all people were naïve. Only j = 1 individuals could attend the event and and therefore are equal to zero.
- Persons with COVID-19-(like) symptoms were banned from participation. All visitors and crew needed a negative PCR test taken within 48 h before attending the event. As the PCR test may pick up low viral loads such as in cases of persons who recently recovered from COVID-19, the ratio of positive tests is higher than the ratio of asymptomatic people only [18], FT,1 = 0.95.
- Fy,0 = 0 if the ventilation meets the requirements of building codes. Fy,1 = 0.90 is applied when ventilation is significantly improved (with a CO2 value below 800 ppm). All venues have been checked prior to the event and during the event, the CO2 value was measured. Because all outdoor events were not completely open (the festival was in a tent, and football stadiums had a large roof), we assumed Fy,2 = Fy,1. These values are based on expert judgment.
- The effectivity of masks was estimated by medical experts. While the effectivity under in vitro conditions can be high [19], a low estimate is realistic for their effectiveness during in-vivo events because masks are not used correctly, and they may be ill-fitting. During all test events, different rules are applied. FM = 0.05 when the mask was used only when people were seated, and FM = 0.1 when the mask was used while people move (but not while they are drinking and eating). During type 2 and 4 events, FM was set at 0, as mask compliance was extremely low to non-existent.
4. Discussion and Concluding Remarks
4.1. Reflection on the Validation of the Model
4.2. Final Conclusion and Recommendations
4.3. Added Value for Decision Makers and Event Planners
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, E.; van Boven, M.; den Hartog, G.; Backer, J.; Klinkenberg, D.; van Hagen, C.; Boshuizen, H.; van Binnendijk, R.; Mollema, L.; van der Klis, F.; et al. Associations between measures of social distancing and SARS-CoV-2 seropositivity: A nationwide population-based study in the Netherlands. Clin. Infect. Dis. 2021, 73, 2318–2321. [Google Scholar] [CrossRef] [PubMed]
- Prem, K.; Cook, A.; Jit, M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput. Biol. 2017, 13, e1005697. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Coletti, P.; Melegaro, A.; Wallinga, J.; Grijalva, C.; Edmunds, J.; Beutels, P.; Hens, N. Systematic Review of Social Contact Surveys to Inform Transmission Models of Close-contact Infections. Epidemiology 2019, 30, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Ale, B.; Bellamy, L.; Roelen, A.; Cooke, R.; Goossens, L.; Hale, A.; Kurowicka, D.; Smith, E. Development of a Causal Model for Air Transport Safety. In Proceedings of the ASME 2005 International Mechanical Engineering Congress and Exposition, Engineering/Technology Management, Orlando, FL, USA, 5–11 November 2005; pp. 107–116. [Google Scholar] [CrossRef] [Green Version]
- Alimohamadi, Y.; Taghdir, M.; Sepandi, M. Estimate of the Basic Reproduction Number for COVID-19: A Systematic Review and Meta-analysis. J. Prev. Med. Public Health 2020, 53, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenqing, H.; Grace, Y.; Yayuan, Z. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: Meta-analysis and sensitivity analysis. Med. Virol. 2020, 92, 2543–2550. [Google Scholar] [CrossRef]
- Locatelli, I.; Trächsel, B.; Rousson, V. Estimating the basic reproduction number for COVID-19 in Western Europe. PLoS ONE 2021, 16, e0248731. [Google Scholar] [CrossRef] [PubMed]
- De Ziekte COVID-19. Available online: https://www.rivm.nl/coronavirus-covid-19/ziekte (accessed on 4 June 2021).
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, E13–E22. [Google Scholar] [CrossRef]
- Backer, J.; Klinkenberg, D.; Wallinga, J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill 2020, 25, 2000062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhouib, W.; Maatoug, J.; Ayouni, I.; Zammit, N.; Ghammen, R.; Fredj, S.; Ghannem, H. The incubation period during the pandemic of COVID-19: A systematic review and meta-analysis. Syst. Rev. 2021, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- RIVM. Available online: https://www.rivm.nl/coronavirus-covid-19/actueel/wekelijkse-update-epidemiologische-situatie-covid-19-in-nederland (accessed on 4 June 2021).
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef] [PubMed]
- The Netherlands in Figures by Statistics Netherlands (CBS). Available online: https://opendata.cbs.nl/#/CBS/en/ (accessed on 13 September 2021).
- Jonkman, S. Loss of Life Estimation in Flood Risk Assessment—Theory and Applications. PhD Thesis, Delft University, Delft, The Netherlands, 2007. [Google Scholar]
- Maaskant, B.; Jonkman, S.; Kok, M. Analyse Slachtofferaantallen VNK-2 en Voorstellen voor Aanpassingen van Slachtofferfuncties; Rapport PR1669.10; HKV Lijn in Water: Lelystad, The Netherlands, 2009. [Google Scholar]
- CRA. Hoogwaterbeschermings Programma: Van ‘Sober en Doelmatig’ Naar ‘Slim en Doelmatig’; Board of Government Advisors: The Hague, The Netherlands, 2020.
- Zhang, Z.; Bi, O.; Fang, S.; Wei, L.; Wang, X.; He, J.; Wu, Y.; Liu, X.; Gao, W.; Zhang, R.; et al. Insight into the practical performance of RT-PCR testing for SARS-CoV-2 using serological data: A cohort study. Lancet Microbe 2021, 2, e79–e87. [Google Scholar] [CrossRef]
- Ueki, H.; Furusawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Kabata, H.; Nishimura, H.; Kawaoka, Y. Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2. mSphere 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Stobernack, T.; Kolen, B.; Znidarsic, L.; Donders, S.; Kamphorst, I.; van Rijn, M.; Bonthuis, D.; Cloquet, M.; Schram, M.; Scharloo, R.; et al. Health risks of mass gathering events during the COVID-19 pandemic. Lancet Infect. Dis. 2022, submitted.
- RIVM. Epidemiologische Situatie van SARS-CoV-2 in Nederland; National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2021. [Google Scholar]
- Goldstein, E.; Lipsitch, M.; Cevik, M. On the Effect of Age on the Transmission of SARS-CoV-2 in Households, Schools, and the Community. J. Infect. Dis. 2021, 223, 362–369. [Google Scholar] [CrossRef] [PubMed]
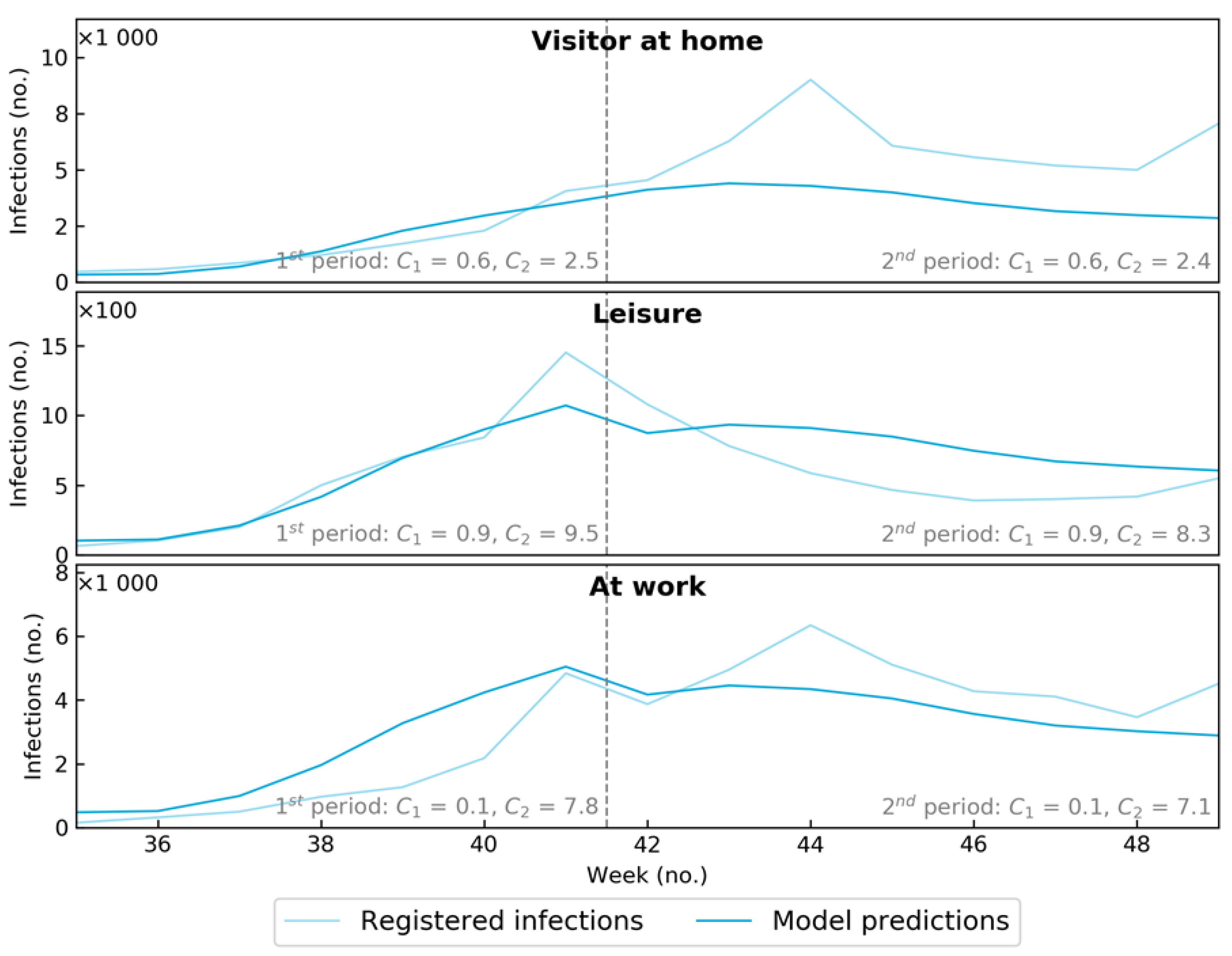
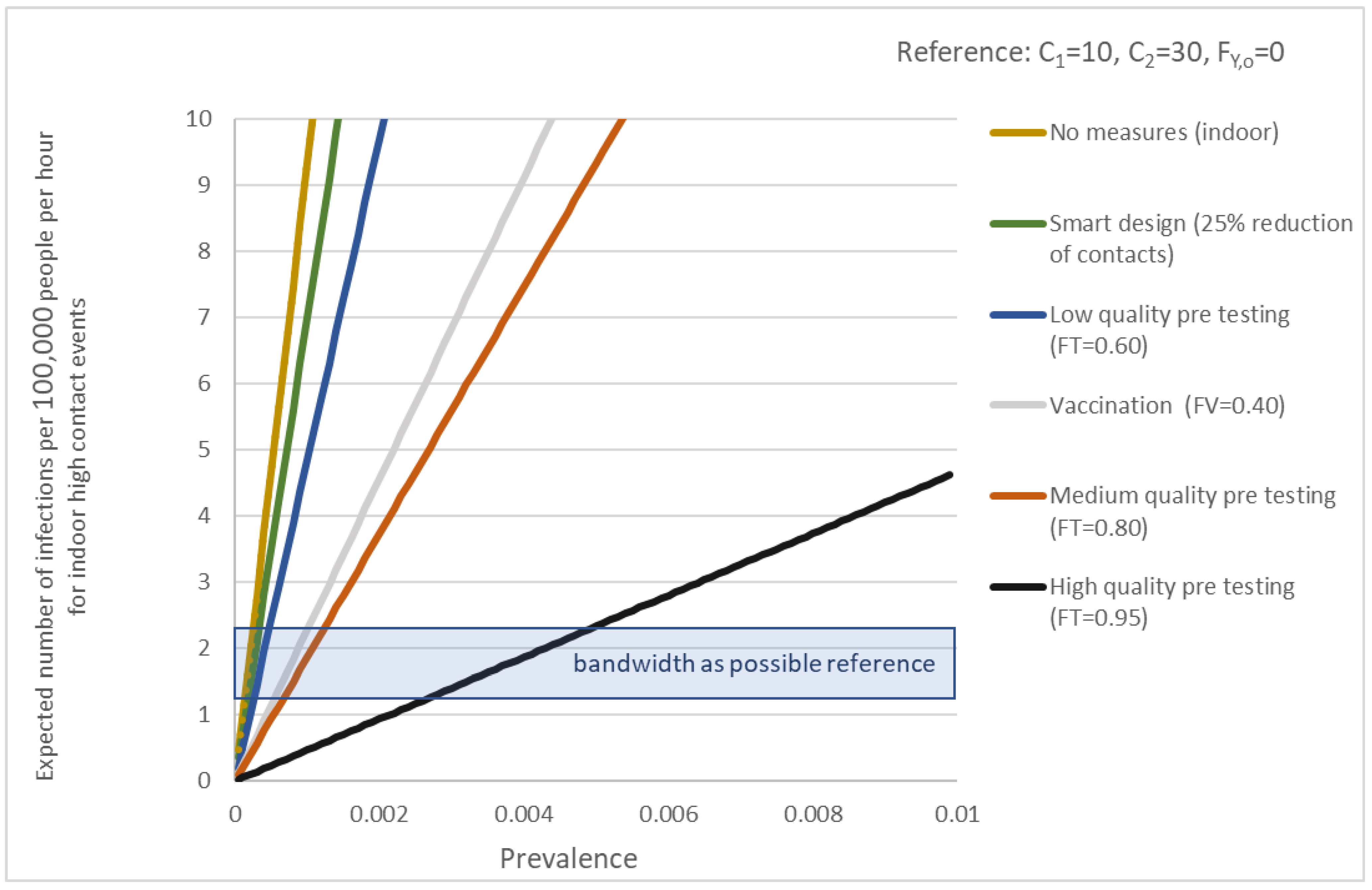
| Setting | No. Positive PCR Tests (Source RIVM) | Hours at Location | Average Infections per Hour |
|---|---|---|---|
| At home | 186,772 | 1.74 × 1010 | 1.08 × 10−5 |
| Visitor | 59,882 | 1.09 × 109 | 5.49 × 10−5 |
| Leisure | 8530 | 1.49 × 108 | 5.72 × 10−5 |
| At work | 46,881 | 1.00 × 109 | 4.69 × 10−5 |
| Between 2 and 1.5 m | Between 1.5 and 0.5 m | Less than 0.5 m | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 min | 1 < min < 15 | >15 min | <1 min | 1 < min < 15 | >15 min | <1 min | 1 < min < 15 | >15 min | C1 | C2 | Fy,x | |
| Visitor | 0.31 | 0.92 | 2.42 | 0.31 | 0.10 | 0.14 | 0.35 | 0.41 | 0.89 | 0.58 | 2.47 | 0.71 |
| Leisure | 1.28 | 0.49 | 3.18 | 1.11 | 0.06 | 0.00 | 1.13 | 0.06 | 1.72 | 0.89 | 8.88 | 0.53 |
| At work | 0.36 | 0.38 | 1.68 | 0.24 | 0.09 | 0.02 | 0.24 | 0.17 | 0.11 | 0.10 | 7.47 | 0.90 |
| Setting | Model | C1 | C2 | R Absolute Fy,2 = 100 and Fy,1 = 50 |
R Absolute Fy,2 = 95 and Fy,1 = 90 | Difference |
|---|---|---|---|---|---|---|
| Low contact | Normal ventilated (reference) | 5.0 | 30.0 | 3.07 × 10−4 | 3.07 × 10−4 | 0% |
| Low contact | Well ventilated | 5.0 | 30.0 | 2.33 × 10−4 | 1.74 × 10−4 | −25% |
| Low contact | Outdoor | 5.0 | 30.0 | 1.59 × 10−4 | 1.67 × 10−4 | 5% |
| High contact | Normal ventilated (reference) | 12.5 | 50.0 | 6.45 × 10−4 | 6.45 × 10−4 | 0% |
| High contact | Well ventilated | 12.5 | 50.0 | 5.22 × 10−4 | 4.23 × 10−4 | −19% |
| High contact | Outdoor | 12.5 | 50.0 | 3.99 × 10−4 | 4.11 × 10−4 | 3% |
| Setting | Model | C1 | C2 | R absolute | A1, A2 (×1000) | difference |
| Low contact (reference) | 1.5 m for C1, 10 m for C2 | 5.0 | 30.0 | 3.07 × 10−4 | 6.38, 0.98 | 0.0% |
| Low contact | 2.0 m for C1, 10 m for C2 | 8.9 | 30.0 | 2.21 × 10−4 | 2.71, 0.67 | −28.1% |
| Low contact | 1.5 m for C1, 8 m for C2 | 5.0 | 19.2 | 3.07 × 10−4 | 6.38, 1.54 | 0.0% |
| High contact (reference) | 1.5 m for C1, 10 m for C2 | 12.5 | 50.0 | 6.45 × 10−4 | 6.38, 0.98 | 0.0% |
| High contact | 2.0 m for C1, 10 m for C2 | 22.2 | 50.0 | 4.69 × 10−4 | 2.71, 0.67 | −27.3% |
| High contact | 1.5 m for C1, 8 m for C2 | 12.5 | 32.0 | 6.45 × 10−4 | 6.38, 1.54 | 0.0% |
| Type I | Type II | Type III | Type IV | |
|---|---|---|---|---|
| People at event () (without crew) (persons) | 815 | 2341 | 1692 | 2960 |
| Average duration () of event (hours) | 4.4 | 4.3 | 3 | 7 |
| Average prevalence () at events | 0.0056 | 0.0061 | 0.0056 | 0.0077 |
| Number of Pretests (incl crew) (persons) | 1198 | 3078 | 2033 | 3890 |
| Positive pre-test (persons) | 11 (0.9%) | 18 (0.6%) | 12 (0.6%) | 26 (0.7%) |
| After (5 d) tests (incl crew) (persons) | 926 | 2603 | 1689 | 3168 |
| Positive after test (persons) | 1 (0.1%) | 14 (0.5%) | 4 (0.2%) | 26 (0.8%) |
| Possible infections at event (realization) (persons) | 0 | 4 | 0 | 12 |
| Confirmed infections at event (realization) (persons) | 0 | 0 | 0 | 4 |
| Expected infections () at event (model) (persons) | 0.04 | 0.28 | 0.05 | 0.54 |
| Expected Infections () at event without measures (model) (persons) | 0.86 | 5.56 | 1.14 | 10.81 |
| Individual risk ) per hour at event | 1.12 × 10−5 | 2.62 × 10−5 | 1.00 × 10−5 | 2.61 × 10−5 |
| Minimal individual risk per hour at event | 1.4 × 10−5 | 1.5 × 10−5 | 8.04 × 10−6 | 1.6 × 10−5 |
| Maximum individual risk per hour at event | 8.5 × 10−6 | 4.3 × 10−5 | 1.6 × 10−5 | 3.6 × 10−5 |
| Average individual risk ) per hour at home | 1.06 × 10−5 | 1.14 × 10−5 | 1.06 × 10−5 | 1.45 × 10−5 |
| Average individual risk )per hour having a visitor | 4.50 × 10−5 | 4.82 × 10−5 | 4.48 × 10−5 | 6.13 × 10−5 |
| Individual risk per hour ) at event without measures | 1.25 × 10−4 | 5.10 × 10−4 | 2.24 × 10−4 | 5.22 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolen, B.; Znidarsic, L.; Voss, A.; Donders, S.; Kamphorst, I.; van Rijn, M.; Bonthuis, D.; Clocquet, M.; Schram, M.; Scharloo, R.; et al. SARS-CoV-2 Risk Quantification Model and Validation Based on Large-Scale Dutch Test Events. Int. J. Environ. Res. Public Health 2022, 19, 7238. https://doi.org/10.3390/ijerph19127238
Kolen B, Znidarsic L, Voss A, Donders S, Kamphorst I, van Rijn M, Bonthuis D, Clocquet M, Schram M, Scharloo R, et al. SARS-CoV-2 Risk Quantification Model and Validation Based on Large-Scale Dutch Test Events. International Journal of Environmental Research and Public Health. 2022; 19(12):7238. https://doi.org/10.3390/ijerph19127238
Chicago/Turabian StyleKolen, Bas, Laurens Znidarsic, Andreas Voss, Simon Donders, Iris Kamphorst, Maarten van Rijn, Dimitri Bonthuis, Merit Clocquet, Maarten Schram, Rutger Scharloo, and et al. 2022. "SARS-CoV-2 Risk Quantification Model and Validation Based on Large-Scale Dutch Test Events" International Journal of Environmental Research and Public Health 19, no. 12: 7238. https://doi.org/10.3390/ijerph19127238
APA StyleKolen, B., Znidarsic, L., Voss, A., Donders, S., Kamphorst, I., van Rijn, M., Bonthuis, D., Clocquet, M., Schram, M., Scharloo, R., Boersma, T., Stobernack, T., & van Gelder, P. (2022). SARS-CoV-2 Risk Quantification Model and Validation Based on Large-Scale Dutch Test Events. International Journal of Environmental Research and Public Health, 19(12), 7238. https://doi.org/10.3390/ijerph19127238







