Investigation of Synergistic Effects and Kinetics on Co-Pyrolysis of Alternanthera philoxeroides and Waste Tires
Abstract
:1. Introduction
2. Experimental Method and Equipment
2.1. Materials
2.2. Thermogravimetric Experiment
2.3. Kinetic Parameter Analysis
2.3.1. Model-Free Methods
2.3.2. Model-Fitting Method (Coats–Redfern (CR))
3. Results and Discussion
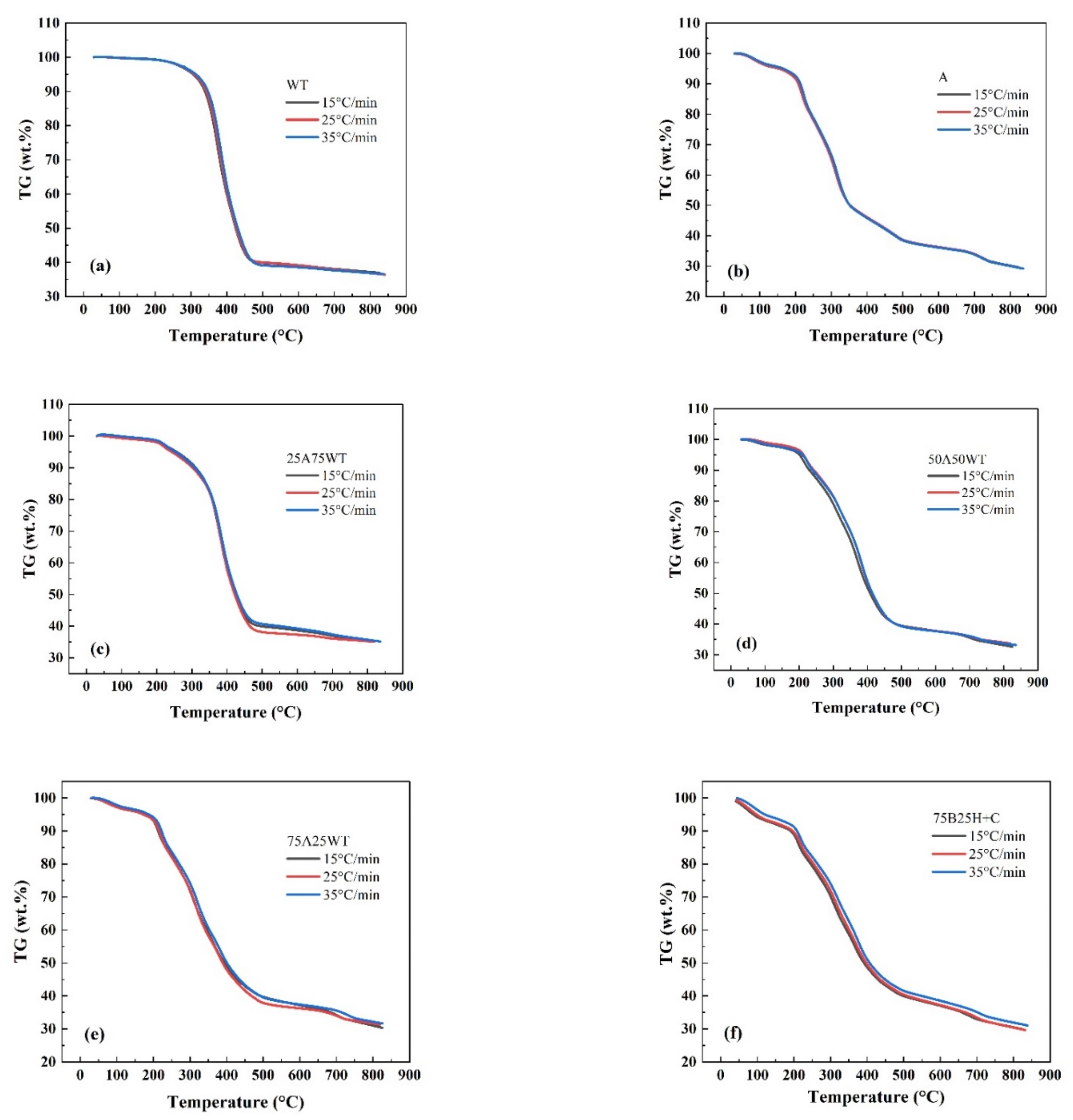
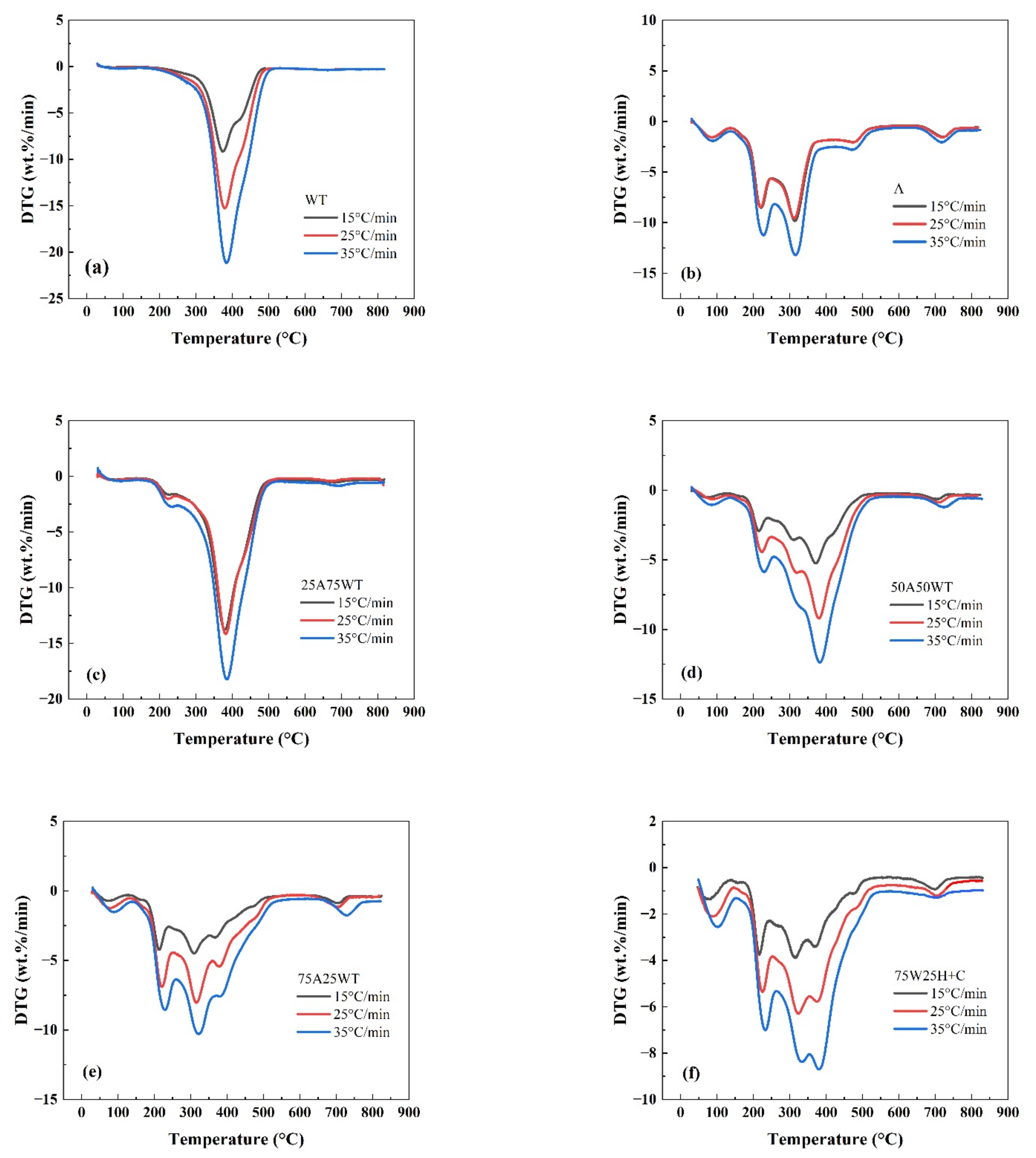
3.1. Effect of Heating Rate on Pyrolysis Process
3.2. The Effect of Sample Mixing Ratio and Catalyst on the Pyrolysis Process
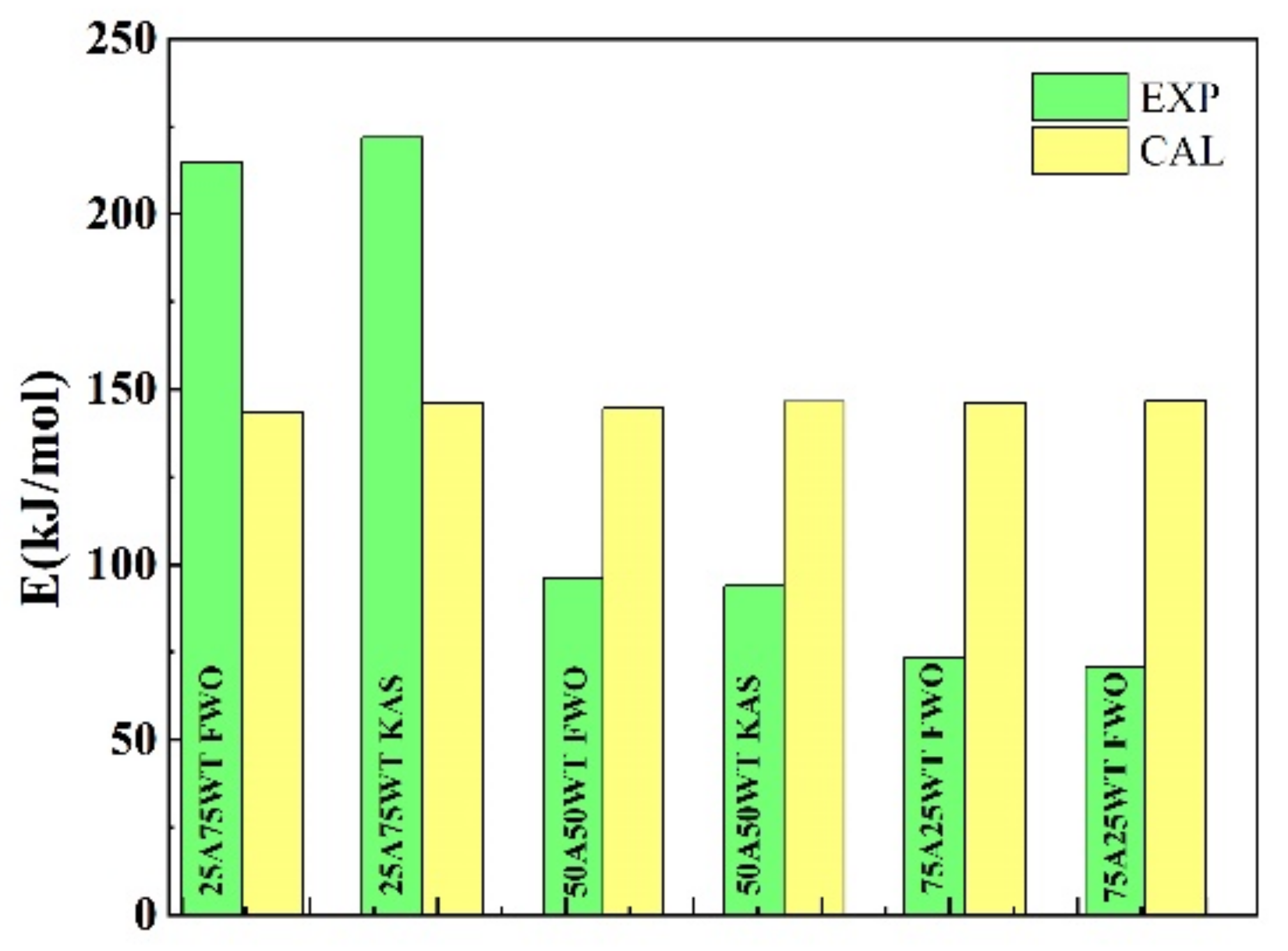
3.3. The Analysis of the Synergistic Effect of the Blends
3.4. Kinetic Analysis
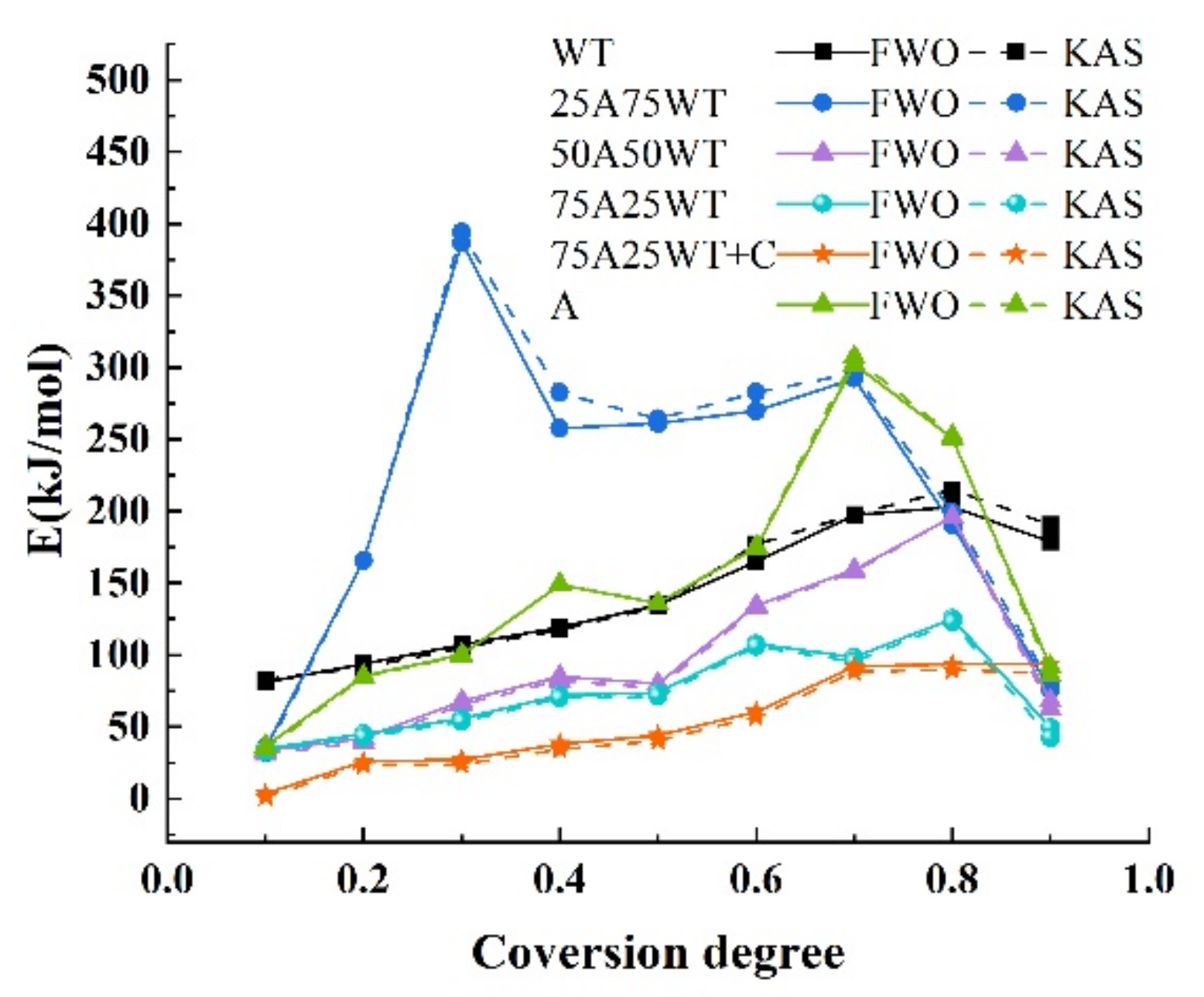
3.4.1. Model-Free Method
3.4.2. Model-Fitting Method (Coats–Redfern)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Parameters | |||
| Conversion degree | The temperature of maximum mass loss rate | ||
| Heating rate | The temperature of initial mass loss | ||
| Pre-exponential factor | Weight loss of Alternanthera philoxeroides | ||
| The maximum mass loss rate | Calculated weight loss | ||
| Apparent activation energy | Weight loss obtained from the TG curve | ||
| The average activation energy of Alternanthera philoxeroides | Weight loss of waste tires | ||
| Calculated activation energy | The initial weight of the sample | ||
| The average activation energy of waste tires | The final weight of the sample | ||
| ) | Weight of the sample at a time | ||
| Correlation coefficient | Percentage of Alternanthera philoxeroides in the mixture | ||
| Reaction temperature (K) | Percentage of waste tires in the mixture | ||
| Abbreviations | |||
| AP | Alternanthera philoxeroides | DTG | The first derivative of TG (loss rate) |
| CFP | Catalyst fast pyrolysis | WTS | Waste tires |
| CR | Coats–Redfern | 25A75WT | 25% of Alternanthera philoxeroides and 75% of waste tires |
| FWO | Flynn–Wall–Ozawa | 50A50WT | 50% of Alternanthera philoxeroides and 50% of waste tires |
| KAS | Kissinger–Akahira–Sunose | 75A25WT | 75% of Alternanthera philoxeroides and 25% of waste tires |
| TGA | Thermogravimetric analyzer | 75A25WT+C | 75% of Alternanthera philoxeroides and 25% of waste tires + 5 mg of HZSM-5 catalyst |
| TG | Weight loss | ||
References
- Lu, Q.; Zhang, Z.; Ye, X.; Li, K.; Cui, M.; Yang, Y. Catalytic Fast Pyrolysis of Alkali-Pretreated Bagasse for Selective Preparation of 4-Vinylphenol. J. Anal. Appl. Pyrolysis 2019, 143, 104669. [Google Scholar] [CrossRef]
- Qi, W.; Yang, W.; Xu, Q.; Xu, Z.; Wang, Q.; Liang, C.; Liu, S.; Ling, C.; Wang, Z.; Yuan, Z. Comprehensive Research on the Influence of Nonlignocellulosic Components on the Pyrolysis Behavior of Chinese Distiller’s Grain. ACS Sustain. Chem. Eng. 2020, 8, 3103–3113. [Google Scholar] [CrossRef]
- Wu, K.; Ying, W.; Shi, Z.; Yang, H.; Zheng, Z.; Zhang, J.; Yang, J. Fenton Reaction-Oxidized Bamboo Lignin Surface and Structural Modification to Reduce Nonproductive Cellulase Binding and Improve Enzyme Digestion of Cellulose. ACS Sustain. Chem. Eng. 2018, 6, 3853–3861. [Google Scholar] [CrossRef]
- Maheshwari, J.K. Alligator Weed in Indian Lakes. Nature 1965, 206, 1270. [Google Scholar] [CrossRef]
- Liu, W.-J.; Zeng, F.-X.; Jiang, H.; Yu, H.-Q. Total Recovery of Nitrogen and Phosphorus from Three Wetland Plants by Fast Pyrolysis Technology. Bioresour. Technol. 2011, 102, 3471–3479. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K. Value Added Liquid Products from Waste Biomass Pyrolysis Using Pretreatments. Sci. Total Environ. 2015, 538, 145–151. [Google Scholar] [CrossRef]
- Yanik, J.; Kornmayer, C.; Saglam, M.; Yüksel, M. Fast Pyrolysis of Agricultural Wastes: Characterization of Pyrolysis Products. Fuel Processing Technol. 2007, 88, 942–947. [Google Scholar] [CrossRef]
- Bu, Q.; Morgan, H.M., Jr.; Liang, J.; Lei, H.; Ruan, R. Catalytic Microwave Pyrolysis of Lignocellulosic Biomass for Fuels and Chemicals. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 69–123. ISBN 2468-0125. [Google Scholar]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Konda, N.M.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The Multi-Scale Challenges of Biomass Fast Pyrolysis and Bio-Oil Upgrading: Review of the State of Art and Future Research Directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. An Overview of Catalysts in Biomass Pyrolysis for Production of Biofuels. Biofuel Res. J. 2018, 5, 872–885. [Google Scholar] [CrossRef] [Green Version]
- Fong, M.J.B.; Loy, A.C.M.; Chin, B.L.F.; Lam, M.K.; Yusup, S.; Jawad, Z.A. Catalytic Pyrolysis of Chlorella Vulgaris: Kinetic and Thermodynamic Analysis. Bioresour. Technol. 2019, 289, 121689. [Google Scholar] [CrossRef]
- Lorenzetti, C.; Conti, R.; Fabbri, D.; Yanik, J. A Comparative Study on the Catalytic Effect of H-ZSM5 on Upgrading of Pyrolysis Vapors Derived from Lignocellulosic and Proteinaceous Biomass. Fuel 2016, 166, 446–452. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhou, G.; Feng, Y.; Wang, Y.; Yu, G.; Deng, S.; Huang, J.; Wang, B. Enhancing the Production of Renewable Petrochemicals by Co-Feeding of Biomass with Plastics in Catalytic Fast Pyrolysis with ZSM-5 Zeolites. Appl. Catal. A Gen. 2014, 481, 173–182. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Nie, J.; Jin, B.; Shao, S.; Xiao, G. Catalytic Pyrolysis of Black-Liquor Lignin by Co-Feeding with Different Plastics in a Fluidized Bed Reactor. Bioresour. Technol. 2015, 192, 68–74. [Google Scholar] [CrossRef]
- Ryu, H.W.; Kim, D.H.; Jae, J.; Lam, S.S.; Park, E.D.; Park, Y.-K. Recent Advances in Catalytic Co-Pyrolysis of Biomass and Plastic Waste for the Production of Petroleum-like Hydrocarbons. Bioresour. Technol. 2020, 310, 123473. [Google Scholar] [CrossRef]
- Ng, Q.H.; Chin, B.L.F.; Yusup, S.; Loy, A.C.M.; Chong, K.Y.Y. Modeling of the Co-Pyrolysis of Rubber Residual and HDPE Waste Using the Distributed Activation Energy Model (DAEM). Appl. Therm. Eng. 2018, 138, 336–345. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Zhang, B.; Song, Z.; Qian, X. Pyrolysis Characteristics of Waste Tire in an Analytical Pyrolyzer Coupled with Gas Chromatography/Mass Spectrometry. Energy Fuels 2015, 29, 3181–3187. [Google Scholar] [CrossRef]
- Duan, P.; Jin, B.; Xu, Y.; Wang, F. Co-Pyrolysis of Microalgae and Waste Rubber Tire in Supercritical Ethanol. Chem. Eng. J. 2015, 269, 262–271. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical Routes for the Valorization of Waste Polyolefinic Plastics to Produce Fuels and Chemicals. A Review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Jana, A.; Mondal, S.; Jana, B.; Sadhukhan, A.K.; Gupta, P. Pyrolysis of Three Different Categories of Automotive Tyre Wastes: Product Yield Analysis and Characterization. J. Anal. Appl. Pyrolysis 2018, 135, 379–389. [Google Scholar] [CrossRef]
- Li, D.; Lei, S.; Rajput, G.; Zhong, L.; Ma, W.; Chen, G. Study on the Co-Pyrolysis of Waste Tires and Plastics. Energy 2021, 226, 120381. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.; He, Y.; Evrendilek, F.; Chen, Z.; Liu, J. Co-Pyrolytic Performances, Mechanisms, Gases, Oils, and Chars of Textile Dyeing Sludge and Waste Shared Bike Tires under Varying Conditions. Chem. Eng. J. 2022, 428, 131053. [Google Scholar] [CrossRef]
- Mao, Q.; Hu, J.; Zhao, Y.; Yan, S.; Yang, H.; Chen, H. Synergistic Effect during Biomass and Waste Plastics Co-Pyrolysis. J. Fuel Chem. Technol. 2020, 48, 286–292. [Google Scholar]
- Bhattacharjee, N.; Biswas, A.B. Pyrolysis of Alternanthera Philoxeroides (Alligator Weed): Effect of Pyrolysis Parameter on Product Yield and Characterization of Liquid Product and Bio Char. J. Energy Inst. 2018, 91, 605–618. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Biswas, A.B. Value-Added Fuels from the Catalytic Pyrolysis of Alternanthera Philoxeroides. Fuel 2021, 295, 120629. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, X.; Zeng, Z.; Dai, L.; Zhang, S.; Jiang, L.; Wu, Q.; Yang, X.; Liu, Y.; Zhang, B. Catalytic Co-Pyrolysis of Alternanthera Philoxeroides and Peanut Soapstock via a New Continuous Fast Microwave Pyrolysis System. Waste Manag. 2019, 88, 102–109. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Role of Isoconversional Methods in Varying Activation Energies of Solid-State Kinetics: II. Nonisothermal Kinetic Studies. Thermochim. Acta 2005, 436, 101–112. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Zhu, X.; Qian, M.; Yadavalli, G.; Wu, J.; Chen, S. Thermal Behavior and Kinetic Study for Catalytic Co-Pyrolysis of Biomass with Plastics. Bioresour. Technol. 2016, 220, 233–238. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General Treatment of the Thermogravimetry of Polymers. J. Res. Natl. Bur. Stand. A Phys. Chem. 1966, 70, 487. [Google Scholar] [CrossRef]
- Abdelouahed, L.; Leveneur, S.; Vernieres-Hassimi, L.; Balland, L.; Taouk, B. Comparative Investigation for the Determination of Kinetic Parameters for Biomass Pyrolysis by Thermogravimetric Analysis. J. Therm. Anal. Calorim. 2017, 129, 1201–1213. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Naqvi, M.; Chen, W.-H.; Ceylan, S.; Rashid, H.; Ahmad, J.; Taqvi, S.A. Pyrolysis of High Ash Sewage Sludge: Kinetics and Thermodynamic Analysis Using Coats-Redfern Method. Renew. Energy 2019, 131, 854–860. [Google Scholar] [CrossRef]
- Kumar, A.; Mylapilli, S.V.P.; Reddy, S.N. Thermogravimetric and Kinetic Studies of Metal (Ru/Fe) Impregnated Banana Pseudo-Stem (Musa Acuminate). Bioresour. Technol. 2019, 285, 121318. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.A.; Khairy, M. Effect of Heating Rate on the Chemical Kinetics of Different Biomass Pyrolysis Materials. Biofuels 2015, 6, 157–170. [Google Scholar] [CrossRef]
- Fisher, T.; Hajaligol, M.; Waymack, B.; Kellogg, D. Pyrolysis Behavior and Kinetics of Biomass Derived Materials. J. Anal. Appl. Pyrolysis 2002, 62, 331–349. [Google Scholar] [CrossRef]
- Lah, B.; Klinar, D.; Likozar, B. Pyrolysis of Natural, Butadiene, Styrene–Butadiene Rubber and Tyre Components: Modelling Kinetics and Transport Phenomena at Different Heating Rates and Formulations. Chem. Eng. Sci. 2013, 87, 1–13. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Devolatilization of Biomass Fuels and Biomass Components Studied by TG/FTIR Technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. [Google Scholar] [CrossRef]
- Williams, P.T.; Besler, S. The Influence of Temperature and Heating Rate on the Slow Pyrolysis of Biomass. Renew. Energy 1996, 7, 233–250. [Google Scholar] [CrossRef]
- Czajka, K.M. The Impact of the Thermal Lag on the Interpretation of Cellulose Pyrolysis. Energy 2021, 236, 121497. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of Pyrolysis Characteristics and Kinetics of Palm Kernel Shell Using TGA–FTIR and Model-Free Integral Methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Salvilla, J.N.V.; Ofrasio, B.I.G.; Rollon, A.P.; Manegdeg, F.G.; Abarca, R.R.M.; de Luna, M.D.G. Synergistic Co-Pyrolysıs of Polyolefin Plastics with Wood and Agricultural Wastes for Biofuel Production. Appl. Energy 2020, 279, 115668. [Google Scholar] [CrossRef]
- Wang, C.; Bi, H.; Lin, Q.; Jiang, X.; Jiang, C. Co-Pyrolysis of Sewage Sludge and Rice Husk by TG–FTIR–MS: Pyrolysis Behavior, Kinetics, and Condensable/Non-Condensable Gases Characteristics. Renew. Energy 2020, 160, 1048–1066. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, B.; Liu, C. Mechanism of Synergistic Effects and Kinetics Analysis in Catalytic Co-Pyrolysis of Water Hyacinth and HDPE. Energy Convers. Manag. 2021, 228, 113717. [Google Scholar] [CrossRef]
- Quan, C.; Xu, S.; An, Y.; Liu, X. Co-Pyrolysis of Biomass and Coal Blend by TG and in a Free Fall Reactor. J. Therm. Anal. Calorim. 2014, 117, 817–823. [Google Scholar] [CrossRef]
- Kai, X.; Li, R.; Yang, T.; Shen, S.; Ji, Q.; Zhang, T. Study on the Co-Pyrolysis of Rice Straw and High Density Polyethylene Blends Using TG-FTIR-MS. Energy Convers. Manag. 2017, 146, 20–33. [Google Scholar] [CrossRef]
- Kai, X.; Yang, T.; Shen, S.; Li, R. TG-FTIR-MS Study of Synergistic Effects during Co-Pyrolysis of Corn Stalk and High-Density Polyethylene (HDPE). Energy Convers. Manag. 2019, 181, 202–213. [Google Scholar] [CrossRef]
- Cai, H.; Zou, H.; Liu, J.; Xie, W.; Kuo, J.; Buyukada, M.; Evrendilek, F. Thermal Degradations and Processes of Waste Tea and Tea Leaves via TG-FTIR: Combustion Performances, Kinetics, Thermodynamics, Products and Optimization. Bioresour. Technol. 2018, 268, 715–725. [Google Scholar] [CrossRef]
- Xu, S.; Cao, B.; Uzoejinwa, B.B.; Odey, E.A.; Wang, S.; Shang, H.; Li, C.; Hu, Y.; Wang, Q.; Nwakaire, J.N. Synergistic Effects of Catalytic Co-Pyrolysis of Macroalgae with Waste Plastics. Process Saf. Environ. Prot. 2020, 137, 34–48. [Google Scholar] [CrossRef]




| A. philoxeroides | |
|---|---|
| Proximate analysis: | |
| Moisture | 4.58 |
| Ash | 17.35 |
| Volatile | 54.68 |
| Fixed carbon | 27.97 |
| Composition analysis | |
| Hemicellulose | 33.87 |
| Lignin | 25.68 |
| Cellulose | 27.51 |
| Ultimate analysis (wt%): | |
| C | 39.13 |
| H | 5.09 |
| O | 35.9 |
| N | 2.53 |
| Sample | 1st Stage | 2nd Stage | 3rd Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | _ | _ | _ | _ | _ | _ | _ | _ | 241.0 | 510.0 | 379.5 | 15.28 |
| A | 141.6 | 245.7 | 220.4 | 8.48 | 262.5 | 367.6 | 313.5 | 9.55 | 386.7 | 542.0 | 474.3 | 2.1 |
| 25A75WT | 179.0 | 243.2 | 221.7 | 2 | _ | _ | _ | _ | 248.6 | 530.0 | 379.5 | 14.2 |
| 50A50WT | 156.7 | 244.0 | 223.8 | 4.44 | 258.4 | 333.2 | 319.2 | 5.9 | 344.5 | 531.0 | 379.8 | 9.2 |
| 75A25WT | 148.2 | 248.2 | 220.7 | 6.69 | 255.0 | 358.9 | 312.7 | 8 | 361.7 | 528.0 | 377.9 | 5.43 |
| 75A25WT+C | 146.2 | 239.7 | 223.0 | 5.35 | 253.4 | 351.6 | 321.69 | 6.3 | 355.0 | 506.0 | 374.1 | 5.78 |
| FWO | KAS | FWO | KAS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 0.1 | 80.9 | 0.955 | 82.8 | 0.961 | 25A75WT | 0.1 | 35.2 | 0.985 | 32.0 | 0.969 |
| 0.2 | 93.5 | 0.972 | 91.2 | 0.978 | 0.2 | 165.6 | 0.998 | 165.8 | 0.999 | ||
| 0.3 | 106.9 | 0.954 | 105.0 | 0.964 | 0.3 | 386.8 | 0.990 | 394.2 | 0.996 | ||
| 0.4 | 119.3 | 0.976 | 117.4 | 0.984 | 0.4 | 257.5 | 0.992 | 282.4 | 0.997 | ||
| 0.5 | 134.8 | 0.969 | 133.4 | 0.978 | 0.5 | 261.0 | 0.952 | 264.3 | 0.964 | ||
| 0.6 | 164.9 | 0.975 | 177.0 | 0.985 | 0.6 | 269.7 | 0.961 | 282.6 | 0.974 | ||
| 0.7 | 197.2 | 0.971 | 197.6 | 0.980 | 0.7 | 292.3 | 0.968 | 296.0 | 0.978 | ||
| 0.8 | 202.9 | 0.988 | 215.1 | 0.975 | 0.8 | 190.6 | 0.982 | 198.9 | 0.990 | ||
| 0.9 | 178.7 | 0.985 | 190.5 | 0.970 | 0.9 | 75.5 | 0.934 | 79.6 | 0.966 | ||
| Average | 142.1 | 145.5 | Average | 214.9 | 221.7 | ||||||
| 50A50WT | 0.1 | 33.3 | 0.978 | 31.0 | 0.984 | 75A25WT | 0.1 | 34.4 | 0.986 | 32.4 | 0.991 |
| 0.2 | 42.0 | 0.999 | 39.2 | 0.995 | 0.2 | 45.0 | 0.987 | 42.8 | 0.993 | ||
| 0.3 | 67.9 | 0.999 | 65.2 | 0.995 | 0.3 | 55.8 | 0.982 | 53.5 | 0.989 | ||
| 0.4 | 85.0 | 0.981 | 82.2 | 0.966 | 0.4 | 71.9 | 0.951 | 69.7 | 0.960 | ||
| 0.5 | 79.9 | 0.999 | 76.8 | 0.999 | 0.5 | 73.6 | 0.955 | 71.1 | 0.964 | ||
| 0.6 | 134.3 | 0.990 | 132.8 | 0.996 | 0.6 | 107.6 | 0.964 | 105.8 | 0.973 | ||
| 0.7 | 159.4 | 0.997 | 158.0 | 0.992 | 0.7 | 98.3 | 0.999 | 95.3 | 0.999 | ||
| 0.8 | 196.0 | 0.978 | 196.0 | 0.986 | 0.8 | 125.1 | 0.968 | 122.7 | 0.976 | ||
| 0.9 | 68.0 | 0.993 | 62.6 | 0.997 | 0.9 | 49.2 | 0.967 | 42.3 | 0.968 | ||
| Average | 96.2 | 93.8 | Average | 73.5 | 70.6 | ||||||
| 75A25WT | 0.1 | 3.3 | 0.982 | 1.2 | 0.970 | A | 0.1 | 36.5 | 0.954 | 34.3 | 0.964 |
| +C | 0.2 | 25.9 | 0.960 | 23.3 | 0.963 | 0.2 | 85.3 | 0.962 | 84.8 | 0.972 | |
| 0.3 | 27.1 | 0.994 | 23.9 | 0.998 | 0.3 | 100.1 | 0.977 | 99.6 | 0.985 | ||
| 0.4 | 37.9 | 0.976 | 34.2 | 0.980 | 0.4 | 148.9 | 0.996 | 149.3 | 0.999 | ||
| 0.5 | 43.8 | 0.967 | 40.2 | 0.972 | 0.5 | 136.1 | 0.976 | 135.9 | 0.985 | ||
| 0.6 | 60.4 | 0.979 | 56.8 | 0.985 | 0.6 | 174.4 | 0.974 | 175.5 | 0.983 | ||
| 0.7 | 91.7 | 0.995 | 88.3 | 0.988 | 0.7 | 302.3 | 0.976 | 307.2 | 0.985 | ||
| 0.8 | 93.6 | 0.998 | 89.4 | 0.992 | 0.8 | 250.7 | 0.990 | 252.3 | 0.996 | ||
| 0.9 | 93.6 | 0.975 | 87.1 | 0.981 | 0.9 | 91.8 | 0.999 | 85.7 | 0.999 | ||
| Average | 53.1 | 49.4 | Average | 147.4 | 147.2 | ||||||
| 25A75WT | 50A50WT | 75A25WT | |
|---|---|---|---|
| FWO | 143.5 | 144.8 | 146.0 |
| KAS | 146.0 | 146.4 | 146.8 |
| WT | 24.235 | 0.995 |
| A | 4.257 | 0.989 |
| 25A75WT | 17.717 | 0.993 |
| 50A50WT | 7.108 | 0.992 |
| 75A25WT | 3.982 | 0.988 |
| 75A25WT+C | 1.754 | 0.991 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shujaa Aldeen, A.; Wang, J.; Zhang, B.; Tian, S.; Xu, Z.; Zhang, H. Investigation of Synergistic Effects and Kinetics on Co-Pyrolysis of Alternanthera philoxeroides and Waste Tires. Int. J. Environ. Res. Public Health 2022, 19, 7101. https://doi.org/10.3390/ijerph19127101
Shujaa Aldeen A, Wang J, Zhang B, Tian S, Xu Z, Zhang H. Investigation of Synergistic Effects and Kinetics on Co-Pyrolysis of Alternanthera philoxeroides and Waste Tires. International Journal of Environmental Research and Public Health. 2022; 19(12):7101. https://doi.org/10.3390/ijerph19127101
Chicago/Turabian StyleShujaa Aldeen, Awsan, Jiapeng Wang, Bo Zhang, Shuying Tian, Zhixiang Xu, and Huiyan Zhang. 2022. "Investigation of Synergistic Effects and Kinetics on Co-Pyrolysis of Alternanthera philoxeroides and Waste Tires" International Journal of Environmental Research and Public Health 19, no. 12: 7101. https://doi.org/10.3390/ijerph19127101
APA StyleShujaa Aldeen, A., Wang, J., Zhang, B., Tian, S., Xu, Z., & Zhang, H. (2022). Investigation of Synergistic Effects and Kinetics on Co-Pyrolysis of Alternanthera philoxeroides and Waste Tires. International Journal of Environmental Research and Public Health, 19(12), 7101. https://doi.org/10.3390/ijerph19127101






